Summary
We report the generation and initial characterization of a mouse line expressing tamoxifen-inducible improved Cre (iCre) recombinase (iCre-ERT2) under the regulation of NPHS2 (podocin) gene promoter. The resulting transgenic mouse line was named podocin-iCreERT2 mice. The efficiency of iCre activity was confirmed by crossing podocin-iCreERT2 with the ROSA26 reporter mouse. By using the floxed ROSA reporter mice, we found that tamoxifen specifically induced recombination in the kidneys. In the absence of tamoxifen, recombination was undetectable in podocin-iCreERT2;ROSA26 mice. However, following intraperitoneal injection of tamoxifen, selective recombination was observed in the podocytes of adult animals. We further examined the efficiency of recombination by assessing various tamoxifen exposure regimens in adult mice. These results suggest that podocin-iCre-ERT2 mouse provides an excellent genetic tool to examine the function of candidate genes in podocytes in a spatially and temporally-restricted manner.
Keywords: kidneys, podocin, transgenic mice, Cre-LoxP, ROSA26
Several groups have successfully engineered the generation of podocyte-specific Cre-recombinase lines (Belteki et al., 2005; Bugeon et al., 2003; Eremina et al., 2002; Juhila et al., 2006; Moeller et al., 2003, 2005; Shigehara et al., 2003). Most notably, regulatory elements from the human NPHS2 promoter were used to drive tissue specific Cre recombinase expression in podocytes (Moeller et al., 2003, 2005). Although this model has been useful for tissue-specific gene deletion studies in podocytes, it does not provide for a temporal-restricted expression of the targeted gene, an important disadvantage which has led to its limited use in studying the role of podocytes in pathological conditions of the kidney.
In contrast to bigenic systems for obtaining control over time and tissue of inactivation (Metzger and Chambon, 2001; Sauer, 1998) and overcoming the constitutively on activity of Cre itself (Nagy, 2000), the use of estrogen receptor ligand-binding domain (ERT2) has provided ligand-dependent transient labeling or inactivation capability, recombining either reporter alleles or conditional null or hypomorphic alleles, respectively (Danielian et al., 1993; Feil et al., 1997).
Because we were interested to examine the role of podocytes in pathological conditions in adult mice, we generated a transgenic mouse that expresses Cre recombinase only in podocytes and only following treatment with tamoxifen. To enhance the expression level of Cre recombinase, we used the improved Cre (iCre) gene with a mammalianized codon usage (Shimshek et al., 2002) instead of the prokaryotic Cre to improve Cre recombination efficiency.
To generate the podocin-iCreERT2 recombinase transgenic mouse, we placed the iCreERT2 expression cassette under the regulatory control of the human NPHS2 promoter fragment (podocin) (Fig. 1). We assessed the activity of podocin-iCreERT2 expression cassette by transient transfection of podocyte cell lines with an iCre-responsive LacZ reporter. To this end, CMV-LoxP-Stop-LoxP-LacZ plasmid (CMV-LSL-LacZ), a floxed LacZ reporter plasmid, harboring 3.2 kb LacZ gene was generated (Fig. 2, Panel a). Following transfection of differentiated podocytes with CMV-LSL-LacZ and podocin-iCreERT2, LacZ staining was found in the podocyte cell line only in the presence of tamoxifen (Fig. 2, Panel b).
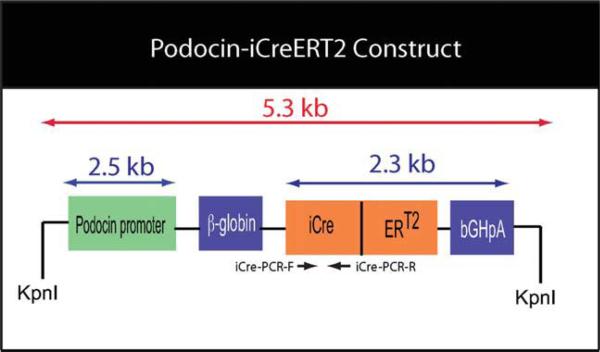
FIG. 1.
Schematic diagram of the podocin-iCreERT2 construct. Human podocin promoter was inserted in the upstream of an iCre-ERT2 expression cassette, which also contained an intron derived from the rabbit β-globin and a bovine growth hormone poly A (bGHpA) sequence. PCR primers, iCre-PCR-F (forward) and iCre-PCR-R (reverse), were used to determine the presence of the iCre transgene in mice.
FIG. 2.
(a) Schematic diagram of the CMV-LoxP-Stop-LoxP-LacZ (CMV-LSL-LacZ) construct. (b) The podocin-iCre-ERT2 construct was tested in podocytes in vitro by using CMV-LSL-LacZ, a floxed LacZ reporter. Podocytes were exposed to tamoxifen for 16 h and evaluated by β-gal staining. (I) control nontransfected podocytes, (II) transfected podocytes with podocin-iCre-ERT2 construct, (III) podocytes transfected with both podocin-iCre-ERT2 and LacZ floxed constructs but only exposed to vehicle for 16 h (IV) podocytes transfected with both constructs and exposed to tamoxifen (100 nM) for 16 h. β-gal activation is manifested by bright blue staining.
Transgenic mice were subsequently generated by microinjection of podocin-iCreERT2 cassette into the pronuclei of fertilized eggs of C57BL/6 mice. The micro-injected eggs were then transferred to pseudopregnant mothers to generate podocin-iCreERT2 founder mice. We identified four founder mouse lines carrying the transgene by polymerase chain reaction (PCR) genotyping. Three founders successfully transmitted the transgene to their offspring after breeding with wild-type mice. These founders were selected for further characterization.
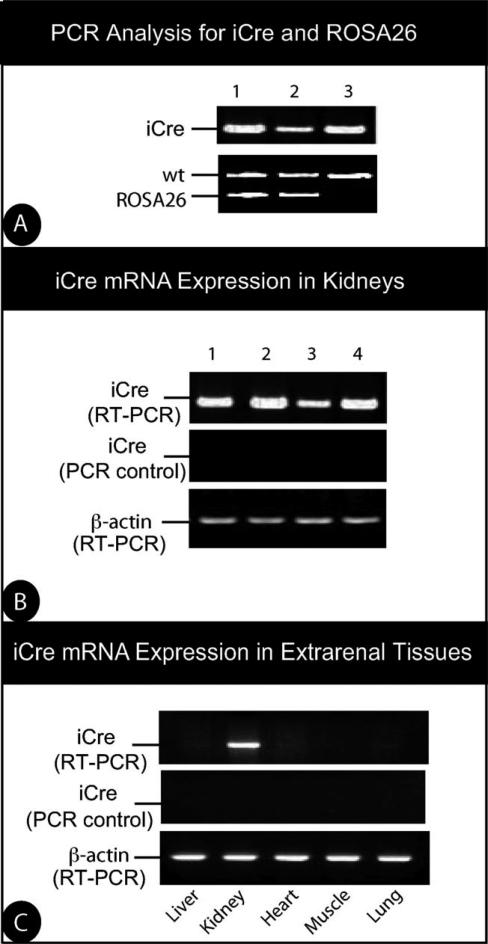
To test the efficiency and specificity of these lines, we crossed podocin-iCreERT2 animals with the ROSA26 reporter mice (R26R) (Soriano, 1999). Breeding of the mice carrying the podocin-iCreERT2 transgene with R26R mice produced podocin-iCreERT2;R26R mice. Mice resulting from these crosses had expected sized litters, mendelian frequency, and showed no signs of embryonic or adult abnormalities. Genotype identification was performed by PCR for both iCre and R26R transgenes in the podocin-iCreERT2;R26R double transgenic mice (Fig. 3, Panel a). RT-PCR of iCre indicated that mice from these lines expressed iCre mRNA in their kidneys (Fig. 3, Panel b). Importantly, RT-PCR analysis of extrarenal tissues collected from these animals did not detect iCre mRNA in the tissues analyzed (Fig. 3, Panel c).
FIG. 3.
iCre expression in kidneys of transgenic mice. (a) PCR analysis of genomic DNA isolated from the tail of 6-week-old podocin-iCreERT2 transgenic mice crossed with R26R mice. Each lane represents DNA from a different mouse. The upper gel shows genotyping for podocin-iCre-ERT2. The bottom gel exhibits genotyping for the presence of ROSA26 transgene. (b) RT-PCR for iCre in the kidneys treated with DNase I. PCR reactions were performed using templates from RT reactions with or without reverse transcriptase to control for DNA contamination (PCR control). Each lane represents DNA from a different mouse. Amplification of β-actin mRNA served as an additional control. (c) RT-PCR of iCre mRNA expression in extrarenal tissues from transgenic mice. Kidney tissue was used as control.
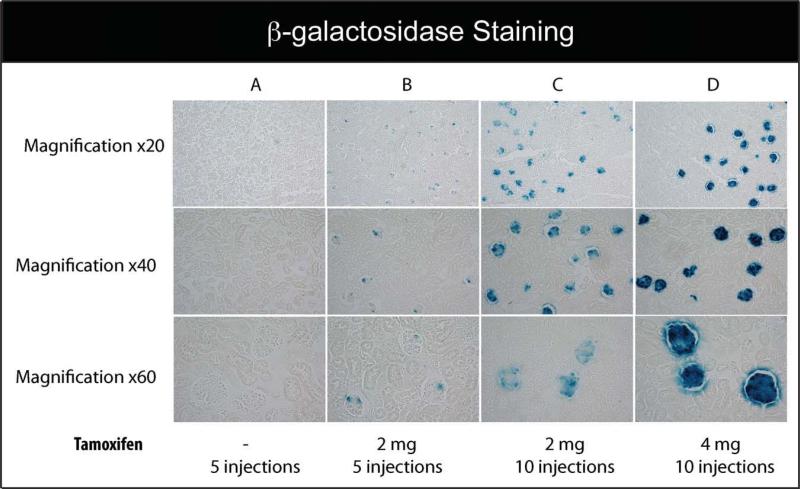
To assay for tamoxifen-dependent induction of podocin-iCreERT2, tamoxifen was administrated to double transgenic mice. Tamoxifen administration was initiated in 8–10 week old mice. A single intraperitoneal (ip) dose of tamoxifen in adult mice resulted, on average, in 10% excision by five days after injection (data not shown). Therefore, to gauge the relative efficiencies of iCre-mediated recombination in tamoxifen-treated mice, several doses of tamoxifen (1, 2, or 4 mg) with two different injection schemes were tested. The first scheme involved injection of tamoxifen ip for five consecutive days before sacrificing the mice. The second scheme involved 10 injections of tamoxifen prior to sacrificing the animals. As shown in Figure 4, we did not observe β-gal activity in the kidney sections of the control double transgenic mice treated with sesame oil (Fig. 4, Panel a). In contrast, kidney sections of the podocin-iCreERT2;R26R double transgenic mice treated with tamoxifen exhibited significant β-gal activity. Tamoxifen injection scheme 2, which involved 10 injections of tamoxifen, was more effective in inducing lacZ expression than scheme 1, which involved five injections of tamoxifen (Fig. 4, Panels b, c, and d).
FIG. 4.
Podocyte-specific LacZ activation in podocin-iCreERT2; R26R double transgenic mice was assessed using X-gal staining. (a) podocin-iCreERT2; R26R adult mice treated with vehicle (6–8 weeks old), (b) adult mice injected ip with tamoxifen (2 mg) for 5 days, (c) adult mice injected ip with tamoxifen (2 mg) for 10 days, and (d) adult mice injected ip with tamoxifen (4 mg) for 10 days. Kidneys were sectioned and stained for β-gal expression. Histological analysis for β-gal staining was evaluated five days after last ip injection. Several magnifications of the kidney sections with β-gal staining in the podocytes are shown with blue staining indicative of LacZ positive cells.
Since ROSA26 locus enables β-gal activity after iCre-mediated excision of the neo-cassette, detection of positive blue staining in tissues indicates that iCre recombinase (iCre-ERT2) is active in turning on the LacZ gene expression in R26R mice. Thus, to determine whether lacZ expression was specifically restricted to podocyte of the kidneys, we performed X-gal and nephrin staining on the same cryosection. Figure 5a indicates that β-gal staining corresponded to the nephrin positive cells.
FIG. 5.
(a) X-gal positive (blue) staining and nephrin positive (green) immunofluorescence staining were performed on the same cryosection. Nephrin positive cells corresponded to β-gal staining in Podocin-iCreERT2, R26R double transgenic mice. (b) Immunostaining for nephrin (brown) merged with whole mount X-gal staining of adult kidneys (light blue). Arrows point to prominent cells (dark blue) that are positive for both β-gal and nephrin.
To further confirm the specific targeting of podocytes, we also performed whole mount X-gal staining in the kidneys of double transgenic adult mice. Tamoxifen was administered to podocin-iCreERT2;R26R double transgenic mice, as previously described (2 mg/day/ mouse for a total of 10 injections). Animals were sacrificed and their kidneys were collected and analyzed by X-gal staining. Immunolocalization of X-gal positive sections with nephrin confirmed that, indeed, β-gal activity colocalizes with nephrin staining (Fig. 5, Panel b). Therefore, these results suggest that we have successfully generated a podocin-iCreERT2 transgenic mouse line that will be useful for achieving podocyte-specific deletion of the floxed genes of interest in a temporal and spatial-restricted manner.
Taken together, we report the generation of a line of transgenic mice expressing iCreERT2 under the control of the podocin promoter. By crossing the podocin-iCreERT2 transgenic mice to R26R reporter mice, we were able to demonstrate that podocin-iCreERT2 transgenic mice can be used to delete the floxed pGK-neo cassette upstream of the LacZ gene, turning on the LacZ reporter gene in podocytes only when tamoxifen treatment was administered, providing a spatiotemporal-restricted control of podocin-iCreERT2 transgenic mice. The efficiency of different doses of tamoxifen used in this study is in keeping with 5–10 injections in the range of 0.5–2 mg tamoxifen/mouse/day reported in previous studies (Kostetskii et al., 2005; Parlakian et al., 2005; Sohal et al., 2001). It is likely that at the lower doses and with fewer days of tamoxifen treatment, the tamoxifen was not able to “penetrate” all cells to activate the estrogen receptor-fused iCre-ERT2, without which the iCreERT2 could not enter nucleus to initiate the loxP recombination. As the dose and frequency of tamoxifen treatment increased, more podocytes exhibited iCreERT2-mediated loxP recombination, as manifested by a significant increase in the intensity of β-galactosidase staining.
In conclusion, the podocin-CreERT2 transgenic mice generated in this study will provide a tamoxifen-inducible form of the iCre-ERT2 that operates exclusively in podocytes. This mouse model will present an invaluable and simple tool in studying gene function in the podocytes of the kidney since it confers control over both spatial and temporal expression of gene products in the kidney using a simple and direct approach, and ultimately will allow to define the physiological and pathological roles of a variety of genes expressed in podocytes.
METHODS
The p2.5Podocin PE plasmid containing a 2.5 kb of genomic DNA sequence of human NPHS2 (podocin) gene promoter was a gift from Dr. Lawrence Holzman (University of Michigan), (Moeller et al., 2003). The floxed ROSA26 reporter mice were kind gifts from Dr. Austin J. Cooney (Baylor College of Medicine), and podocyte cell line was a kind gift of Dr. Jochen Reiser (University of Miami). The purified construct DNA was introduced into the pronuclei of fertilized oocytes from the C57BL/6 mouse through the service of Genetically Engineered Mouse Core Facility at the Baylor College of Medicine. All animal studies were conducted according to the “Principles of Laboratory Animal Care” (NIH publication No. 85023, revised 1985) and the guidelines of the IACUC of Baylor College of Medicine.
Podocin-iCreERT2 Construct
The NPHS2 promoter region was released from the original p2.5PodocinPE plasmid by restriction digestion with NotI and SfoI. The iCre coding sequences (1.1 kb) were obtained from plasmid CC10-iCre (a gift from Dr. Franco DeMayo, Baylor College of Medicine) by digestion with Hind III and Xho I and then inserted into pBluescriptKS, digested with the same enzymes (Bridges et al., 2008; Chen et al., 2007; Lan et al., 2004). The ERT2 and bovine growth hormone polyA (bGHpA) sequences were obtained by digesting the plasmid PNAPP-CreERT2 (a gift from Dr. Ron Davis, Baylor College of Medicine) with Xho I, followed by purification of a 1.2 kb band, and ligation into the iCre/pBS plasmid that had been linearized by digestion with Xho I, resulting in an inframe fusion of the iCre and ERT2 coding sequences. A short human synapsin promoter and rabbit β-globin intron were inserted as a 1 kb EcoR I fragment upstream from the iCre-ERT2 sequences using the unique EcoRI site. The synapsin promoter was removed by digestion with Not I and BamHI (blunt-ended) and replaced with the 2.5 kb NPHS2 promoter (obtained using a Not I and Sfo I digestion) to create the podocin-iCreERT2 construct (Fig. 1). The nucleotide sequences of the transgene vector, podociniCreERT2, were confirmed by sequence analysis (Baylor College of Medicine Sequencing Core). The primer positions for genotyping transgenic mice are indicated by arrows (Fig. 1).
CMV-LoxP-Stop-LoxP-LacZ Construct
LacZ gene was digested with SalI/HindIII and released from pShuttle-CMV-LacZ plasmid (Stratagene, CA). Following its release, LacZ gene was ligated into a SalI/HindIII fragment of the expression vector pRK5 (Genentech, CA) to make the intermediate construct pRK5-LacZ. To construct the remaining part of the vector, a 5.7 kb LoxP-STOP-LoxP expression cassette was digested with SalI and released from Lox-STOP-Lox-TOPO plasmid (Addgene, MA). LoxP-STOP-LoxP was inserted into the unique SalI site of the intermediate vector pRK5-LacZ, resulting in plasmid CMV-LoxP-Stop-LoxP-LacZ. After orientation screening by digestion, the final construct was confirmed by sequencing (Baylor College of Medicine Sequencing Core).
Cell Culture and Transfection
Conditionally immortalized mouse podocytes were cultured as previously described (Mundel et al., 1997). For these studies, differentiated mouse podocytes were grown to 70% confluency on collagen-I-coated dishes. The cells were transfected with 2 μg of LoxP-STOP-LoxP and 2 μg of podocin-iCreERT2 using Lipofectamine 2000 (Invitrogen, CA) according to manufacturer's protocol. Twenty-four hours after transfection, cells were treated with 100 nM tamoxifen (Sigma, MO). Staining was carried out using X-Gal Staining Kit (Genlantis, CA), as instructed by the manufacturer.
Identification of Transgenic Mice
Tail biopsies from 3-week-old mice were obtained, and genomic DNA was isolated by using REDExtract-N-Amp Kit (Sigma, MO). We identified transgenic mice using PCR with the following primers: iCre-Forward: 5′-TCAAC ATGCTGCACAGGAGAT-3′ and iCre-Reverse: 5′-ACCATAGATC AGGCGGTGGGT 3′. PCR reactions were carried out with an initial denaturation of 94°C for 4 min, followed by 30 cycles of reaction (94°C, 30 s; 57°C, 30 s; 72°C, 1 min) with a final extension of 72°C for 10 min. We typically used 10–50 ng of genomic DNA as template per reaction. The expected PCR product was a 500 bp DNA fragment.
Tamoxifen Treatment
Tamoxifen (Sigma, T5648) was prepared in sesame oil at a concentration of 10 mg/ml for intraperitoneal (i.p.) injection. We tested three different dosages (1, 2, or 4 mg) to gauge the relative efficiencies of iCre-mediated recombination in treated mice.
β-Galactosidase Staining
Mice were anesthetized, and whole body fixation was performed by intracardial perfusion with ice-cold 1.5% paraformaldehyde in Phosphate Buffered Saline followed by 20% sucrose in PBS. The kidneys were removed, embedded in Optimal Cutting Temperature, frozen in dry ice, and cut into 10 μm cryosections. Cryosections were postfixed with 4% paraformaldehyde in PBS (pH 7.8) for 5 min and incubated overnight at 30°C in staining solution containing 1 mg/ml 5-bromo-4-chloro-3-indoyl β-d-galactopyranoside (X-Gal; Sigma), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS (pH 7.8). The sections were mounted and examined under a Nikon Eclipse 80i microscope.
Immunofluorescent Staining
Sections were fixed as previously described. Immunostaining for nephrin was carried out with goat anti-nephrin polyclonal antibody (1:100 dilution, R&D, MN) over night. After washing, sections were incubated with Alexa Fluor 594 conjugated donkey anti-goat IgG (1:200 dilution; Molecular Probes, OR). A Nikon Eclipse 80i microscope equipped with epifluorescent optics was used for fluorescence microscopy.
Whole-Mount X-Gal Staining of Kidneys
After fixation, kidneys were removed and postfixed at 4°C in 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM Ethylenediaminetetraacetic acid in PBS, washed three times for 30 min at 37°C with detergent buffer (0.01% sodium deoxycholate, 0.02% Nonidet P-40, 2 mM MgCl2 in PBS, pH 7.3), and then stained overnight in detergent buffer containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal. X-gal stained samples were washed twice in PBS and postfixed overnight in 1% paraformaldehyde at 4°C. After washes with PBS, samples were embedded in paraffin wax and 5 μm sections were prepared. For double staining with nephrin, X-gal stained sections were incubated with goat anti-nephrin polyclonal antibody (1:100 dilution, R&D, MN). Immunohistochemical analysis was performed by using the VectaStain ABC kit (Vector Laboratories, CA).
ACKNOWLEDGMENTS
The authors would like to thank Dr. Lawrence Holzman (University of Philadelphia) for his kind gift of Podocin promoter, and Dr. Austin J. Cooney (Baylor College of Medicine) for providing us with ROSA26R mice. They also thank Dr. Francesco J. DeMayo and the transgenic core laboratory at Baylor College of Medicine for DNA microinjection.
Contract grant sponsor: NIDDK; Contract grant numbers: RO1DK067604; RO1DK078900; Contract grant sponsor: Department of Veterans Affairs (CDA grant)
Abbreviations
- bGHpA
bovine growth hormone polyA
- CMV-LSL-LacZ
CMV-LoxP-Stop-LoxP-LacZ plasmid
- iCre
improved Cre
- iCre-ERT2
improved Cre recombinase
- PCR
polymerase chain reaction
- OCT
optimal cutting temperature
- PBS
phosphate buffered saline
- EDTA
ethylenediaminetetraacetic acid
LITERATURE CITED
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges PJ, Koo Y, Kang DW, Hudgins-Spivey S, Lan ZJ, Xu X, DeMayo F, Cooney A, Ko C. Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor alpha (Esr1) from the ovary and testis. Genesis. 2008;46:499–505. doi: 10.1002/dvg.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeon L, Danou A, Carpentier D, Langridge P, Syed N, Dallman MJ. Inducible gene silencing in podocytes: A new tool for studying glomerular function. J Am Soc Nephrol. 2003;14:786–791. doi: 10.1097/01.asn.0000050222.86847.ea. [DOI] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu T-J, Xie C, Zhang X, O'Keefe RJ, Chen D. Generation of a transgenic mouse model with chondrocytespecific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P, White R, Hoare S, Fawell S, Parker M. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7:232–240. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE. Glomerularspecific gene excision in vivo. J Am Soc Nephrol. 2002;13:788–793. doi: 10.1681/ASN.V133788. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Juhila J, Roozendaal R, Lassila M, Verbeek SJ, Holthofer H. Podocyte cell-specific expression of doxycycline inducible Cre recombinase in mice. J Am Soc Nephrol. 2006;17:648–654. doi: 10.1681/ASN.2005050547. [DOI] [PubMed] [Google Scholar]
- Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- Lan Z-J, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Moeller M, Sanden S, Soofi A, Wiggins R, Holzman L. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Sanden S, Floege J, Kriz W, Holzman LB. An efficient system for tissue-specific overexpression of transgenes in podocytes in vivo. Am J Physiol Renal Physiol. 2005;289:F481–F488. doi: 10.1152/ajprenal.00332.2004. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson G, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: The universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Parlakian A, Charvet C, Escoubet B, Mericskay M, Molkentin JD, Gary-Bobo G, De Windt LJ, Ludosky M-A, Paulin D, Daegelen D, Tuil D, Li Z. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation. 2005;112:2930–2939. doi: 10.1161/CIRCULATIONAHA.105.533778. [DOI] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, H̨bner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]