SUMMARY
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is an important cause of diarrhea, hemorrhagic colitis and hemolytic uremic syndrome in humans worldwide. The two major virulence determinants of EHEC are the Shiga toxins (Stx) and the type III secretion system (T3SS), including the injected effectors. Lack of a good model system hinders the study of EHEC virulence. Here, we investigated whether bovine and human intestinal xenografts in SCID mice can be useful for studying EHEC and host tissue interactions. Fully developed, germ-free human and bovine small intestine and colon were established by subcutaneous transplantation of human and bovine fetal gut into SCID mice. Xenografts were allowed to develop for 3–4 months and thereafter were infected by direct intraluminal inoculation of Stx-negative derivatives of EHEC O157:H7, strain EDL933. The small intestine and colon xenografts closely mimicked the respective native tissues. Upon infection, EHEC induced formation of typical attaching and effacing lesions and tissue damage that resembled hemorrhagic colitis in colon xenografts. By contrast, xenografts infected with an EHEC mutant deficient in T3SS remained undamaged. Furthermore, EHEC did not attach to or damage the epithelium of small intestinal tissue, and these xenografts remained intact. EHEC damaged the colon in a T3SS-dependent manner, and this model is therefore useful for studying the molecular details of EHEC interactions with live human and bovine intestinal tissue. Furthermore, we demonstrate that Stx and gut microflora are not essential for EHEC virulence in the human gut.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) is an emerging zoonotic pathogen that causes acute human gastroenteritis and hemorrhagic colitis (Kaper et al., 2004). In addition, it is associated with Shiga toxins (Stx), which can cause systemic complications including hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP), which can affect the kidneys and the central nervous system, and even cause death (Tarr et al., 2005). EHEC also causes disease in newborn calves and asymptomatically colonizes the gut mucosa of adult bovines, constituting the main reservoir for food and environmental contamination (Chase-Topping et al., 2008). In the infected epithelia, EHEC elicits a histopathology termed attaching and effacing (AE) lesions. This includes intimate attachment of the bacteria to the apical surface of the epithelial cells, disruption of the brush border microvillus, and accumulation of polymerized actin beneath the attached bacteria forming structures termed ‘actin pedestals’ (Kaper et al., 2004). EHEC contains a chromosomal pathogenicity island, termed the locus of enterocyte effacement (LEE), which is essential for virulence and required for formation of AE lesions (Spears et al., 2006). The LEE encodes a type III protein secretion system (T3SS), which is a syringe-like apparatus composed of ∼25 different proteins and hundreds of subunits. The T3SSs are used by EHEC to translocate (inject) protein effectors directly from the cytoplasm of the pathogen into the cytoplasm of the eukaryotic host cell. The delivered effectors subvert specific host–signaling pathways that have a central role in colonization of the host and in provoking the disease. One of these effectors, Tir, transverses the host cell membrane and forms a binding site to the bacterial adhesin intimin. The Tir–intimin interaction leads to intimate attachment and formation of the actin pedestal beneath attached bacteria (Croxen and Finlay, 2010).
Different results have been obtained in natural and model hosts, which raises the question of whether Stx is involved in inflammation and diarrhea in the xenograft models. In a piglet model, Stx was not essential for gut virulence (Tzipori et al., 1987). Similarly, epithelial adhesion and colonization of the bovine terminal rectal mucosa, which is currently considered the prime site for carriage and shedding, was unaffected by the absence of Stx (Sheng et al., 2006). By contrast, in an infant rabbit model, Stx increased the severity and duration of EHEC-induced diarrhea and purified Stx was able to induce inflammation and diarrhea (Ritchie et al., 2003).
Because mice are resistant to EHEC infection, other model system have been adopted, including infection of calves, piglets and young rabbits (Tzipori et al., 1995; Ritchie et al., 2003). An alternative approach is the use of animal pathogens and their corresponding native hosts as model systems. These include Citrobacter rodentium in mice (Mundy et al., 2007) and rabbit enteropathogenic E. coli (REPEC) in rabbits (Cantey et al., 1989). Nevertheless, there is a clear need for model systems that will allow investigation of the virulence properties of EHEC in the context of the complete human intestinal mucosa. In this study, we used the model of intestinal xenografts in SCID mouse (Savidge et al., 1995) to test the capacity of EHEC to interact with the mucosa of its native hosts: humans and calves. We found that human and bovine small intestine and colonic xenografts closely mimic the respective native tissues. Importantly, upon infection, EHEC induced typical AE lesions and tissue damage on the colonic xenografts, but not on the small intestinal epithelium, which remained intact. Accordingly, we developed an infection model that will allow analysis and comparison of the molecular details of EHEC interaction with human and bovine gut mucosa.
RESULTS
Region-specific morphogenesis and cytodifferentiation in intestinal xenografts
Human intestinal xenografts have been previously reported (Savidge et al., 1995; Golan et al., 2009). Bovine intestinal xenografts are described here for the first time. Initially, we tested whether human and bovine xenografts taken from different intestinal regions would develop into the corresponding mature tissues. Upon transplantation, both human and bovine fetal small intestine and colon displayed immature mucosa and villi were present throughout the intestine, including the colon (supplementary material Fig. S1A,B). Approximately 10 weeks after transplantation, the resulting mucosal architecture was entirely dependent upon the selected region of the fetal intestine (small intestine or colon) (supplementary material Fig. S1C,D). Typically, small intestinal xenografts displayed well-developed villi and crypts, whereas the colon displayed characteristic flat crypts (Figs 1 and 2; supplementary material Fig. S2). Furthermore, the xenografts were organized into all four distinct intestinal layers: mucosa (simple columnar epithelium, lamina propria and muscularis mucosa), submucosa, muscularis (both circular and longitudinal fibers), and serosa (Figs 1 and 2; supplementary material Figs S1, S2). These results indicate that the human and bovine xenografts undergo region-specific morphogenesis.
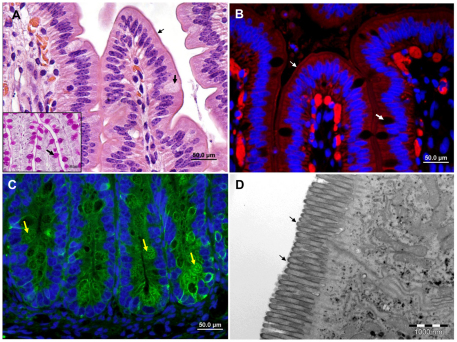
Fig. 1.
Bovine fetal small intestinal xenograft in SCID mice. Representative microscopic images of fully differentiated small intestinal xenografts. (A) H&E and PAS staining of formalin-fixed paraffin sections. (B,C) Fluorescence staining of cryosections with phalloidin-rhodamine (red, B) or anti-cytokeratin antibodies (green, C) combined with DAPI (blue). (D) TEM image of xenograft. Normal, fully developed villous (A,B) and crypt (C) mucosal epithelia are visible. Microvilli are clearly visible on the apical (luminal) border of the villous epithelial cells (A,B,D; small arrows), which are interspaced with a small number of goblet cells (A,B; large arrows) containing PAS-positive mucus (inset A; arrow). Crypts of the small intestinal mucosa with numerous Paneth cells (arrows) are shown in C. Scale bars: 50 μm (A-C); 1 μm (D).
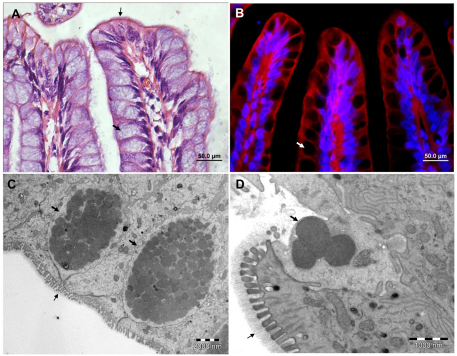
Fig. 2.
Bovine fetal colon xenograft in SCID mice. Representative microscopic images of H&E-stained formalin-fixed paraffin sections (A), fluorescence staining of cryosections with phalloidin-rhodamine (red) combined with DAPI (blue) (B) and TEM (C, D) of fully differentiated colon xenografts. Normal, fully developed colonic mucosa are composed of numerous goblet cells (large arrows) containing characteristic mucus granules (C,D) which are excreted through the luminal orifice of the cell (D). Microvilli are visible on the apical (luminal border) of the epithelial cells (A,C,D; small arrows) and are covered by the glycocalyx layer (D; small arrow). Scale bars: 50 μm (A,B); 2 μm (C); 1 μm (D).
We also examined epithelial cell differentiation and found that the developed xenografts display all major intestinal epithelial cell lineages: enterocytes, goblet cells, Paneth cells and enteroendocrine cells. Moreover, the cell distribution matched that observed in normal tissues (Figs 1 and 2; supplementary material Fig. S2). Enterocytes were abundant in the small intestine but not in the colon, and were a simple columnar shape. Goblet cells were more abundant in the colon, were periodic acid Schiff (PAS)-positive, and appeared as dark gaps in the immunofluorescent images (Figs 1 and 2; supplementary material Fig. S2). Paneth cells were seen exclusively in the small intestine with their secretory granules pointing towards the apical side of the cell and their nuclei towards the basal side (Fig. 1C; supplementary material Fig. S2C). Finally, enteroendocrine cells were seen at the base of the crypts with their secretory granules pointing towards the basal side of the cells (Fig. 1).
Taken together, our results indicate that the intestinal xenografts undergo region-specific morphogenesis and cytodifferentiation that closely resembles the corresponding native tissues. The region-specific morphogenesis and cytodifferentiation were highly reproducible and observed in over 300 examined xenografts. Similar results, using only human xenografts, have also been reported by Savidge and colleagues (Savidge et al., 1995).
Infecting EHEC do not damage or induce AE lesions in bovine or human small intestinal xenografts
We next tested whether the xenograft system can be exploited to study EHEC infection. We used strain 933-TUV, an EHEC O157:H7 derived from EDL933 by deletion of both stx1 and stx2 genes (Li et al., 2004). Cultures were injected directly into the lumen of bovine and human xenografts, and the xenografts were recovered at different time points after infection and processed for histological examination. We routinely implanted two xenografts (bovine or human) on each mouse back. Each xenograft was sampled from at least three sites and analyzed by microscopy (light, fluorescent and electron microscopy). Tissue damage was not detected in either small intestinal or colon xenografts recovered less than 6 hours after infection (12 small intestine and 12 colon xenografts were examined). However, at 8 hours after infection, we noted AE histopathology and considerable mucosal damage in the colon xenografts (see next section). By contrast, infected bovine or human small intestinal xenografts were indistinguishable from the corresponding PBS-injected negative controls (altogether 43 small intestinal xenografts were examined ≥8 hours after infection). The infected small intestinal xenografts displayed normal villous morphology and typical crypt architecture, and lacked any signs of inflammation. Typical examples are shown in Fig. 3 and in supplementary material Fig. S4. Similarly, the small intestinal tissue appeared unaffected, even upon prolonged (24 hour) infection (12 xenografts tested). In some cases, bacteria were found near the small intestinal epithelium, but these were never intimately attached (Fig. 3; supplementary material Fig. S3).
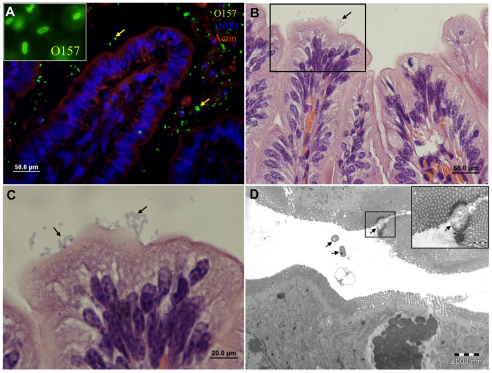
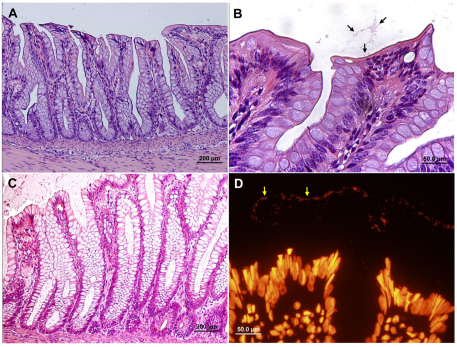
Fig. 3.
EHEC are not pathogenic in the bovine small intestine and only sparsely adhere to the villous epithelium. Representative microscopic images of tissue sections of bovine small intestinal xenografts in SCID mice 8 hours after intraluminal challenge with 4×107 organisms. Xenograft cryosection stained with anti-O157 antibody (green) combined with DAPI (blue) and phalloidin-rhodamine (red) (A), H&E stained formalin-fixed sections (B,C) and TEM image (D). Mucosal epithelium is intact and undamaged and most organisms are not attached to the mucosal epithelium (arrows in A and D), whereas adhering organisms (arrows in C, and D inset) do not induce the characteristic AE lesions. Scale bars: 50 μm (A,B); 20 μm (C); 2 μm (D).
A possible explanation for the lack of interaction between EHEC and the small intestinal cells is that the bacteria cannot grow or are even killed upon injection into the small intestinal xenografts, possibly via the accumulation of antibacterial peptides. However, microscopic analysis detected a large number of EHEC in the small intestinal lumen, suggesting that the bacteria proliferate in the xenograft (Fig. 3; supplementary material Fig. S4). To confirm this, we quantified the bacterial growth within the small intestinal lumen by extracting the organ and counting CFUs. The inoculated EHEC were found to be viable and to grow rapidly in the small intestinal xenografts (supplementary material Fig. S5). Taken together, our results indicate that EHEC do not interact with the small intestinal epithelium.
Infecting EHEC induce formation of AE histopathology and tissue damage in both human and bovine colon xenografts
In parallel to the small intestinal infection experiments, we carried out infection of large intestinal xenografts using identical infection conditions and the same bacterial strain. As mentioned earlier, formation of AE lesions or tissue damage was not detected in colon xenografts recovered less than 6 hours after infection (12 xenografts were examined, data not shown). However, at ≥8 hours after infection, we noted clear formation of AE lesions: 58 xenografts were examined in these experiments and typical results are shown in Figs 4–6. Upon prolonged infection (≥8 hours after challenge) we noted marked mucosal damage, in addition to the AE lesions, in 70% of the xenografts. This was characterized by swollen and pyknotic epithelial cells and hemorrhages, whereas the inflammatory response was surprisingly limited. Typical examples are shown in Figs 5 and 6. Notably, although EHEC exhibited rapid growth in the small intestine, growth in the bovine large intestine was poor (supplementary material Fig. S5). The reason for the slow growth in the colon xenografts is not clear. Nevertheless, this finding stresses the notion that the ability of EHEC to cause AE lesions and tissue damage in the colon is not simply due to better growth and higher viability in the colon xenografts. These results show that EHEC effectively colonizes the colon, inducing the formation of AE lesions and mucosal damage.
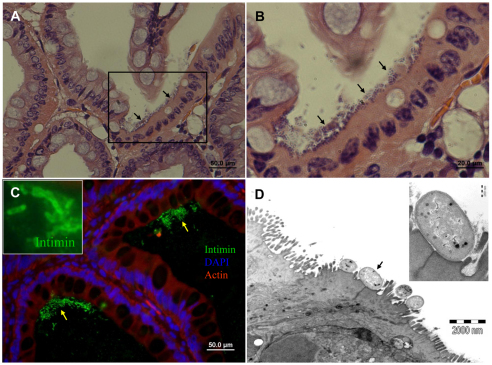
Fig. 4.
EHEC induce formation of AE lesions on bovine colon epithelial cells. Representative microscopic images of tissue sections of bovine colon xenografts in SCID mice 8 hours after intraluminal challenge with 4×107 organisms. H&E staining of formalin-fixed paraffin sections (A,B), fluorescence staining of cryosections with anti-intimin antibody (green) combined with DAPI (blue) and phalloidin-rhodamine (red) (C) and TEM (D). EHEC adhere to mucosal epithelial cells (A-C; arrows) expressing intimin (C and inset image) and induce formation of AE lesions (D). Pedestal formation under adhering bacterial cell (arrow) is demonstrated (D) and better seen in the enlarged inset image. Scale bars:50 μm (A,C); 20 μm (B); 2 μm (D).
Fig. 6.
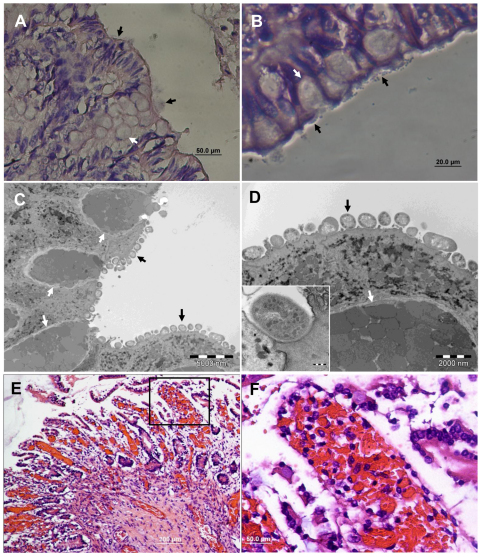
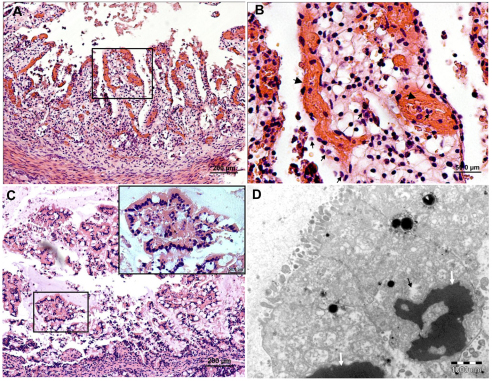
EHEC infecting human colon induce formation of AE lesions and tissue damage. Representative microscopic images of tissue sections of human colon xenografts in SCID mice 8 hours after intraluminal challenge with 4×107 organisms. H&E staining of formalin-fixed paraffin sections (A,B,E,F) and TEM (C,D). Bacteria adhere to mucosal epithelial cells (black arrows in A and B) and induce formation of AE lesions (B). AE lesions and pedestal formation under adhering bacterial cells (black arrows) are demonstrated (C) and better seen in the enlarged image (D and inset image). Goblet cells are indicated by white arrows in all images. Scale bars: 50 μm (A); 20 μm (B); 5000 nm (C); 2000 nm (D). Some colon segments showed severe epithelial damage and hemorrhages only sparsely infiltrated by leukocytes (E) and this is better seen in the boxed area enlarged in F. Scale bars: 200 μm (E); 50 μm (F).
Fig. 5.
Hemorrhagic colitis and epithelial damage induced by EHEC infection of the bovine colon. Microscopic images of tissue sections of bovine colon xenografts in SCID mice 8 hours after intraluminal challenge with 4×107 organisms. H&E staining of formalin-fixed paraffin sections (A–C) and TEM (D). Epithelial damage associated with hemorrhages (B, large arrows) and leukocyte infiltration (B, small arrows). In other infected colon xenografts, epithelial damage is not associated with signs of inflammation (C) and cells appear with condensed pyknotic nuclei, damaged membranes and cytoplasmic vacuolization (D, arrows). Scale bars: 200 μm (A,C); 50 μm (B); 1 μm (D).
EHEC-induced AE lesions and damage in colon xenografts are T3SS-dependent processes
In the experiments described above, we used an EHEC strain that was devoid of an active stx gene and thus the damage seen in the infected colon xenografts was independent of Stx. We next tested whether the induced damage was dependent on T3SS. We compared an EHEC strain expressing active T3SS (933-TUV, designated ‘wild type’) with an isogenic escN::kan mutant that cannot assemble functional T3SS. Both strains were injected at similar concentrations into bovine and human colon xenografts, and we used PBS-injected xenografts as a negative control. In contrast to damage seen in xenografts injected with wild-type EHEC, those injected with the escN mutant remained normal: they displayed typical crypt architecture that lacked any inflammatory infiltrate and showed no evidence of damage. Fifteen colon xenografts were infected with the escN mutant and typical results are shown in Fig. 7. The escN::kan bacteria were found mostly near the epithelium; they were rarely attached and were never intimately attached (Fig. 7). We did not detect any differences between xenografts injected with the escN mutants and the negative controls, which were injected with PBS (data not shown). Overall, these data suggest that EHEC-induced formation of AE lesions and damage to the colon mucosa are dependent on the expression of active T3SS.
Fig. 7.
EHEC-induced formation of AE lesions and tissue damage are T3SS-dependent processes. Representative microscopic images of tissue sections of bovine (A,B) and human (C,D) colon xenografts in SCID mice 8 hours after intraluminal challenge with 4×107 EHEC of a strain with deleted escN gene (encoding a T3SS-associated ATPase), which is unable to assemble a functional T3SS. H&E staining of formalin-fixed paraffin sections (A–C) and fluorescence staining of cryosections with Sytox Orange staining for nucleic acids (D). The bovine (A,B) and human (C,D) colon mucosa remained unaffected, showing normal mucosal architecture and nonadhering organisms in the lumen (B and D, arrows). Scale bars: 200 μm (A,C); 50 μm (B,D).
DISCUSSION
Studies on EHEC pathogenesis are hampered by a lack of suitable animal models that can fully recapitulate the natural disease in humans. To date, in vitro organ culture (IVOC) represents the gold standard model for studying EHEC infection of human intestinal mucosa. Although this system has provided important insight into in vivo colonization processes, it does not recapitulate the natural disease in the context of the live, intact and active gut. Here, we studied the association of EHEC with human gut xenografts implanted in SCID mice. Because of the importance of cattle in the epidemiology of EHEC, we also studied the association of EHEC with bovine xenografts. Although the human gut xenograft model has been described previously (Savidge et al., 1995; Golan et al., 2009), to the best of our knowledge, this is the first report of a bovine gut xenograft model.
We found that the human as well as bovine xenografts faithfully mimic the corresponding tissue of pediatric and calf intestine, respectively: they showed similar organization of the entire organ, including maintenance of embryonic mesenteric polarity and angiogenesis. The xenograft also showed similarity to the original tissue in levels of cytodifferentiation and cellular morphology. The epithelium of the small intestinal xenografts contained the typical cell repertoire, which consists of mainly enterocytes and a small number of goblet cells. In addition, smaller numbers of Paneth and endocrine cells were typically located in the villus crypt. By contrast, the epithelium of colon xenografts contained mainly goblet cells and exhibited typical shallow crypts and short microvilli. Thus, based on anatomy and histology, the xenografts were almost indistinguishable from the corresponding native human and bovine tissues.
One obvious difference between the native tissue and xenografts is the fact that the latter are sealed at both ends, are not connected to the gastrointestinal tract and are essentially devoid of normal microflora. Instead, we frequently found luminal cell debris and mucus in the xenografts. Although it was not attempted here, it should be possible to reconstruct the typical human flora in the xenograft by inoculation with the appropriate mix of microbes, thus creating a model that better mimics the native human intestine. Nevertheless, although the lack of microflora is a disadvantage for some experiments, in others it is actually an advantage, allowing experimentation in a germ-free intestine and thus eliminating possible microflora effects. For instance, in our study, the xenografts were essentially germ-free and thus all of the pathogenic effects on the tissue were directly related to EHEC, because microflora components were not involved. These results show that the presence of microflora is not essential for EHEC virulence, a conclusion that is also supported by a previous study using human IVOC (Chong et al., 2007).
A second obvious difference between the native tissue and the xenograft model in SCID mice is the lack of functional B- and T-cells and an adaptive immune response. Other than this immunodeficiency, SCID mice exhibit normal numbers and functions of nonlymphoid blood cells, including natural killer cells, macrophages and granulocytes. Several studies have demonstrated the innate immune response of the mouse to various enteric pathogens in the xenograft model (Zhang and Stanley, 2004; Golan et al., 2009). Although this model probably closely mimics the acute response to enteric pathogens, it cannot be used to study the adaptive immune response or for vaccine development. Nevertheless, the human or bovine immune systems can be fully reconstituted in this model using previously reported techniques (Shultz et al., 2007).
Inoculation of human and bovine colon xenografts with EHEC led to the formation of typical AE histopathology, which was widespread 8 hours after inoculation. EHEC infection of the colon was associated with tissue damage, which could be excessive, including frequent formation of AE lesions and in some cases, marked mucosal damage as well. Intriguingly, in most cases this mucosal damage was associated with an abnormally low inflammatory response. Notably, all of these phenotypes were strictly dependent on the expression of functional T3SS by the infecting EHEC and were independent of Stx production. Therefore, the xenograft model is expected to be an effective instrument for analyzing the function of T3SS effectors in native tissue. For instance, we recently reported that two of the EHEC T3SS effectors, NleB and NleE, possess anti-inflammatory activity (Nadler et al., 2010). We are now using the xenograft model to test whether these two effectors are involved in repressing inflammation in regions with damaged mucosal epithelium.
Significantly, although EHEC-induced damage and AE lesions were readily found in the colon, they were not seen in the small intestinal segments. Nevertheless, EHEC grew rapidly in the small intestinal xenografts, indicating that the small intestinal human xenografts permit EHEC survival and growth but not colonization. Our results are consistent with those showing that EHEC cannot colonize human small intestinal IVOC (Phillips et al., 2000). To the best of our knowledge, our data are the first to show adhesion of EHEC to the human colon, which has long been believed to be the primary site of colonization. The basis for the tissue tropism displayed by EHEC is not fully understood. Although intimin has been suggested to have a role on the bacterial side (Phillips and Frankel, 2000; Fitzhenry et al., 2002), additional bacterial factors might contribute to this tissue tropism including specific expression of T3SS and intimin in the colon xenografts. The host cell might also direct the tropism by differential display of surface components on the apical side of the enterocytes. Moreover, tropism could be driven by the tissue characteristics of the small intestine (e.g. long microvilli and the composition of the mucus layer, which might provide nonspecific protection). Whatever the case, the dramatic tropism exhibited by EHEC in the xenograft model provides a unique opportunity to dissect the process and elucidate its molecular basis.
In conclusion, we have developed an infection model that allows analysis and comparison of the molecular details of EHEC interaction with the human and bovine gut mucosa. This model is expected to enable investigation of the functions of the different virulence factors in the native tissue, as well as of the mechanism governing tissue tropism. Moreover, the model allows comparison of these disease processes between humans and bovines, the latter being a natural host of this organism that is colonized without virulence (except in neonatal calves).
METHODS
Animals
CB-17 SCID mice (6 to 8 weeks old) were obtained from Harlan Laboratories (Israel). They were housed under SPF conditions in sterilized, filter-topped cages and were fed autoclaved food and water ad libitum. The animal studies were approved by the Ethical Committee of the Hebrew University of Jerusalem, Israel.
Bovine tissue
Bovine fetuses up to 26 weeks (gestational age) were obtained from two different slaughterhouses in Israel. Fetuses were held at 4°C for transport to the laboratory. Upon arrival, the fetuses were washed with RPMI medium and intestines were clipped out into fresh medium, cleaned of all mesentery tissue, and cut into approximately 4 cm sections.
Human tissue
Human intestines up to 16 weeks (gestational age) from fetal tissues destined for disposal following prostaglandin-induced therapeutic abortion were obtained from Hadassah Har Hatzofim Hospital, Jerusalem, Israel. Intestinal tissue was placed in ice-cold RPMI medium for transport to the laboratory. Upon arrival, the tissue was washed in a change of medium, cleaned of all mesentery tissue, and cut into approximately 4 cm sections.
Bovine and human xenografts
Tissue was implanted essentially as previously described (Golan et al., 2009). Briefly, the SCID mice were anesthetized, and a small incision was made in both rear flanks and both suprascapular regions. A subcutaneous tunnel was then produced between the two ipsilateral incisions by blunt dissection. A thin forceps was inserted into the tunnel, and the tissue segment was threaded through it. The tissue was trimmed as necessary, and the incision was closed with 5-0 nylon simple interrupted sutures. The grafts were allowed to develop for up to 16 weeks before manipulation. Bovine and human xenograft survival rates were 86% and 71%, respectively (for tissue implanted within 6 hours of harvesting). Survival rates deteriorated to zero in older tissues.
These studies were approved by the Helsinki Committee of the Hadassah University Hospital (no. 381-23/04/04) and the Ethics Committee for Animal Experimentation of the Hebrew University (no. MD-89-56-4). The mothers undergoing legal abortions gave their written, informed consent for use of the fetal tissues in the study.
Infection of intestinal xenografts
EHEC EDL933-TUV slt1- and slt2-negative and an isogenic escN::kan mutant have been previously described (Li et al., 2004; Shifrin et al., 2008). Bacteria were grown in LB medium overnight at 37°C without shaking and then for another 4 hours in DMEM before inoculation. Between 3×107 and 5×107 bacteria in a volume of 100 μl were injected directly into the lumen of the intestinal xenografts through a 23-gauge needle. The control grafts received 100 μl PBS alone. The mice were sacrificed 8 hours or 12 hours after infection, and the grafts were retrieved for histochemical analysis and total bacterial counts. We analyzed 210 (144 bovine and 66 human) intestinal xenografts originating from 17 bovine and 11 human fetal donors (Table 1).
Table 1.
Summary of bovine and human intestinal xenoafts
Histochemical analysis
Xenograft tissues were trisected for histology, immunofluorescence staining, electron microscopy and total bacterial counts. Samples for histological analysis were fixed in neutral buffered 4% formaldehyde, paraffin embedded, and sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin (H&E) or PAS. Fresh xenograft tissue for fluorescence staining was fixed in 2.5% paraformaldehyde overnight at room temperature, incubated with 15% (w/v) sucrose for 12 hours at 4°C and frozen in Tissue-Tek® (EMS, Hatfield, PA) embedding medium. Serial 10 μm cryosections were stained with either phalloidin-rhodamine (Sigma) and 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) or Sytox Orange nucleic acid stain (Invitrogen). Cytokeratin was stained with FITC bovine anti-cytokeratin antibodies (Sigma C 5992) overnight at 4°C in a humidified chamber. EHEC was stained with rabbit anti-O157 antiserum or anti-intimin antiserum followed by secondary detection with goat anti-rabbit IgG-FITC (Sigma). Sections were mounted with VectaShield (Vector Laboratories, Burlingame, CA) and imaged with a Nikon Eclipse E400 epifluorescence microscope with Olympus DP70 camera.
Total bacterial counts
Xenograft tissue was weighed and homogenized on ice in 1 ml sterile PBS immediately after removal. For total bacterial counts, homogenates were plated as serial tenfold dilutions on LB agar plates and incubated overnight at 37°C to determine CFU count.
Mean CFU counts were calculated at 8 hours and 12 hours after challenge and compared by the non-parametric Mann-Whitney two-independent-sample test for comparison of means using SPSS 10.0.1 software (SPSS, Chicago, IL).
Transmission electron microscopy
Samples for transmission electron microscopy (TEM) were fixed with a mixture of 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, for 2 hours and then washed with 0.1 M phosphate buffer. After osmification, dehydration and embedding (Epon), the tissue was sectioned using an LKB-ultrotome 8800 III and observed with a Tecnai 12 (Phillips, Eindhoven, The Netherlands) TEM equipped with MegaView II CCD camera and AnalySIS version 3.0 software.
Supplementary Material
Acknowledgments
This study was supported by the Israel Science Foundation (F.I.R.S.T. 256/06 to I.R., S.Y. and N.Y.S.) and by grant number 5969 from the Ministry of Health, State of Israel. I.R. is an Etta Rosensohn Professor of Bacteriology.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests
AUTHOR CONTRIBUTIONS
N.Y.S. and I.R. conceived and designed the experiments. L.G. performed the experiments. E.G. developed the animal model. N.Y.S., I.R. and L.G. analyzed the data. S.Y. contributed the human fetal tissues. N.Y.S., I.R. and L.G. wrote the paper.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.005777/-/DC1
REFERENCES
- Cantey J.R., Inman L.R., Blake R.K. (1989). Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J. Infect. Dis. 160, 136–141 [DOI] [PubMed] [Google Scholar]
- Chase-Topping M., Gally D., Low C., Matthews L., Woolhouse M. (2008). Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 6, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y., Fitzhenry R., Heuschkel R., Torrente F., Frankel G., Phillips A.D. (2007). Human intestinal tissue tropism in Escherichia coli O157: H7-initial colonization of terminal ileum and Peyer’s patches and minimal colonic adhesion ex vivo. Microbiology 153, 794–802 [DOI] [PubMed] [Google Scholar]
- Croxen M.A., Finlay B.B. (2010). Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 [DOI] [PubMed] [Google Scholar]
- Fitzhenry R.J., Pickard D.J., Hartland E.L., Reece S., Dougan G., Phillips A.D., Frankel G. (2002). Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50, 180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan L., Livneh-Kol A., Gonen E., Yagel S., Rosenshine I., Shpigel N.Y. (2009). Mycobacterium avium paratuberculosis invades human small intestinal goblet cells and elicits inflammation. J. Infect. Dis. 199, 350–354 [DOI] [PubMed] [Google Scholar]
- Kaper J.B., Nataro J.P., Mobley H.L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 [DOI] [PubMed] [Google Scholar]
- Li M., Rosenshine I., Tung S.L., Wang X.H., Friedberg D., Hew C.L., Leung K.Y. (2004). Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl. Environ. Microbiol. 70, 5274–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R., Schuller S., Girard F., Fairbrother J.M., Phillips A.D., Frankel G. (2007). Functional studies of intimin in vivo and ex vivo: implications for host specificity and tissue tropism. Microbiology 153, 959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler C., Baruch K., Kobi S., Mills E., Haviv G., Farago M., Alkalay I., Bartfeld S., Meyer T.F., Ben-Neriah Y., Rosenshine I. (2010). The Type III secretion effector NleE inhibits NF-κB activation. PLoS Pathog. 6, e1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.D., Frankel G. (2000). Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181, 1496–1500 [DOI] [PubMed] [Google Scholar]
- Phillips A.D., Navabpour S., Hicks S., Dougan G., Wallis T., Frankel G. (2000). Enterohaemorrhagic Escherichia coli O157:H7 target Peyer’s patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J.M., Thorpe C.M., Rogers A.B., Waldor M.K. (2003). Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immunol. 71, 7129–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge T.C., Morey A.L., Ferguson D.J., Fleming K.A., Shmakov A.N., Phillips A.D. (1995). Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation 58, 361–371 [DOI] [PubMed] [Google Scholar]
- Sheng H., Lim J.Y., Knecht H.J., Li J., Hovde C.J. (2006). Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immunol. 74, 4685–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin Y., Peleg A., Ilan O., Nadler C., Kobi S., Baruch K., Yerushalmi G., Berdichevsky T., Altuvia S., Elgrably-Weiss M., et al. (2008). Transient shielding of intimin and the type III secretion system of enterohemorrhagic and enteropathogenic Escherichia coli by a group 4 capsule. J. Bacteriol. 190, 5063–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L.D., Ishikawa F., Greiner D.L. (2007). Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 [DOI] [PubMed] [Google Scholar]
- Spears K.J., Roe A.J., Gally D.L. (2006). A comparison of enteropathogenic and enterohaemorrhagic Escherichia coli pathogenesis. FEMS Microbiol. Lett. 255, 187–202 [DOI] [PubMed] [Google Scholar]
- Tarr P.I., Gordon C.A., Chandler W.L. (2005). Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K.I., Robins-Browne R.M., O’Brien A.D., Lior H., Cohen M.L., Smithers J., Levine M.M. (1987). Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immunol. 55, 3117–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Gunzer F., Donnenberg M.S., de Montigny L., Kaper J.B., Donohue-Rolfe A. (1995). The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immunol. 63, 3621–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Stanley S.L., Jr (2004). Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell Microbiol. 6, 535–554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.