Abstract
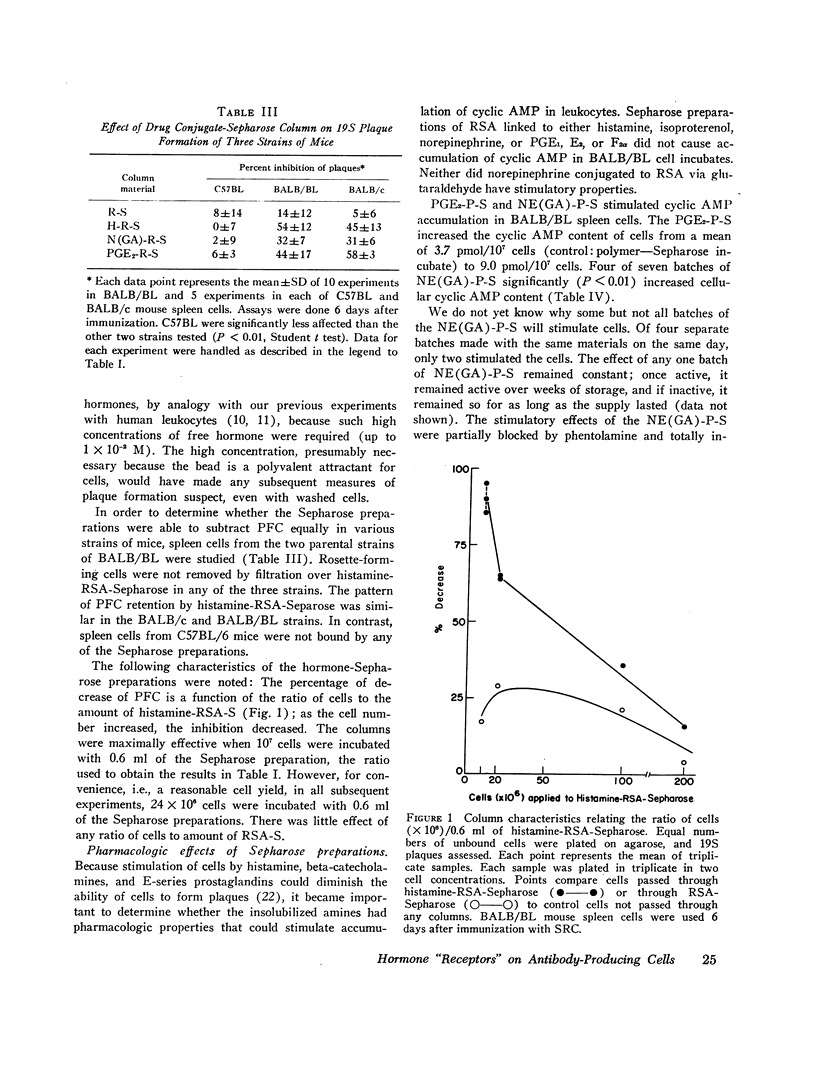
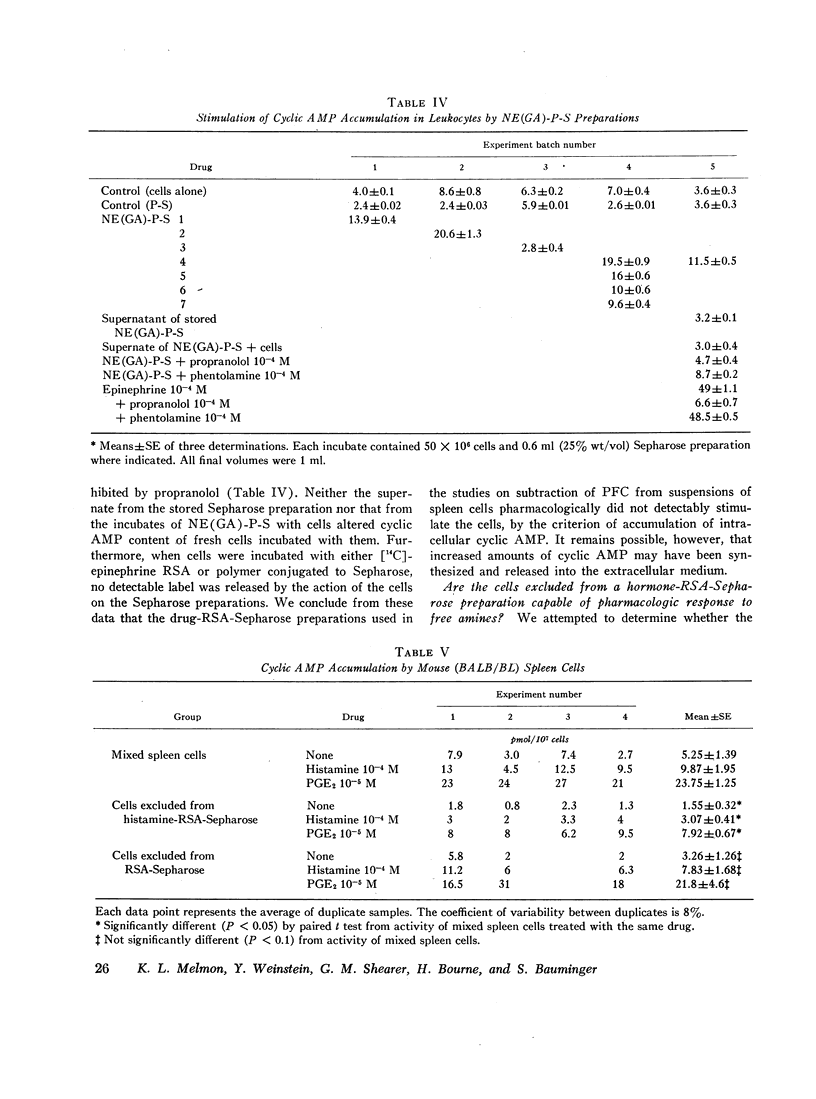
Spleen cells from mice immunized with sheep red cells were separated by differential adherence to insolubilized histamine, catecholamines, and prostaglandins. The hormones were insolubilized by linking them to Sepharose beads through a protein carrier. We measured hemolytic plaque formation (per million splenic leukocytes) of cells which passed through columns of hormone-carrier-Sepharose beads (i.e., those cells that failed to bind). As compared with control (no column) cells, the number of plaque-forming cells was substantially reduced by passage through histamine, epinephrine, isoproterenol, and prostaglandin-E2 columns. Plaque-forming cells were not significantly reduced by passage through carrier Sepharose (another control) or norepinephrine- and prostaglandin-F2α-carrier Sepharose columns. Thus, the ability of an insolubilized hormone preparation to subtract plaque-forming cells roughly correlated with the presence of pharmacologic receptors for the corresponding free hormones, as judged by stimulation of cyclic AMP accumulation in the same cells, reported previously. Both 19S and 7S plaque-forming cells were subtracted by columns prepared from pharmacologically active hormones, but none of the insolubilized hormones stimulated accumulation of intracellular cyclic AMP. The cell membrane phenomenon that allows adherence to a given hormone-carrier-bead column may be identical with the cell receptor.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Schild H. O. Inhibition by sympathomimetic amines of histamine release by antigen in passively sensitized human lung. Nature. 1969 Dec 6;224(5223):1028–1029. doi: 10.1038/2241028a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3',5'-adenosine monophosphate in human leukocytes. J Immunol. 1972 Mar;108(3):695–705. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L. Adenyl cyclase in human leukocytes: evidence for activation by separate beta adrenergic and prostaglandin receptors. J Pharmacol Exp Ther. 1971 Jul;178(1):1–7. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L., Lichtenstein L. M. Histamine augments leukocyte adenosine 3',5'-monophosphate and blocks antigenic histamine release. Science. 1971 Aug 20;173(3998):743–745. doi: 10.1126/science.173.3998.743. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Henney C. S., Lichtenstein L. M. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971 Aug;107(2):610–612. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Orange R. P., Austen K. F. Pharmacologic inhibition of the antigen-induced release of histamine and slow reacting substance of anaphylaxis (SRS-A) from monkey lung tissues mediated by human IgE. J Immunol. 1971 May;106(5):1267–1273. [PubMed] [Google Scholar]
- Kooman W. J., Orange R. P., Austen K. F. Immunochemical and biologic properties of rat IgE. 3. Modulation of the IgE-mediated release of slow-reacting substance of anaphylaxis by agents influencing the level of cyclic 3',5'-adenosine monoposphate. J Immunol. 1970 Nov;105(5):1096–1102. [PubMed] [Google Scholar]
- Leskowitz S., Jones V. E., Zak S. J. Immunochemical study of antigenic specificity in delayed hypersensitivity. V. Immunization with monovalent low molecular weight conjugates. J Exp Med. 1966 Feb 1;123(2):229–237. doi: 10.1084/jem.123.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein J., Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972 Aug 25;177(4050):707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Yaron A., Ben-Efraim S., Sober H. A. Immunogenicity of a series of alpha,N-DNP-L-lysines. Biochemistry. 1965 Aug;4(8):1638–1645. doi: 10.1021/bi00884a028. [DOI] [PubMed] [Google Scholar]
- Segal S., Globerson A., Feldman M., Haimovich J., Givol D. Specific blocking in vitro of antibody synthesis by affinity labelling reagents. Nature. 1969 Sep 27;223(5213):1374–1375. doi: 10.1038/2231374a0. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G. Cluster formation in vitro by mouse spleen cells and sheep erythrocytes. J Immunol. 1968 Dec;101(6):1264–1270. [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G., Connell M. S., Priore R. L. Cellular differentiation of the immune system of mice. I. Separate splenic antigen-sensitive units for different types of anti-sheep antibody-forming cells. J Exp Med. 1968 Sep 1;128(3):437–457. doi: 10.1084/jem.128.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Melmon K. L., Weinstein Y., Sela M. Regulation of antibody response by cells expressing histamine receptors. J Exp Med. 1972 Nov 1;136(5):1302–1307. doi: 10.1084/jem.136.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulica A., Haimovich J., Sela M. Detection of antibody-like structures on cell surfaces with chemically modified bacteriophages. J Immunol. 1971 Mar;106(3):721–731. [PubMed] [Google Scholar]
- Truffa-Bachi P., Wofsy L. Specific separation of cells on affinity columns. Proc Natl Acad Sci U S A. 1970 Jul;66(3):685–692. doi: 10.1073/pnas.66.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Melmon K. L., Bourne H. R., Sela M. Specific leukocyte receptors for small endogenous hormones. Detection by cell binding to insolubilized hormone preparations. J Clin Invest. 1973 Jun;52(6):1349–1361. doi: 10.1172/JCI107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. The relationship of antibody-forming cells to rosette-forming cells. Immunology. 1971 Aug;21(2):233–245. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]



