Abstract
The ideal vaccine to protect against toxoplasmosis in humans would include antigens that elicit a protective T helper cell type 1 immune response, and generate long-lived IFN-γ-producing CD8+ T cells. Herein, we utilized a predictive algorithm to identify candidate HLA-A02 supertype epitopes from T. gondii proteins. Thirteen peptides elicited production of IFN-γ from PBMC of HLA-A02 supertype persons seropositive for T. gondii infection but not from seronegative controls. These peptides displayed high-affinity binding to HLA-A02 proteins. Immunization of HLA-A*0201 transgenic mice with these pooled peptides, with a universal CD4+ epitope peptide called PADRE, formulated with adjuvant GLA-SE, induced CD8+ T cell IFN-γ production and protected against parasite challenge. Peptides identified in this study provide candidates for inclusion in immunosense epitope-based vaccines.
Keywords: Toxoplasma gondii, HLA-A2 Epitope, vaccine
1. Introduction
Toxoplasmosis refers to a disease caused by the parasite Toxoplasma gondii (T. gondii). The active infection destroys tissues, especially brain and eye in the fetus, newborn infant, immune compromised persons and those with retinal disease. In the presence of a normal immune response, the parasite remains as a chronic cryptic, latent brain infection, that can recrudesce and thus cause eye damage throughout the life of the host[1–3]. About four thousand cases of newly active retinal disease are diagnosed in the U.S. each year[4]. Importantly, this parasite chronically infects 30–50% of human population worldwide, with unknown consequences of this chronic infection of the brain in 2–3 billion persons throughout the world[5]. There is speculation concerning, and limited evidence of association of seropositivity with certain neurologic disease. This work suggests that T. gondii infection might contribute to certain motor, cognitive, and behavioral abnormalities[6]. Currently, additional medicines for the active infection without associated hypersensitivity and toxicity, curative medicines for the currently untreatable latent bradyzoite form of the parasite, and a vaccine to prevent infection with this parasite are being sought[7–11]. There have been previous studies, which indicated that peptide formulations are effective at inducing immune responses to this and other infectious agents in murine and human hosts[12–16]. Thus, we considered that it might be feasible to create an immunosense vaccine consisting of peptides created from immunogenic parasite proteins using HLA supermotif, immunosense approaches.
Human cells have a major histocompatibility complex (MHC) Class I processing pathway in which the proteasome in the cytosol degrades proteins from T. gondii into chains of eight to ten amino acids. These peptides associated with MHC Class I molecules then travel through the endoplasmic reticulum, and are presented at the surface of all cells so that the T cell receptors of cytotoxic T cells (CTLs) and IFN-γ producing CD8+ T cells can recognize the MHC molecule and bound peptide[17]. Thus, IFN-γ producing CD8+ T cells are able to identify the peptides as self or non-self. Cells that present non-self peptides are killed and/or elicit IFN-γ[18]. In this manner, CD8+ T lymphocytes play a major role in protection against T. gondii by secreting IFN-γ which activates macrophages to inhibit replication, kill the parasite, and induce lysis of infected cells. Thus, this obligate intracellular parasite loses its intracellular niche[19].
Since CD8+ T cells recognize target cells by peptide epitopes presented in the context of MHC Class I molecules, it is of great interest to identify MHC Class I restricted peptide epitopes from specific T. gondii antigens to facilitate creating vaccines that stimulate cell-mediated immune responses. The identification of CD8+ T cell responses would also provide peptide antigens that could be used for directly monitoring CD8+ T cell responses resulting from vaccination[20]. To date, no T. gondii specific HLA-A02 restricted peptides have been proven to function in protection against T. gondii.
The goals of the present study were to: (1) to identify HLA-A02 restricted epitopes from T. gondii; (2) to evaluate some of them as components in a candidate vaccine by immunizing HLA-A*0201 transgenic mice to determine whether they could provide protection against T. gondii challenge, and (3) to evaluate the effect of adjuvants in poly-epitope immunizations as a proof of principle for this supermotif immunosense vaccine. Accordingly, we screened GRA10, GRA15, SAG2C, SAG2D, SAG2X, SAG3, SRS9, BSR4, SPA, and MIC proteins from T. gondii for CD8+ T cell epitopes by using an HLA motif algorithm in the immunoepitope database (IEDB). This was intended to predict potential epitopes that would bind to the HLA-A02 supertype family, which is present in 50% of the population worldwide, irrespective of ethnicity[21]. Ten nonamer T.gondii derived peptides derived from the amino acid sequence of SAG2C38–46, SAG2D180–189, SAG2X44–52, SAG2X351–359, SAG3136–144, SAG3375–383, SPA12–20, SPA82–90, MIC19–17, and MICA2P11–19 were identified via bioinformatic and affinity binding assays and tested in ELISpot assays with peripheral blood mononuclear cells from HLA-A*0201 T. gondii-seropositive individuals herein. In an earlier study SAG1, SUSA1, GRA2, GRA3, GRA6, GRA7, ROP2, ROP16, and ROP18 were screened and GRA624–32 (VVFVVFMGV), GRA629–37 (FMGVLVNSL), and GRA325–33 (FLVPFVVFL) also had been found to elicit IFN-γ from PBMC from seropositive but not seronegative persons[22]. HLA-A*0201 transgenic mice immunized with the newly identified peptides, and the three HLA-A02 peptides identified previously[22] which were all pooled. They were administered with the CD4+ helper T cell peptide PADRE and the adjuvant, GLA-SE, which induced high levels of IFN-γ production and protected mice against challenge from type II parasites.
2. Methods
2.1. Bioinformatic prediction of CD8+ T cell epitopes
ARB, SMM, and ANN algorithms from immunoepitope database (IEDB) http://www.iedb.org/ were used to predict binding affinity to HLA-A*0201[23–25] of T. gondii specific peptides. Protein sequences from GRA10, GRA15, SAG2C, SAG2D, SAG2X, SAG3, SRS9, BSR4, SPA, and MIC were screened for nonamer or decamer CD8+ T cell epitopes based on their predicted binding affinity to HLA-A*02 supertype molecules. A total of 29 unique peptides that had IC50 <50nM using the bioinformatic algorithm were selected. Protein sequences were from ToxoDB 5.1. Population coverages of peptides were predicted[26].
2.2. Peptides and GLA-SE
All peptides were synthesized by Synthetic Biomolecules (San Diego, CA) and were >90% pure. Peptides were first dissolved in DMSO and then diluted in PBS or media. GLA-SE was synthesized by Infectious Diseases Research Institute (IDRI, Seattle, WA).
2.3. MHC-peptide binding assays
Quantitative assays to measure binding of peptides to HLA Class I molecules are based on inhibition of binding of a radiolabeled standard peptide. Assays were performed as described[25,27]. Briefly, 1-10nM of radiolabeled peptides were co-incubated with 1M-1nM purified MHC and 1–3µM human β2-microglobulin. After 2 days, binding of radiolabeled peptide to corresponding MHC Class I molecule was determined by capturing MHC-peptide complexes on Greiner Lumitrac 600 microplates coated with W6/32 antibody and measuring bound counts per minutes using a Topcount microscintillation counter. Concentration of peptide yielding 50% inhibition of binding of radiolabeled probe peptide (IC50) then was calculated. Under conditions used, where [radiolabeled probe] < [MHC] and IC50 ≥ [MHC], measured IC50 values are reasonable approximations of true Kd values.
2.4. Mice
HLA-A*0201 Kd transgenic mice were produced at Pharmexa-Epimmune (San Diego, CA) and bred at the University of Chicago. All studies were conducted with Institutional Animal Care and Use Committee at the University of Chicago approval.
2.5. Enzyme-linked immunospot (ELISpot) assay
Cryopreserved peripheral blood mononuclear cells (PBMC), obtained in accordance with institutional and NIH guidelines, from both T. gondii-seropositive and seronegative individuals were used in ELISpot assays to study human PBMCs. PBMCs were isolated from heparinized blood samples by density gradient centrifugation using Histopaque (Sigma-Aldrich), then washed twice with PBS, and cryopreserved in AIM-V medium (Gibco) containing 20% FCS and 10% DMSO.
2×105 PBMCs per well were incubated at 37°C, 5% CO2 in AIM-V medium, pulsed by peptide or peptide pools at 10µg/ml in MSIPS4W10 Multiscreen HTS-IP 96-well plates (Millipore, Bedford, MA) previously coated with 50µl of 15µg/ml anti-human IFN-γ (1-D1K) monoclonal antibody (mAb) in sterile PBS overnight, and then processed as described[22]. After washing with sterile PBS and blocking with RPMI-1640 medium containing 10% FCS at room temperature for 2–3 hr, plates were successively incubated with 100µl of 1ng/ml biotinylated anti-human IFN-γ mAb (7B6-1) for 2 hr and streptavidin-conjugated alkaline phosphatase at a 1:1000 dilution for 1 hr at room temperature. Wells were washed with PBS between incubation stages. Spots were developed using 5-bromo-4-chloro-3-indolyl-phosphate/p-nitro blue tetrazolium chloride (BCIP/NBT) and quenched by extensive washing with distilled water. After drying, plates were counted using an automated ELISpot reader (CTL ImmunoSpot). Media containing an equivalent concentration of DMSO was used to measure background response levels, and 5µg/ml of T. gondii lysate antigen (TLA) was used as a positive control.
ELISpot assays for production of IFN-γ by murine splenocytes were performed similarly, except that anti-mouse IFN-γ mAb (AN18) and biotinylated α-mouse IFN-γ mAb (R4-6A2) were used as cytokine-specific capture and detection antibodies instead. 5 × 105 splenocytes were plated per well as described[22]. All antibodies and reagents used for ELISpot assays were obtained from Mabtech (Cincinnati, OH). Cells were plated in at least 3 replicate wells for each condition. Results were expressed as number of spot forming cells (SFCs) per 106 PBMCs or per 106 murine splenocytes.
2.6. Immunizations and challenge
Age-matched female HLA-A*0201 mice were inoculated subcutaneously (s.c.) at the base of the tail using a 30-gauge needle with pooled CD8+ T cell peptides as shown in Table 2B, with pan DR epitope (PADRE), a universal helper T lymphocyte epitope emulsified in 3-deacylated monophosphoryl lipid A (GLA-SE). Controls were injected with PBS. Mice were boosted once at a 2-week interval. Mice were challenged intraperitoneally with 10,000 Pru-FLUC tachyzoites 10–14 days after the last immunization.
Table 2.
Binding capactiy and affinity.
| A. A2-Supertype binding capacity of T. gondii derived epitopes. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A02-supertype | Supertype alleles bound (≤ 500nM) |
||||||||
| Sequence | Length | Protein | Position | HLA- A*0201 |
HLA- A*0202 |
HLA- A*0203 |
HLA-A*0206 | HLA- A*6802 |
|
| FLSLSLLVI | 9 | SAG2C | 38 | 736 | 173 | 83 | >10,000 | 9834 | 2 |
| FMIAFISCFA | 10 | SAG2D | 180 | 32 | 56 | 56 | 112 | 397 | 5 |
| FVIFACNFV | 9 | SAG2X | 44 | 34 | 11 | 6.5 | 5.6 | 1.2 | 5 |
| FMIVSISLV | 9 | SAG2X | 351 | 16 | 1.8 | 1 | 6.2 | 48 | 5 |
| FLLGLLVHV | 9 | SAG3 | 375 | 6.4 | 37 | 4.6 | 9.9 | 173 | 5 |
| FLTDYIPGA | 9 | SAG3 | 136 | 0.7 | 7.1 | 7.8 | 1.2 | 2095 | 4 |
| ITMGSLFFV | 9 | SPA | 12 | 0.16 | 0.64 | <0.5 | 0.26 | 1.1 | 5 |
| GLAAAVVAV | 9 | SPA | 82 | 9.6 | 31 | 1.3 | 55 | 263 | 5 |
| VLLPVLFGV | 9 | MIC1 | 9 | 1.7 | 1.5 | 0.42 | 1.3 | 1142 | 4 |
| FAAAFFPAV | 9 | MICA2P | 11 | 1.4 | 1.4 | 6 | 1.3 | 2.7 | 5 |
| B. Binding affinity of identified peptides and immunogenicity in human and transgenic mice. | ||||||
|---|---|---|---|---|---|---|
| Peptides1 | Peptide Sequences |
Predicted IC50 nM2 |
Affinity3 HLA- A* 0201 |
Elicit IFN-γ4 in Seropositive human |
Seronegative human |
Immunogenicity5 in mice |
| GRA624–32 | VVFVVFMGV | 76.3 | 14 | + | − | − |
| GRA629–37 | FMGVLVNSL | 26.6 | 18 | + | − | + |
| GRA325–33 | FLVPFVVFL | 8.6 | <0.10 | + | − | + |
| SAG2C38–46 | FLSLSLLVI | 34.1 | 736 | + | − | + |
| SAG2D180–189 | FMIAFISCFA | 15.6 | 32 | + | − | + |
| SAG2X44–52 | FVIFACNFV | 4.5 | 34 | + | − | + |
| SAG2X351–359 | FMIVSISLV | 27.5 | 16 | + | − | + |
| SAG3375–383 | FLLGLLVHV | 2.3 | 6.40 | + | − | + |
| SAG3136–144 | FLTDYIPGA | 2.8 | 0.70 | + | − | + |
| SPA12–20 | ITMGSLFFV | 10.7 | 0.16 | + | − | + |
| SPA82–90 | GLAAAVVAV | 27.8 | 9.60 | + | − | + |
| MIC19–17 | VLLPVLFGV | 7.3 | 1.70 | + | − | + |
| MICA2/P11–19 | FAAAFFPAV | 12.5 | 1.40 | + | − | − |
Peptides derived from proteins and the position within the proteins.
Peptides binding affinity to HLA-A02 were predicted using the ARB algorithms from immunoepitope database (IEDB).
Binding affinity was determined by MHC binding assay.
PBMC cells from four T. gondii-seropositive HLA-A02 humans and four seronegative ones were stimulated with peptides, the T cells that produce IFN-γ were tested by ELISPOT assay.
Splenic T cells were isolated from HLA-A*0201 mice 10 to 14 days after peptide immunization and tested for their ability to generate IFN-γ in response to peptide.
2.7. Ex vivo preparation of murine splenocytes
Three to five mice from each group were euthanized 7 to 14 days after the last immunization. Spleens were harvested, pressed through a 70µm screen to form a single-cell suspension, and depleted of erythrocytes with ASK lysis buffer (160mM NH4Cl, 10mM KHCO3, 100µM EDTA). Remaining splenocytes were washed twice with Hank’s Balanced Salt Solution (HBSS) and resuspended in complete RPMI medium (RPMI-1640 supplemented with 2mM L-GlutaMax [Invitrogen], 100U/ml penicillin, 100µg/ml streptomycin, 1mM sodium pyruvate, 50µM β-mercaptoethanol, and 10% FCS) before use in subsequent in vitro assays.
2.8. In vivo bioluminescence imaging
Mice infected with Pru-FLUC tachyzoites were imaged at day 7 post-challenge using the in vivo imaging system (IVIS; Xenogen, Alameda, CA) as previously described[22]. Briefly, mice were injected i.p. with 200µl of D-luciferin and immediately anesthetized in an O2-rich induction chamber with 2% isoflurane. After 12 min, mice were imaged in ventral positions and photonic emissions were assessed using Living image® 2.20.1 software (Xenogen). Data were presented as pseudocolor representations of light intensity and mean photons/s/region of interest (ROI).
2.9. Statistical analyses
Statistical analyses for all in vitro assays were performed using a 2-tailed student’s T test. Peptides were considered immunogenic in mice if they induced IFN-γ spot formation from immunized mice that was significant (P<0.05) relative to spot formation from control mice. Peptides and peptide pools were considered immunogenic in humans if they induced IFN-γ spot formation from PBMCs that was significant (P<0.05) relative to spot formation from the same cells incubated with media containing an equivalent concentration of DMSO. Each ELISpot experiment was replicated a minimum of twice. P values of ≤ 0.05 were considered significant.
3. Results
3.1. Identification of candidate HLA-A*0201-restricted CD8+ T cell epitopes that elicit IFN-γ from seropositive persons
To identify candidate epitopes from Toxoplasma gondii that elicit IFN-γ from CD8+ T cells, the peptides derived from GRA10, GRA15, SAG2C, SAG2D, SAG2X, SAG3, SRS9, BSR4, SPA, and MIC were screened for potential supertype epitopes using the ARB, SMM, and ANN algorithms from immunoepitope database (IEDB) on the basis of their predicted binding affinity to HLA-A02 molecules. A total of 29 unique peptides IC50 <50nM of all ranked nonameric or decameric peptides were selected (Table 1).
Table 1.
Predicted peptide candidates utilized for screening CD8+ T cells1.
| HLA-A*0201 | ANTIGEN | PEPTIDE SEQUENCES |
LENGTH | LOCATION | PREDICTED IC50 |
PEPTIDE POOL |
|---|---|---|---|---|---|---|
| HLA-A*0201 | BSR4 | LLAVCMSGV | 9 | 21–29 | 34.3 | P1 |
| HLA-A*0201 | GRA15 | FNMNFYIIGA | 10 | 211–220 | 28.8 | |
| HLA-A*0201 | GRA10 | YLGYCALLPL | 10 | 686–695 | 8.1 | |
| HLA-A*0201 | GRA10 | KLMRQYDMMV | 10 | 323–332 | 11.6 | |
| HLA-A*0201 | GRA10 | RLQEIIALA | 9 | 189–197 | 27.8 | |
| HLA-A*0201 | GRA10 | FLAGSQVPG | 10 | 54–63 | 35.2 | |
| HLA-A*0201 | SAG2C | FMIAFISCFA | 10 | 348–357 | 15.6 | P2 |
| HLA-A*0201 | SAG2C | FLSLSLLVI | 9 | 38–46 | 34.1 | |
| HLA-A*0201 | SAG2C | SLPLSPFTV | 9 | 147–155 | 40.6 | |
| HLA-A*0201 | SAG2D | FMIAFISCFA | 10 | 180–189 | 15.6 | |
| HLA-A*0201 | SAG2x | FMIVSISLV | 9 | 1:351-359 | 4.5 | |
| HLA-A*0201 | SAG2x | VLSSSFMIV | 9 | 1:346-354 | 27.5 | |
| HLA-A*0201 | SAG2x | FVIFACNFV | 9 | 1:44-52 | 40.1 | P3 |
| HLA-A*0201 | SAG2x | CLPLYLFVI | 9 | 1:38-46 | 42.2 | |
| HLA-A*0201 | SAG3 | FLLGLLVHV | 9 | 375–383 | 2.3 | |
| HLA-A*0201 | SAG3 | FLTDYIPGA | 9 | 136–144 | 2.8 | |
| HLA-A*0201 | SAG3 | FLVGCSLTV | 9 | 306–314 | 5 | |
| HLA-A*0201 | SRS9 | VSGFVVAS | 8 | 390–397 | 34.8 | |
| HLA-A*0201 | SRS9 | KLMAVCIGGI | 10 | 20–29 | 37.9 | P4 |
| HLA-A*0201 | SPA | ITMGSLFFV | 9 | 12–20 | 10.7 | |
| HLA-A*0201 | SPA | KLADVLPSA | 9 | 236–244 | 12.3 | |
| HLA-A*0201 | SPA | FLCDMDIATL | 10 | 208–217 | 14.1 | |
| HLA-A*0201 | SPA | VLALIFVGV | 9 | 20–28 | 20.1 | |
| HLA-A*0201 | SPA | GLAAAVVAV | 9 | 82–90 | 27.8 | |
| HLA-A*0201 | MIC1 | VLLPVLFGV | 9 | 9–17 | 7.3 | P5 |
| HLA-A*0201 | MIC4 | YLIGSGFSA | 9 | 540–548 | 11.8 | |
| HLA-A*0201 | MIC6 | MMPSGVPMA | 9 | 80–88 | 22.5 | |
| HLA-A*0201 | MIC8 | YVMRYSDYV | 9 | 747–755 | 8 | |
| HLA-A*0201 | MICA2P | FAAAFFPAV | 9 | 11–19 | 12.5 |
Peptides derived from GRA10, GRA15, SAG2C, SAG2D, SAG2X, SAG3, SRS9, BSR4, SPA, MIC1, MICA2P were screened for potential supertype epitopes using the ARB algorithms from immunoepitope database at http://www.iedb.org/ on the basis of their predicted binding affinity to HLA-A02. A total of 29 unique peptides IC50 < 50nM (lower score, higher predicted binding affinity) of all ranked nonameric or decameric peptides were selected.
To determine which of these peptides would be recognized in the context of a Toxoplasma infection, peripheral blood mononuclear cells from T. gondii-seropositive HLA-A*0201 individuals were tested for response to these peptides in pools or individually by using an IFN-γ ELISpot assay. Candidate peptides were considered immunogenic if they induced IFN-γ spot formation that was significant compared to an irrelevant HLA-A*0201-restricted peptide. As shown in Figure 1A, there were three peptide pools which stimulated significant response by human peripheral blood mononuclear cells (PBMC) derived from HLA-A02 seropositive individuals. Then the ability of individual peptides in each of these peptide pools was tested to identify those that could stimulate significant T cell response by PBMC in HLA-A02 seropositive humans. Using this approach, we identified 10 peptides recognized by HLA-A02 PBMC from seropositive humans herein (Fig. 1). These were: one from SAG2C38–46 (FLSLSLLVI); one from SAG2D180–189 (FMIAFISCFA); two from SAG2X (SAG2X44–52, FVIFACNFV; SAG2X351–359 FMIVSISLV); two from SAG3 (SAG3375–383, FLLGLLVHV; SAG3136–144, FLTDYIPGA); two from sporozoite antigen SPA (SPA12–20, ITMGSLFFV; SPA82–90, GLAAAVVAV; one from MIC19–17 (VLLPVLFGV); and one from MICA2P11–19 (FAAAFFPAV). All these identified peptides stimulated significant IFN-γ production by peripheral blood cells from 4 HLA-A*0201 persons infected with T. gondii, but not from 4 seronegative controls as shown in Figure 2.
Figure 1. IFN-γ ELISpot assay with pools of peptides that are predicted to bind avidly to HLA A02.
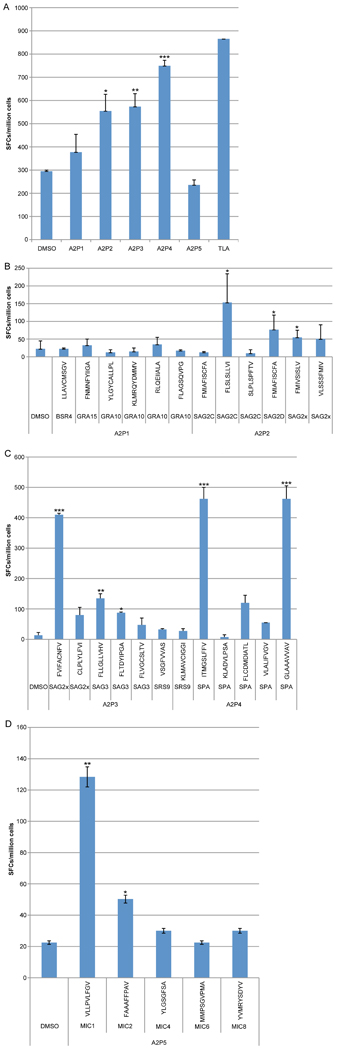
(A) Twenty-nine HLA-A*02-restricted peptides were divided in 5 pools with 8 to 9 peptides per pool and tested for reactivity in PBMCs isolated from HLA-matched T. gondii-seropositive individuals in an ex vivo ELISpot assay. A2P1 to A2P5: peptide pools as described in Table 1. IFN-γ ELISpot assay with individual peptides in pool 1 and pool 2 (B), pool 3 and pool 4 (C), pool 5 (D), These graphs show the spot count of each peptide that bound to HLA supertype HLA-A02 tested for reactivity with PBMCs isolated from HLA-A02 T. gondii-seropositive individuals. The peptides are listed by their sequence and antigen. * P<0.05; ** P<0.01; *** P<0.001.
Figure 2. A02 peptide pool was tested in HLA-A02 restricted human cells.
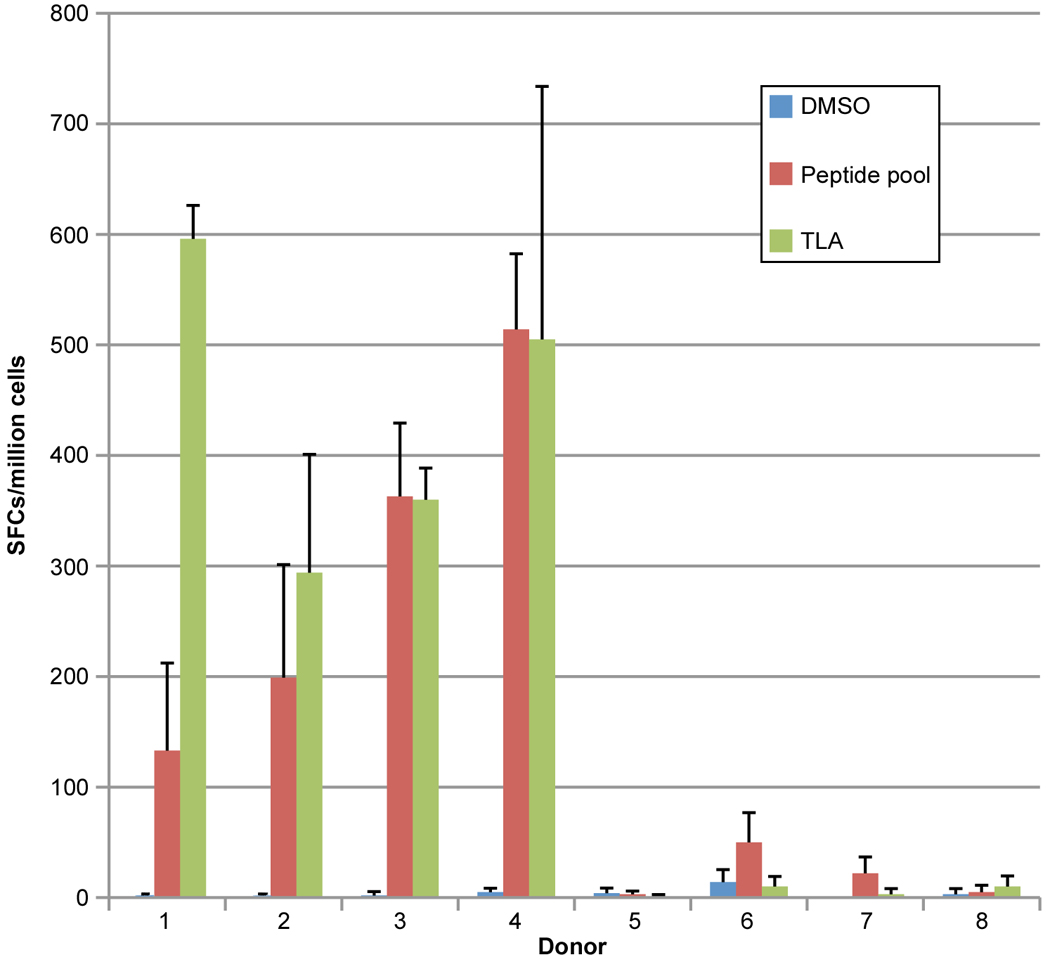
PBMC cells from four T. gondii-seropositive HLA-A02+ (1–4) and four seronegative (5–8) persons were stimulated with a peptide pool that contain the peptides in table 2B and Toxoplasma lysate antigen (TLA); Frequency of SFCs was measured using an ELISpot assay. All differences between unstimulated and peptide or TLA stimulated PBMCs were significant (p<0.05) for seropositive (1 and 3: p<0.001; 2 and 4: p<0.05) but not seronegative persons (p>0.05).
3.2. MHC binding assay demonstrates A2-supertype binding capacity of peptides recognized by HLA A2 positive PBMCs
The binding affinity of these identified peptides for MHC A2 molecules was proven empirically with a MHC binding assay. All of the peptides identified herein were bound with high affinity to the HLA-A02, all binding 4 or more alleles within the respective supertype except one binding 2 alleles (Table 2A). There are nine out of ten peptides that have HLA-A*0201 binding affinity that bound with a Kd of less than 50nM. Specifically, SAG2X44–52, SAG2X351–359, SPA112–20, and MICA2P11–19, bound with a Kd of 50nM or less for all five tested HLA-A02 alleles. SAG3375–383, SAG3136–144, SPA12–20, and MIC19–17 bound 4 HLA-A02 alleles with affinities under 50nM. SAG2C38–46 bound HLA-A*0201 with > 50nM affinity, but bound HLA-A*0202 and HLA-A*0203 allele in the 50 to 500nM range (intermediate binding). The data shows that all these identified peptides bound proteins of the HLA-A02 supertype as predicted, which would be predictive of candidates for eliciting IFN-γ from CD8+ T cells.
3.3. CD8+ T cell responses against peptides after immunization in HLA transgenic mice
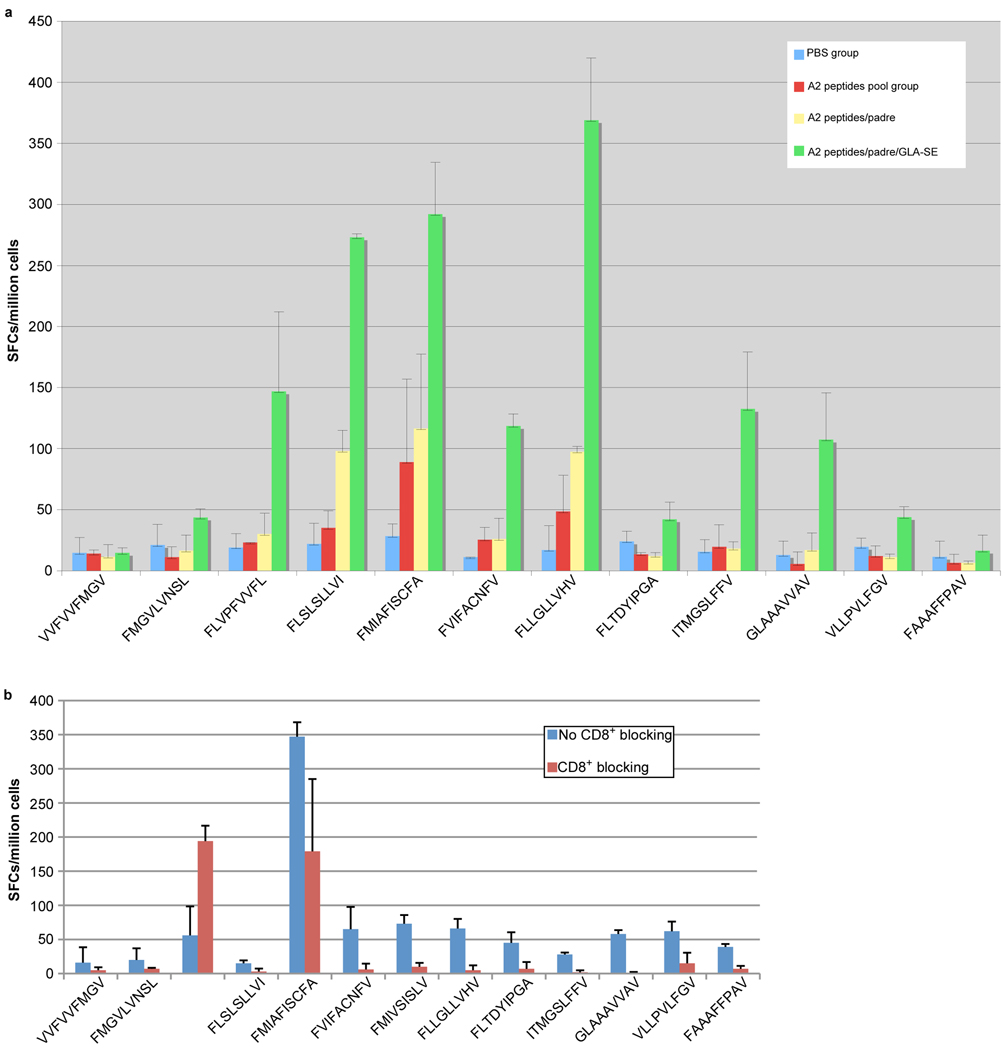
To determine whether the peptides identified were immunogenic in vivo, HLA-A*0201 transgenic mice were immunized with pools of peptides that included peptides identified above as well as three other previously identified CD8+ T-cell epitopes, VVFVVFMGV (GRA624–32), FMGVLVNSL (GRA629–37), and FLVPFVVFL (GRA325–33) [22]. A summary of peptide binding and immunogenicity activity is provided in Table 2B. HLA-A*0201 transgenic mice were immunized with; (1) CD8+ epitope peptide pools, (2) CD8+ epitope peptide pools plus PADRE, and (3) CD8+ epitope peptides pool plus PADRE emulsified with GLA-SE. Eleven to fourteen days post immuniztion, spleen cells were isolated and exposed to each individual peptide. A peptide was considered immunogenic if it induced IFN-γ spot formation that was significantly higher in the immunization group when compared with the group immunization with PBS. Figure 3A shows representative data for IFN-γ spot formation from four immunization groups which were stimulated by individual peptides. After the immunizations with the two pooled peptides, SAG2C38–46 (FLSLSLLVI) and SAG2D180–189 (FMIAFISCFA) stimulated T cells from immunized HLA-A*0201 transgenic mice to produce significant IFN-γ production in vitro. However, when PADRE, a universal CD4+ epitope peptide, was added to the immunizing peptides, five peptides (SAG2C38–46 FLSLSLLVI; SAG2D180–189 FMIAFISCFA; SAG2X44–52 FVIFACNFV; SAG2X351–359 FMIVSISLV; SAG3375–383 FLLGLLVHV) induced significant IFN-γ production from T cells of immunized mice. More strikingly, all peptides except GRA624–32 (VVFVVFMGV) and MICA2P11–19 (FAAAFFPAV) were found to be immunogenic when MLA also was used as an adjuvant to immunize HLA-A*0201 mice (Fig. 3). To assess relative contributions of CD8+ T cells to overal IFN-γ response, the activity of splenic CD8+ T cells was blocked using antibody specific to the CD8+ T cell receptor (Fig. 3B). IFN-γ spot formation was significantly reduced in the presence of antibody to CD8 incubated with the spleen cells from mice immunized with the pooled peptides plus PADRE and MLA when the cells were stimulated by all of the peptides except GRA624–32 and MICA2P11–19.
Figure 3. CD8+ T cell responses against peptides in HLA-A*0201 mice following peptide pool immunization.
(A) HLA-A*0201 mice were immunized with PBS, peptide pool, peptide pool with PADRE, peptide pool with PADRE in GLA-SE. Splenic T cells were isolated 10–14 days post immunization and exposed to each peptide in an ex vivo IFN-γ ELISpot assay. (B) CD8 blocking assay was performed in peptide pool plus PADRE and GLA-SE group. Frequency of SFCs was measured in the presence or absence of CD8+ antibody. *, p<0.05. Exact p-values are shown in Table S1.
3.4. Peptide immunizations are protective against Toxoplasma challenge in HLA-A*0201 transgenic mice
We next addressed whether the epitopes that were identified could confer protection against parasite challenge in HLA-A*0201 mice. Groups of HLA-A*0201 mice (n=5–9 mice per group) were immunized with peptide pools with PADRE emulsified in MLA. Mice injected with PBS served as controls. Ten days after the second immunization, mice were challenged with 10,000 type II Prugniaud (Pru) strain that expresses the Firefly luciferase (FLUC) gene and imaged at 7 days post-challenge using the in vivo imaging system (IVIS; Xenogen, Alameda, CA) as previously described. Mice were imaged in ventral positions and photonic emissions were assessed using Living image® 2.20.1 software (Xenogen) (Fig. 4). Data were presented as pseudocolor representations of light intensity and mean photons/s/region of interest (ROI). As shown in Figure 4A, the number of luciferase expressing parasites in the immunized HLA-A* 0201 mice was significantly reduced compared to the unimmunized mice. As shown in Figure 4B, a majority, 4 of 5 (80%) mice immunized with the peptides plus PADRE emulsified in MLA adjuvant survived parasite challenge. In contrast, only 1 of 5 (20%) unimmunized mice survived parasite challenge.
Figure 4. Replication of T. gondii luciferase expressing parasites was significantly reduced in HLA-A*0201 mice immunized with peptide pool with adjuvant 7 days after challenge.
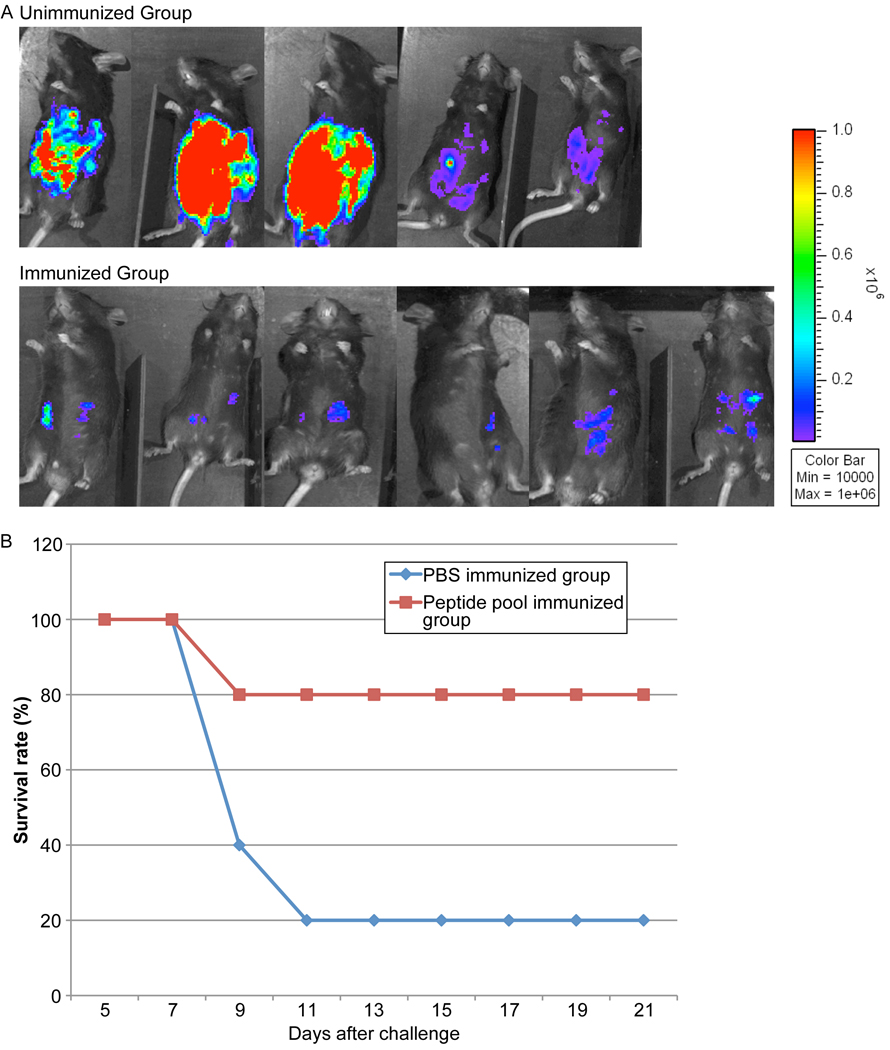
These experiments were performed twice. The results were similar in each experiment. (A) HLA-A02 transgenic mice immunized with peptide pool and adjuvant were protected compared with control mice inoculated with PBS when they were challenged with 10,000 Pru(Fluc)-T. gondii luciferase expressing parasites. There were two replicate experiments. The first experiment is shown. (B) HLA-A02 transgenic mice survival curve after challenge with Type II parasites. Two weeks after last immunization, the transgenic mice immunized with peptide pool and adjuvant or injected with PBS were infected with 10,000 Pru(Fluc) parasites. The survival rates of the two groups were recorded. Differences in survival were significant (p<0.05). This figure shows data from mice in both of the replicate experiments combined (N=9 control and 9 immunized mice).
3.5. Population coverage prediction
An algorithm[26] was developed to calculate projected population coverage of a T cell epitope-based vaccine using MHC binding or T cell restriction data and HLA gene frequencies. We used this web based tool found at http://www.iedb.org/ to predict population coverage of these HLA-A02 epitope peptides based vaccine. The population coverage calculation results in Table 3 showed that the population coverage is varied in different regions: these peptides covered 23.82% population in Australia; 47.01% in Europe; 26.87% in North America; 39.29% in South America; 21.40% in North-East Asia; 12.35% in South-East Asia; 26.78% in Oceania; and 18.27% in Sub-Saharan Africa; and 40.36% for others. The average population coverage is 25.61% ± 13.31%.
Table 3.
Prediction of population coverage.
| Population/Area | Class I | ||
|---|---|---|---|
| Coverage1 (%) | Average Hit2 | PC903 | |
| Australia | 23.82 | 3.10 | 1.71 |
| Europe | 47.01 | 6.11 | 2.45 |
| North Africa | 0.00 | 0.00 | 0.00 |
| North America | 26.87 | 3.49 | 1.78 |
| North-East Asia | 21.40 | 2.78 | 1.65 |
| Oceania | 26.78 | 3.48 | 1.78 |
| Other | 40.36 | 5.25 | 2.18 |
| South America | 39.29 | 5.11 | 2.14 |
| South-East Asia | 12.35 | 1.61 | 1.48 |
| Sub-Saharan Africa | 18.27 | 2.37 | 1.59 |
| Average | 25.61 | 3.33 | 1.68 |
| (Standard deviation) | (13.31) | (1.73) | (0.63) |
Projected population coverage (IEDB[www.iedb.org])
Average number of epitope hits/HLA combinations recognized by the population
Minimum number of epitope hits/HLA combinations recognized by 90% of the population
4. Discussion
In this study, we screened eleven T. gondii protein amino acid sequences for CD8+ T cell epitopes by using an HLA motif algorithm to predict potential epitopes corresponding to the HLA-A02 supertype family, which is represented in 47% of the Europeans and 25% of the world population. We screened peptides from tachyzoite, bradyzoite or sporozoite proteins which were either seen to elicit immune response or were known to be secreted and present in the cytoplasm and thus able to access the MHC Class I pathway[28–30]. These proteins included GRA10, GRA15, SAG2C, SAG2D, SAG2X, SAG3, SRS9, BSR4, SPA, MIC1 or MICA2P of the type II T. gondii strain, ME49, whose sequences came from the ToxoDB website http://toxodb.org/toxo/. These peptides were further selected based on a high MHC allele binding score in a bioinformatic analysis (IC50 < 50nM). Ten nonamer or decamer peptides derived from the amino acid sequence of SAG2C, SAG2D, SAG2X, SAG3, SPA, MIC1, and MICA2P were identified via ELISpot assays with peripheral blood mononuclear cells from HLA-A* 0201 T. gondii-seropositive individuals. In addition, we included three peptides for analysis that had earlier been identified from VVFVVFMGV (GRA624–32), FMGVLVNSL (GRA629–37), FLVPFVVFL (GRA325–33)[22]. HLA-A*0201 transgenic mice immunized with these peptide pools plus adjuvant could induce peptide-specific high IFN-γ production and protect mice against challenge from type II parasites.
The peptide epitopes reported here to elicit IFN-γ are primarily derived from SAG2, SPA and MIC proteins of type II T. gondii. Sequence analysis demonstrated that the SAG1 related sequence (SRS) protein family that is expressed in a stage-specific manner is divided into two major branches, the SAG1-like sequence family and SAG2-like sequence family[31]. The tachyzoite surface is dominated by SAG2A, SAG3, SRS1, SRS2, and SRS3. Some of these proteins have been shown to be involved in invasion and attachment[32]. SAG3 was considered as one member of the redundant system of T. gondii receptors that act as ligands mediating host cell recognition and attachment[33]. Two peptides, FLLGLLVHV and FLTDYIPGA, derived from SAG3375–383 and SAG3136–144 were identified as epitopes that elicit IFN-γ from CD8+ T cells. HLA-binding assay demonstrates that these two peptides have good binding affinity for the HLA-A*0201 supertype. Not only do they have a high binding affinity for HLA-A*0201 allele, but they also bound well to three or four other HLA-A02 alleles.
SAG2C, -D, -X, -Y, encoding bradyzoite surface molecules belonging to the SAG2 family are important for persistence of cysts in the brain. A significantly lower cyst number was seen in mice infected with Pru ΔSAG2CDXY parasites[34]. In our study, there was one peptide derived from SAG2C38–46, one peptide derived from SAG2D180–189, and two peptides derived from SAG2X44–52 and SAG2X351–359 that elicited IFN-γ production from CD8+ T cells from seropositive HLA-A02 individuals, but not seronegative HLA-A02 persons. HLA-binding assay demonstrated that these peptides have good binding affinity for five HLA-A02 alleles, except SAG2C38–46 which only bound two alleles with affinity ≤ 50nM.
The complete life cycle of T. gondii encompasses both the sexual stage (sporozoite) and asexual stage (tachyzoite and bradyzoite). Of the three, the infective stage of T. gondii, sporozoites in oocysts are resistant and persist in the environment after excretion by cats. Sporulation creates highly infectious oocysts that have been linked to large scale outbreaks of toxoplasmosis. Sporozoite surface antigen (SPA)[35] is the dominant surface coat protein expressed on the surface of sporozoites. In our study, two peptides derived from this protein, SPA12–20 and SPA82–90, were found to have the ability to stimulate human CD8+ T cells to produce IFN-γ and bound with high affinity all the tested HLA-A02 supertype allele peptides. MIC1-3 knockout T. gondii was considered as a good candidate for a vaccine against T. gondii-induced abortion in sheep [36] and although MIC knockout parasites could be a vaccine, MICs have been found to be immunogenic in mice. Herein, two peptides MIC19–17 and MICA2P11–19 which have high binding affinity for HLA-A02 supertype were identified as CD8+ T cell epitopes that could elicit IFN-γ from T. gondii seropositive HLA-A02 persons. However, MICA2P11–19 was not found to be immunogenic in HLA-A*0201 transgenic mice even when GLA-SE was used as an adjuvant.
The relationship between A2 binding affinity and the immunogenicity of a series of A2 motif-containing peptides was examined previously [37–39]. MHC affinity plays an important role in determining immune responsiveness. Only peptides with a relatively high binding affinity for MHC of 500nM or less usually are immunogenic [39]. In our study, all the identified peptides have binding affinities lower than 500nM with 11 out of 13 peptides exhibiting very high A2 binding affinities.
Furthermore, epitopes selected from many gene products would increase the breadth of the immune response. The combination of more epitopes from different proteins has the potential to make immunization more robust and broaden its coverage. Inclusion of epitopes derived from bradyzoites and sporozoites have the potential to contribute to control against bradyzoites from cysts or against sporozoites from oocysts early during acquisition of infection. T cells effective against bradyzoite epitopes could reduce number of bradyzoites immediately upon cyst rupture. Thus, these may enhance the biologically relevant efficacy in humans as well as reach higher population coverage.
These peptides were proven to be immunogenic when these peptides were formulated with a universal CD4+ peptide, PADRE [40,41] and adjuvants[42] for immunization of HLA-A*0201 transgenic mice. PADRE, a synthetic nonnatural Pan HLA DR binding epitope peptide that binds promiscuously to variants of the human MHC Class II molecule DR and murine Class II, and is effective in human and HLA transgenic mice, can augment CD8+ T cell effector functions by inducing CD4+ T helper cells. Our results demonstrate that the spleen cells from mice immunized with peptides alone cannot activate T cells to secrete IFN-γ, but when the CD4+ T helper cell epitope PADRE was added in the immunogen, the secretion of peptide-specific IFN-γ was elicited. This indicated that the PADRE epitope delivers help for a MHC Class I restricted peptide-specific CTL response to T. gondii. Our results suggest that in a vaccine to prevent T.gondii infection, both CD4+ and CD8+ epitopes should be targeted in order to drive a protective immune response.
However, low-molecular-weight synthetic peptide antigens are not highly immunogenic by themselves. 3-deacylated monophosphoryl lipid A (GLA-SE) is a detoxified derivative of the lipopolysaccharide (LPS) from Salmonella minesota R595, which is a Toll-like receptor 4 (TLR4) agonist as formulated is a potent activator of Th1 responses [43]. In our study, a robust response was observed when GLA-SE was included in this preparation used for immunize mice. High amounts of IFN-γ production were elicited from mice immunized with pooled peptides plus PADRE and GLA-SE when the spleen cells were stimulated by the individual peptides, and the immunized mice were subsequently protected against high parasite burden with type II parasite challenge and had a significantly enhanced survival rate.
Our studies herein provide a conceptual foundation, reagents and proof of principle for an immunosense approach to protection that will facilitate answering many questions in the future. Experiments of interest include determining: whether such preparations are also protective against sporozoite/oocyst and bradyzoite/cyst challenge; duration of protection; whether additional booster immunizations using adjuvanted peptides or other adjuvants, or adding additional peptides, or prime boosts with either this method and delivery as DNA can make protection even more robust; efficacy of peptides recognized by other supermotifs; whether the peptides can be presented linked into polypeptides which can be more easily manufactured and easily used in vaccine preparations; whether such polypeptides delivered and/or formulated differently might be as or more robust in relationship to full length proteins from which they are derived; whether there is protection not only measured as survival and reduction of parasite burden, but also as protection against congenital and ocular infections; whether similar protective immune responses will be produced in humans.
These studies also provide reagents to define whether persons infected with genetically different parasites throughout the world who have the HLA A2 supermotif (~50% of people) will recognize these epitopes. In addition, our data suggest that these peptides could form part of the basis for diagnostic reagents measuring T cell function. This work provides a murine model to study mechanisms of immune responses that are directed toward eptiopes recognized by humans in HLA transgenic mice. This work also demonstrates that the paradigm we developed for epitope discovery and proof of principle studies for vaccines potentially could be utilized for discovery of vaccine constituents that might prevent disease due to other pathogens. This work also demonstrates efficacy and safety of a robust and promising new adjuvant [43] to elicit CD8 T cells. A number of these studies of potential interest mentioned above have been initiated.
Above all, this study was focused on the identification of HLA-A02-specific epitopes by selecting and testing peptides based on HLA-A*0201 binding motif. Thirteen peptides were identified via ELISpot assays with peripheral blood mononuclear cells from HLA-A02 seropositive persons. Eleven peptides with the help of CD4+ T cell epitopes and adjuvancy of GLA-SE were proven to be immunogenic in transgenic mice using an assay measuring ability to elicit IFN-γ and protect the mice against parasite challenge. The peptides that elicit immune responses in HLA-A02 persons and protect HLA-A02 transgenic mice against T. gondii are candidates for use in vaccines to protect humans against T. gondii infection.
Supplementary Material
ACKNOWLEDGEMENTS
We thank C. Oseroff for helpful suggestions, P. Terasaki for HLA-typing, J. Boothroyd and S. Kim for the luciferase expressing parasite, S. Reed, T. Vedvick and IDRI for the GLA-SE adjuvant, and families and collaborating physicians/scientists in the NCCCTS who made this work possible. We thank J. McCammon, M. Sautter and M. Dean-Carpentier for assistance in preparing this manuscript. We gratefully acknowledge support of this work by gifts from the Fin Charity Trust, R. Blackfoot, R. Thewind, A. Akfortseven, S. Gemma, S. Jackson, A.K. Bump, the Rooney Aldens, the Dominique Cornwell and Peter Mann Family Foundation, the Morel, Rosenstein, Kapnick, Taub, Latsko, Schilling and Kiewit families, and Toxoplasmosis Research Institute. This work also was supported by DMID-NIAID U01 AI77887 (RM), the China scholarship Council, a grant from the National Natural Science Foundation Project of China (No. 30700693), the Intramural Research Program (NIH, NIAID [MG]) and The Research to Prevent Blindness Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Hua Cong, Ernest J. Mui, William H. Witola, John Sidney, Jeff Alexander, Alessandro Sette, Ajesh Maewal, and Rima McLeod contributed to concept, design, experiments, analysis, writing, and final approval of this manuscript. The authors declare no competing interests. The funding sources had no influence in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
REFERENCES
- 1.Boyer K, Marcinak J, McLeod R, editors. Toxoplasma gondii (Toxoplasmosis) 3 ed. New York: Churchill Livingstone; 2007. [Google Scholar]
- 2.Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol. 2010;128:28–32. doi: 10.1001/archophthalmol.2009.354. [DOI] [PubMed] [Google Scholar]
- 3.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–344. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82:464–465. doi: 10.4269/ajtmh.2010.09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson MH, Hutchison WM. The prevalence and source of Toxoplasma infection in the environment. Adv Parasitol. 1989;28:55–105. doi: 10.1016/s0065-308x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- 6.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Estrada C, Prada CF, Fernandez-Rubio C, Rojo-Vazquez F, Balana-Fouce R. DNA topoisomerases in apicomplexan parasites: promising targets for drug discovery. Proc Biol Sci. 2010;277:1777–1787. doi: 10.1098/rspb.2009.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hencken CP, Jones-Brando L, Bordon C, Stohler R, Mott BT, Yolken R, et al. Thiazole, oxadiazole, and carboxamide derivatives of artemisinin are highly selective and potent inhibitors of Toxoplasma gondii. J Med Chem. 2010;53:3594–3601. doi: 10.1021/jm901857d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes CD, Silva NM, Ferro EA, Sousa RA, Firminot ML, Bernardes ES, et al. Azithromycin reduces ocular infection during congenital transmission of toxoplasmosis in the Calomys callosus model. J Parasitol. 2009;95:1005–1010. doi: 10.1645/GE-1765.1. [DOI] [PubMed] [Google Scholar]
- 10.Machado AV, Caetano BC, Barbosa RP, Salgado AP, Rabelo RH, Garcia CC, et al. Prime and boost immunization with influenza and adenovirus encoding the Toxoplasma gondii surface antigen 2 (SAG2) induces strong protective immunity. Vaccine. 2010;28:3247–3256. doi: 10.1016/j.vaccine.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaubert KL, Price DA, Salkowitz JR, Sewell AK, Sidney J, Asher TE, et al. Generation of robust CD8(+) T cell responses against subdominant epitopes in conserved regions of HIV-1 by repertoire mining with mimotopes. Eur J Immunol. 2010 doi: 10.1002/eji.200940079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciesielski MJ, Ahluwalia MS, Munich SA, Orton M, Barone T, Chanan-Khan A, et al. Antitumor cytotoxic T-cell response induced by a survivin peptide mimic. Cancer Immunol Immunother. 2010;59:1211–1221. doi: 10.1007/s00262-010-0845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T cell responses in patients with complete remission from acute myeloid leukemia (AML) Blood. 2010 doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Wang R, Wu Y, Zhang D, He Z, Pan W. Epitope mapping of PfCP-2.9, an asexual blood-stage vaccine candidate of Plasmodium falciparum. Malar J. 2010;9:94. doi: 10.1186/1475-2875-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gritzapis AD, Voutsas IF, Lekka E, Papamichail M, Baxevanis CN. Peptide vaccination breaks tolerance to HER-2/neu by generating vaccine-specific FasL(+) CD4(+) T cells: first evidence for intratumor apoptotic regulatory T cells. Cancer Res. 2010;70:2686–2696. doi: 10.1158/0008-5472.CAN-09-2517. [DOI] [PubMed] [Google Scholar]
- 17.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 19.Jongert E, Lemiere A, Van Ginderachter J, De Craeye S, Huygen K, D'Souza S. Functional characterization of in vivo effector CD4(+) and CD8(+) T cell responses in acute Toxoplasmosis: an interplay of IFN-gamma and cytolytic T cells. Vaccine. 2010;28:2556–2564. doi: 10.1016/j.vaccine.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008;36:W509–W512. doi: 10.1093/nar/gkn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, et al. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008;4(2) doi: 10.1186/1745-7580-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan TG, Mui E, Cong H, Witola WH, Montpetit A, Muench SP, et al. Identification of T. gondii epitopes, adjuvants, and host genetic factors that influence protection of mice and humans. Vaccine. 2010;28:3977–3989. doi: 10.1016/j.vaccine.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 24.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, Sette A. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum Immunol. 2001;62:1200–1216. doi: 10.1016/s0198-8859(01)00319-6. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 26.Bui HH, Sidney J, Li W, Fusseder N, Sette A. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinformatics. 2007;8:361. doi: 10.1186/1471-2105-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulukota K, Sidney J, Sette A, DeLisi C. Two complementary methods for predicting peptides binding major histocompatibility complex molecules. J. Mol. Biol. 1997;267:1258–1267. doi: 10.1006/jmbi.1997.0937. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira da Silva Mda F, Barbosa HS, Gross U, Luder CG. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol Biosyst. 2008;4:824–834. doi: 10.1039/b800520f. [DOI] [PubMed] [Google Scholar]
- 29.Ahn HJ, Kim S, Nam HW. Host cell binding of GRA10, a novel, constitutively secreted dense granular protein from Toxoplasma gondii. Biochem Biophys Res Commun. 2005;331:614–620. doi: 10.1016/j.bbrc.2005.03.218. [DOI] [PubMed] [Google Scholar]
- 30.Kim SK, Karasov A, Boothroyd JC. Bradyzoite-specific surface antigen SRS9 plays a role in maintaining Toxoplasma gondii persistence in the brain and in host control of parasite replication in the intestine. Infect Immun. 2007;75:1626–1634. doi: 10.1128/IAI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung C, Lee CY, Grigg ME. The SRS superfamily of Toxoplasma surface proteins. Int J Parasitol. 2004;34:285–296. doi: 10.1016/j.ijpara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Manger ID, Hehl AB, Boothroyd JC. The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1. Infect Immun. 1998;66:2237–2244. doi: 10.1128/iai.66.5.2237-2244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000;37:574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- 34.Saeij JP, Arrizabalaga G, Boothroyd JC. A cluster of four surface antigen genes specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxoplasma gondii persistence. Infect Immun. 2008;76:2402–2410. doi: 10.1128/IAI.01494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford J, Lamb E, Wasmuth J, Grujic O, Grigg ME, Boulanger MJ. Structural and functional characterization of SporoSAG: a SAG2-related surface antigen from Toxoplasma gondii. J Biol Chem. 2010;285:12063–12070. doi: 10.1074/jbc.M109.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mevelec MN, Ducournau C, Bassuny Ismael A, Olivier M, Seche E, Lebrun M, et al. Mic1-3 Knockout Toxoplasma gondii is a good candidate for a vaccine against T. gondii-induced abortion in sheep. Vet Res. 2010;41:49. doi: 10.1051/vetres/2010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 38.Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS One. 2010;5:e9862. doi: 10.1371/journal.pone.0009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo RT, Sette A, Grey HM, Appella E, Sakaguchi K, Zhu NZ, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994;152:3913–3924. [PubMed] [Google Scholar]
- 40.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snocke K, Serra HM, Kubo RT, Sette A, et al. Development of high potency universsal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 41.Alexander J, Oseroff C, Dahlberg C, Qin M, Ishioka G, Beebe M, et al. A decaepitope polypeptide primes for multiple CD8+ IFN-gamma and Th lymphocyte responses: evaluation of multiepitope polypeptides as a mode for vaccine delivery. J Immunol. 2002;168:6189–6198. doi: 10.4049/jimmunol.168.12.6189. [DOI] [PubMed] [Google Scholar]
- 42.Golkar M, Shokrgozar MA, Rafati S, Musset K, Assmar M, Sadaie R, et al. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine. 2007;25:4301–4311. doi: 10.1016/j.vaccine.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 43.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A Defined Tuberculosis Vaccine Candidate Boosts BCG and Protects Against Multidrug-Resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001094. 53ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.