Abstract
Objective
Men with type 2 diabetes have lower concomitant prostate-specific antigen (PSA) levels; however, the influence of metabolic conditions on PSA changes over time remains unknown. Therefore, the goal of this study was to assess associations between type 2 diabetes and hypertension and changes in serum PSA levels.
Methods
In 1990, a randomly selected cohort of Caucasian men, ages 40-79, from Olmsted County, MN completed questionnaires ascertaining demographic characteristics, current medical conditions and medications biennially, with 633 men undergoing blood draws. Men with a physician diagnosis of diabetes or hypertension at baseline, or who reported using medications to treat these conditions prior to baseline were considered exposed. Men with at least two serum PSA measurements (n=569) were included in this analysis. Linear mixed models were used to estimate the annual percent change in serum PSA levels associated with diabetes and hypertension, adjusting for baseline age.
Results
The overall mean change in serum PSA levels was 3.6% per year and increased with age (p=0.009). Men with diabetes experienced less annual change in serum PSA levels (1.1%) than did non-diabetic men (3.7%), adjusting for age (p=0.02). Age-adjusted change in serum PSA levels differed little by hypertension status (3.7% vs. 3.6%; p=0.49).
Conclusions
Our results suggest that Caucasian men with type 2 diabetes experience smaller increases in serum PSA levels as they age compared to men without diabetes. Additional research is needed to elucidate whether this difference results in a relatively lower incidence of prostate cancer or less cancer detection among diabetic men.
Keywords: serum PSA levels, diabetes, hypertension, prostate cancer
INTRODUCTION
Prostate cancer is the most common non-cutaneous cancer in U.S. men, with an estimated 192,280 new cases diagnosed in 2009.1 While only 15% of men diagnosed with prostate cancer will ultimately die from it, the prevalence of clinically diagnosed disease remains high. Currently, prostate-specific antigen (PSA) is the most common screening test for prostate cancer with 48% of Caucasian men ages 50-79 receiving an annual test.2 The low specificity of PSA testing and questionable benefit of PSA screening on prostate cancer mortality highlight the need for better detection strategies for prostate cancer.3 Knowledge about the influence of concomitant comorbidities on serum PSA concentrations may improve the discriminant value of this test and reduce the number of unnecessary biopsies and subsequent overdiagnosis of indolent cancers.
Type 2 diabetes and hypertension, two increasingly prevalent chronic diseases in the U.S., are arguably reaching epidemic proportions. It is estimated that 1 in 3 U.S. adults suffer from high blood pressure and 11% of U.S. men have type 2 diabetes.4,5 Many studies have investigated the association between type 2 diabetes and prostate cancer, with the majority of evidence supporting an inverse association; the reported reduction in risk ranges from 10-40% in diabetics.6,7 Previous findings also suggest that the effect of diabetes on prostate-cancer risk varies with the duration of diabetes; men with newly diagnosed diabetes have an increased risk, but as their diabetes progresses, their risk of prostate cancer declines.8 Furthermore, diabetes is associated with serum PSA levels; men with diabetes have approximately 10 to 20% lower concurrent serum PSA levels than do men without diabetes.9,10 Similarly, elevated hemoglobin A1C levels are also inversely associated with serum PSA levels, and men who use insulin and oral glucose medications have lower serum PSA levels than do men who do not use medications to treat diabetes.11 Taken together, these findings suggest that diabetes influences prostate-cancer risk and concurrent serum PSA levels.
Studies evaluating the relationship between hypertension and prostate-cancer risk are more limited, with previous results suggesting that men with hypertension are more likely than men without hypertension to be diagnosed with prostate cancer.12 Research by Han and colleagues suggests that high blood pressure is positively associated with concurrent serum PSA levels.13
Although the results from these epidemiologic studies suggest that diabetes and hypertension may be associated with prostate cancer and concurrently influence serum PSA concentrations, they are limited by their cross-sectional designs. The effect of these conditions on serum PSA levels over time has yet to be characterized. This is important as the change in serum PSA levels has been demonstrated to be more reliable in the detection of prostate cancer than single serum PSA measurements.14,15 The increasing prevalence of hypertension and diabetes coupled with the increasing speculation regarding the reliable detection of prostate cancer with serum PSA levels make it crucial to gain a better understanding as to how these metabolic conditions influence prostate cancer detection. Therefore, the goal of this study was to determine the associations between type 2 diabetes and hypertension and longitudinal changes in serum PSA levels over 15 years of follow-up, using data from The Olmsted County Study (OCS) of Urinary Symptoms and Health Status among Men.
MATERIALS AND METHODS
The OCS of Urinary Symptoms and Health Status among Men is a longitudinal study of Caucasian men, residing in Olmsted County, MN.16,17 In 1990, a random sample of men 40-79 years old, as enumerated by the Rochester Epidemiology Project, was screened for inclusion.18 Men with a history of prostate or bladder surgery, urethral surgery or stricture, or medical or neurological conditions that affect normal urinary function were excluded. Also, men with diabetes who suffered from end-organ damage were excluded at baseline. Eligible men (n=3,874) were invited to take part in the study, and 2,115 (55%) agreed to participate. Participants completed a previously validated baseline questionnaire that ascertained information on urinary symptoms, medical histories, and various demographic and behavioral characteristics. A 25% random subset of the total cohort was invited to participate in a detailed urologic clinical examination, which included transrectal ultrasonography to determine prostate volume and serum PSA measurements. Of the 537 randomly selected men, 475 (88%) agreed to participate in the clinical portion of the study.
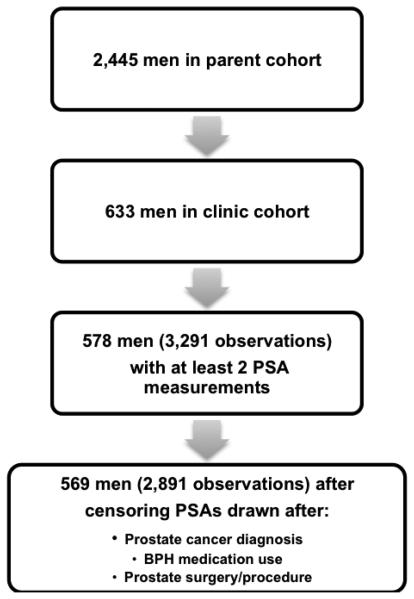
Since 1990, the cohort has been actively followed biennially using a questionnaire similar to the one used at baseline. During the second and third rounds of visits, men who did not participate in the follow-up were replaced by randomly selected eligible men from the community (n=332 total cohort; n=158 clinic cohort). After the third round, the study has been maintained as a fixed cohort. Of the 633 men in the clinic cohort, men with at least two serum PSA measurements were included, and only serum PSA measurements obtained before prostate cancer diagnosis, BPH medication use or prostate surgery/procedure were included. As a result, 569 men with 2,891 observations were included in this analysis (Figure 1).
Figure 1.
Sample size information
Measurements
Information on self-reported physician-diagnosed type 2 diabetes, and high blood pressure was collected at baseline via questionnaire. Men who reported using antihypertensive medication prior to baseline or who reported a physician diagnosis of hypertension at baseline were considered hypertensive for this analysis. In addition, men who reported a physician diagnosis of diabetes at baseline or who used medication to treat diabetes prior to baseline were considered diabetic. Prostate volume, measured via transrectal ultrasonographic imaging, and serum PSA measurements were collected at each round of follow-up during the clinic examination.
Potential confounders and effect modifiers assessed in these analyses include family history of prostate cancer based on a self-reported first-degree relative with physician-diagnosed prostate cancer, household income, years of education, age at baseline blood draw, prostate volume, and body mass index (BMI). Height and weight were measured by a trained research assistant, and BMI was calculated by dividing the weight in kilograms by the height in meters squared. Men with a BMI greater than or equal to 30 kg/m2 were considered obese, based on the definition established by the World Health Organization (WHO).5
Statistical Analysis
Linear mixed effects regression models were used to estimate the annual percent change in serum PSA levels by regressing each measure on time from initial blood draw and adjusting for 10-year baseline age groups. Interaction terms with time were included to allow for different slopes across these age groups. An overall annual change in serum PSA levels for each man was estimated by combining the average longitudinal change (fixed effects) with the individual changes (random effects). Additional models included terms for diagnosis of diabetes or hypertension and interaction terms to compare intercepts and slopes among those with and without a diagnosis. Because of the skewed distribution, serum PSA levels were natural log-transformed, and therefore, annual changes represent percent changes per year assuming an exponential growth curve. Two-stage analysis was used to validate estimates of slopes from the mixed models. All statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC). This study was approved by the Mayo Clinic Institutional Review Board (06-002171).
RESULTS
The 569 men in this sample were followed for a median of 8.4 years after baseline blood draw. The majority of men (91.2%) did not report a family history of prostate cancer. Twenty-five men (4.4%) reported type 2 diabetes at baseline, and 149 (26.2%) reported being hypertensive at baseline. The majority of men were not considered obese at baseline, as 425 (75%) men had a BMI less than 30 kg/m2 (Table 1).
Table 1.
Demographic and medical history characteristics of 569 men
| Characteristic | N (%) |
|---|---|
| Age, years | |
| 40-49 | 243(42.7) |
| 50-59 | 151(26.5) |
| 60-69 | 103(18.1) |
| 70+ | 72(12.7) |
| Marital Status | |
| Single, divorced, widowed, separated | 56(9.8) |
| Married/living together | 511(89.8) |
| Education | |
| Less than high school graduate | 60(10.5) |
| Finished high school/some college | 267(46.9) |
| College degree and beyond | 239(42.0) |
| Salary | |
| <$25,000 | 98(17.2) |
| $25,000-$44,999 | 148(26.0) |
| $45,000-$64,999 | 153(26.9) |
| $65,000+ | 143(25.1) |
| Family history of prostate cancer | |
| No | 519(91.2) |
| Yes | 50(8.8) |
| Diabetes at baseline | |
| No | 544(95.6) |
| Yes | 25(4.4) |
| Hypertension at baseline | |
| No | 420(73.8) |
| Yes | 149(26.2) |
| BMI at baseline, kg/m2 | |
| <25 | 151(26.5) |
| 25-29 | 274(48.2) |
| 30-34 | 118(20.7) |
| ≥35 | 26(4.6) |
Total N for some characteristics is less than 569 due to missing data
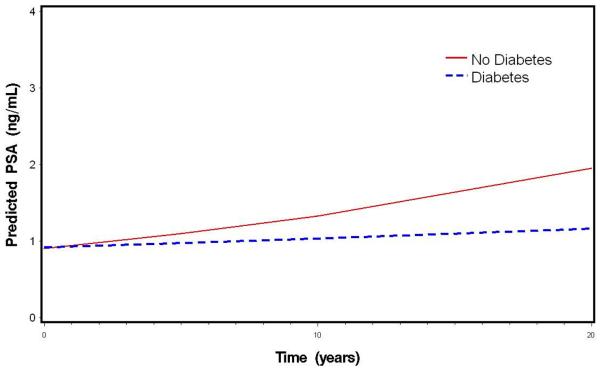
In general, serum PSA levels increased over time as men aged in this cohort. Table 2 displays the annual percent changes in serum PSA levels from the linear mixed models. Overall, serum PSA levels in this cohort increased by 3.58% per year (p<0.001). The annual percent change in serum PSA levels increased with age (p=0.009), with men ages 70 and over at baseline experiencing the greatest annual percent increase in serum PSA levels (4.66%), followed by men ages 60-69 (4.64%), ages 50-59 (3.94%), and ages 40-49 (2.59%) (Table 2). After adjusting for baseline age, baseline (intercept) serum PSA values were not different across diabetes status (p=0.65), or hypertension status (p=0.12) (results not shown). After adjusting for age, men with diabetes at baseline experienced less of an annual increase in serum PSA levels than did men without diabetes (1.11 vs 3.68%; p=0.02) (Table 2). Annual age-adjusted percent change in serum PSA levels did not differ by hypertension status (3.67% vs. 3.55%; p=0.49). These results did not change after adjustment for baseline obesity or baseline prostate volume (data not shown). Figure 2 displays the median predicted values in serum PSA levels during follow-up, by diabetes status.
Table 2.
Age-adjusted mean slopes in 569 men from mixed models
| Annual percent change in PSA |
|
|---|---|
| Baseline Characteristic | Mean, std. dev. |
| Overall * | 3.58, 2.96 |
| p-value | <.0001 |
| Age | |
| 40-49 | 2.59, 2.79 |
| 50-59 | 3.94, 2.91 |
| 60-69 | 4.64, 3.04 |
| 70+ | 4.66, 2.46 |
| p-value | 0.009 |
| Diabetes * | |
| No | 3.68, 2.94 |
| Yes | 1.11, 2.76 |
| p-value | 0.02 |
| Hypertension * | |
| No | 3.55, 2.96 |
| Yes | 3.67, 2.97 |
| p-value | 0.49 |
Age-adjusted slopes from mixed models.
Figure 2.
Age-adjusted median predicted PSA over time by baseline diabetes status
COMMENT
In this prospective cohort study of Caucasian men, ages 40-79, serum PSA levels increased at a rate of 3.6% per year. Older men had more rapid increases in serum PSA levels compared to younger men, and men without diabetes had more rapid increases serum PSA levels compared to men with diabetes. Hypertension, however, was not associated with rate of change in serum PSA levels.
Although there are no other studies evaluating the impact of diabetes on changes in serum PSA levels over time, our finding that the change in serum PSA levels was associated with diabetes is consistent with previous cross-sectional findings suggesting that serum PSA levels are lower among diabetic men than among non-diabetic men. Specifically, Muller et al.11 found men with elevated and highly elevated hemoglobin A1C levels had 15% and 29% lower serum PSA levels, respectively. Men who were on insulin treatment and oral diabetic medications also had lower serum PSA concentrations.11 Using the National Health and Nutrition Examination Surveys, Werny and colleagues found 22% lower average serum PSA levels among men with type 2 diabetes.9 These findings were further replicated by Fukui et al.10 who observed 10 to 16% lower average serum PSA levels among male Japanese diabetics, ages 50-79 years. Our results suggest that men with diabetes have slower increases in serum PSA levels over time, and this might account for the lower serum PSA levels observed among diabetics in cross-sectional studies.
As noted in the introduction, the association between diabetes and serum PSA levels is hypothesized to vary with the duration of diabetes. Several studies have found an inverse relation between diabetes duration and serum PSA levels.8,9 It is plausible that as the duration of diabetes increases, the action of insulin decreases, resulting in subsequent drops in serum PSA levels. This is supported by findings that later-stage diabetes is characterized by insulin resistance and lower levels of circulating insulin, which have been associated with lower prostate-cancer risk and serum PSA levels.8,19 A lower risk in later-stage diabetes may be attributable to the androgen regulation of PSA levels. PSA cleaves insulin growth factor binding protein 3 (IGFBP-3), a major binding protein for insulin growth factor 1 (IGF-1), which is involved in insulin signaling and associated with an increase in prostate-cancer risk.20,21 Previous findings that show use of diabetic medication is associated with serum PSA levels also support this hypothesis, as diabetic-medication use may be a proxy for diabetes severity.11
It remains to be demonstrated whether or not decreases in serum PSA levels drive the lower risk of prostate cancer observed among diabetic men in previous studies.6,22-24 If smaller increases in serum PSA levels among diabetics result in less detection of prostate cancer among asymptomatic cases, it might suggest a detection bias among diabetic men, similar to that thought to exist among obese men due to their lower serum PSA levels and increased prostate volumes.25,26 Furthermore, if the smaller increase in serum PSA levels among diabetic men delays the diagnosis of prostate cancer, men with diabetes may be more likely to be diagnosed with later-stage disease. As such, the potential impact of diabetes on prostate-cancer detection warrants further investigation in future studies.
Hypertension was not associated with change in serum PSA levels over time in this cohort. Findings from previous studies suggest that hypertension is positively associated cross-sectionally with serum PSA levels and longitudinally with the risk of prostate cancer.12,19 Beebe-Dimmer et al.12 found a positive association between hypertension and prostate cancer. Han et al.13 found that diastolic blood pressure was positively associated with serum PSA levels in a sample of 38,356 Korean men. It is possible that androgen-mediated prostate-cancer growth is stimulated by increased sympathetic nervous-system activity subsequent to elevated blood pressure.27 The lack of an association between hypertension and change in serum PSA levels over time in our study may be in part due to the non-specificity of hypertensive medications or to hypertensive status being defined only at baseline. It is possible that some nonhypertensive men were prescribed medication because of cardiovascular disease. It is also plausible that men with hypertension in this cohort were being treated during follow-up and therefore, the effect of hypertension on serum PSA levels is attenuated, resulting in a null association when compared to men without hypertension.
While this study characterizes whether diabetes and hypertension influence change in serum PSA levels using a prospective cohort study with 15 years of follow-up, there are several potential limitations that need to be considered. First, the baseline measures of diabetes and hypertension do not account for changes in these conditions during follow-up, which might have influenced subsequent serum PSA levels. However, it is likely that not accounting for additional diabetics over time is attenuating the difference in change in serum PSA levels among this cohort. Additionally, age at diagnosis of diabetes and hypertension were not queried of men, thereby limiting our ability to make inferences about the progression of these conditions and serum PSA levels. While we are limited in our reliance on self-report of the metabolic conditions, diabetes diagnosis was validated among self-reported cases in a larger cohort study of diabetes in Olmsted County from 1950 to 200028 and most studies show a concordance between self-report and medical records for chronic conditions such as diabetes and hypertension.29,30 Although the long follow-up period lends itself to problems associated with attrition, previous work in this cohort found that participant dropout was not associated with diabetes, hypertension, or serum PSA levels after adjustment for age, thus suggesting the potential impact of this bias on these results may be limited.31 Finally, because this is a Caucasian sample of men, generalizing these findings to other racial groups may not be appropriate.
CONCLUSIONS
In conclusion, our results suggest that type 2 diabetes may decrease the rate at which serum PSA levels change over time. Lower levels of serum PSA as men age potentially influences their detection of prostate cancer. Thus, it is plausible that the presence of diabetes may lead to fewer prostate cancers being detected among this group. As screening guidelines are revised for prostate cancer, it may be prudent to take into consideration the presence of metabolic conditions. Future research should investigate the longitudinal impact of these metabolic conditions on prostate-cancer detection in larger, more diverse samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Cancer I Estimated new cases and deaths of prostate cancer. 2007 [Google Scholar]
- 2.Society AC Cancer Facts & Figures 2010. 2010 [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease C and Prevention Health, United States, 2007. 2007 [Google Scholar]
- 5.Division of N and Physical A Overweight and Obesity. 2007 [Google Scholar]
- 6.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 7.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–8. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. American Journal of Epidemiology. 2005;161:147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 9.Werny DM, Saraiya M, Gregg EW. Prostate-specific antigen values in diabetic and nondiabetic US men, 2001-2002. Am J Epidemiol. 2006;164:978–83. doi: 10.1093/aje/kwj311. [DOI] [PubMed] [Google Scholar]
- 10.Fukui M, Tanaka M, Kadono M, Imai S, Hasegawa G, Yoshikawa T, Nakamura N. Serum prostate-specific antigen levels in men with type 2 diabetes. Diabetes Care. 2008;31:930–1. doi: 10.2337/dc07-1962. [DOI] [PubMed] [Google Scholar]
- 11.Muller H, Raum E, Rothenbacher D, Stegmaier C, Brenner H. Association of diabetes and body mass index with levels of prostate-specific antigen: implications for correction of prostate-specific antigen cutoff values? Cancer Epidemiol Biomarkers Prev. 2009;18:1350–6. doi: 10.1158/1055-9965.EPI-08-0794. [DOI] [PubMed] [Google Scholar]
- 12.Beebe-Dimmer JL, Dunn RL, Sarma AV, Montie JE, Cooney KA. Features of the metabolic syndrome and prostate cancer in African-American men. Cancer. 2007;109:875–881. doi: 10.1002/cncr.22461. [DOI] [PubMed] [Google Scholar]
- 13.Han JH, Choi NY, Bang SH, Kwon OJ, Jin YW, Myung SC, Chang IH, Kim TH, Ahn SH. Relationship between serum prostate-specific antigen levels and components of metabolic syndrome in healthy men. Urology. 2008;72:749–54. doi: 10.1016/j.urology.2008.01.084. discussion 754-5. [DOI] [PubMed] [Google Scholar]
- 14.Loeb S, Roehl KA, Helfand BT, Kan D, Catalona WJ. Can prostate specific antigen velocity thresholds decrease insignificant prostate cancer detection? J Urol. 2010;183:112–6. doi: 10.1016/j.juro.2009.08.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers AJ, Wolters T, Savage CJ, Cronin AM, O'Brien MF, Pettersson K, Roobol MJ, Aus G, Scardino PT, Hugosson J, et al. Prostate-Specific Antigen Velocity for Early Detection of Prostate Cancer: Result from a Large, Representative, Population-based Cohort. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J.Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Guess HA, Panser L, Girman CJ, Chute CG, Oesterling JE, Lieber MM. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch.Fam.Med. 1993;2:729–735. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Parekh N, Lin Y, Marcella S, Kant AK, Lu-Yao G. Associations of lifestyle and physiologic factors with prostate-specific antigen concentrations: evidence from the National Health and Nutrition Examination Survey (2001-2004) Cancer Epidemiol Biomarkers Prev. 2008;17:2467–72. doi: 10.1158/1055-9965.EPI-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djavan B, Bursa B, Seitz C, Soeregi G, Remzi M, Basharkhah A, Wolfram R, Marberger M. Insulin-like growth factor 1 (IGF-1), IGF-1 density, and IGF-1/PSA ratio for prostate cancer detection. Urology. 1999;54:603–6. doi: 10.1016/s0090-4295(99)00280-0. [DOI] [PubMed] [Google Scholar]
- 21.Djavan B, Waldert M, Seitz C, Marberger M. Insulin-like growth factors and prostate cancer. World J Urol. 2001;19:225–33. doi: 10.1007/s003450100220. [DOI] [PubMed] [Google Scholar]
- 22.Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–45. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398–403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123:899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 25.Freedland SJ, Partin AW. Obesity and prostate cancer detection and progression. Rev.Urol. 2004;6:214–216. [PMC free article] [PubMed] [Google Scholar]
- 26.Freedland SJ, Platz EA. Obesity and Prostate Cancer: Making Sense out of Apparently Conflicting Data. Epidemiol Rev. 2007 doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 27.Gann PH, Daviglus ML, Dyer AR, Stamler J. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epidemiol.Biomarkers Prev. 1995;4:611–616. [PubMed] [Google Scholar]
- 28.Burke JP, O'Brien P, Ransom J, Palumbo PJ, Lydick E, Yawn BP, Joseph Melton L, III, Leibson CL. Impact of Case Ascertainment on Recent Trends in Diabetes Incidence in Rochester, Minnesota. American Journal of Epidemiology. 2002;155:859–865. doi: 10.1093/aje/155.9.859. [DOI] [PubMed] [Google Scholar]
- 29.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage. 2005;28:102–10. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 30.El Fakiri F, Bruijnzeels MA, Hoes AW. No evidence for marked ethnic differences in accuracy of self-reported diabetes, hypertension, and hypercholesterolemia. J Clin Epidemiol. 2007;60:1271–9. doi: 10.1016/j.jclinepi.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Gades NM, Jacobson DJ, McGree ME, Lieber MM, Roberts RO, Girman CJ, Jacobsen SJ. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC.Med Res Methodol. 2006;6:58. doi: 10.1186/1471-2288-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]