Abstract
Cyclic AMP inhibits the expression of nitric oxide synthase in hepatocytes but the mechanism for this effect is incompletely understood. Cyclic AMP can activate several intracellular signaling pathways in hepatocytes including Protein Kinase A (PKA), cAMP regulated guanine nucleotide exchange factors (cAMP-GEF’s), and calcium-mediated Protein Kinases. There is considerable overlap and cross-talk between many of these signaling pathways, however, and how these cascades regulate hepatocyte iNOS is not known. We hypothesized that Akt mediates the effect of cAMP on hepatocyte iNOS expression. Hepatocytes cultured with cytokines and dbcAMP increased Akt phosphorylation up to 2 hours of culture. Akt phosphorylation was inhibited by the PI3K inhibitor LY294002 (10 μM), farnyltranferase inhibitor FTI-276, or transfection with a dominant negative Akt. The cyclic AMP- induced suppression of cytokine-stimulated iNOS was partially reversed by LY294002 and FTI-276. LY294002 also increased NFκB nucleus translocation by Western blot analysis in nuclear extracts. Cyclic AMP increased phosphorylation of Raf1 at serine 259 which was blocked by LY294002 and associated with decreased MAPK P44/42 phosphorylation. However, inhibition of MAPK P44/42 signaling with PD98059 failed to suppress cytokine-induced hepatocyte iNOS expression and did not enhance the inhibitory effect of dbcAMP on iNOS production. A constitutively active MAPK P44/42 plasmid had no effect on cytokine-stimulated NO production. These data demonstrate that dbcAMP regulates hepatocyte iNOS expression through an Akt-mediated signaling mechanism that is independent of MAPK P44/42.
Keywords: Akt, Nitric Oxide Synthase, cyclic Adenosine Monophosphate, hepatocyte
1. Introduction
Hepatocyte nitric oxide synthase expression is an integral part of the response to a variety of proinflammatory stimuli such as hemorrhagic shock and sepsis. Excessive nitric oxide (NO) from induced NOS (iNOS) can produce hepatic injury and hepatic dysfunction while global elimination of all NO synthesis in vivo inhibits vascular perfusion and other important physiologic and cellular pathways. Therefore, fully understanding the regulation and function of NO is necessary to understand hepatic physiology and function in shock and sepsis. A more thorough understanding of the role of NO synthesis in these states is essential if this pathway is to be manipulated for therapeutic purposes.
We have shown that glucagon regulates hepatic NOS both in vitro and in vivo. Glucagon receptors lead to calcium mobilization and stimulation of adenylate cyclase to activate a number of downstream signaling cascades, protein kinases, and phosphatases that ultimately regulate intracellular gene expression. In hepatocytes, the regulation of iNOS by glucagon appears to be due primarily to the actions of the second messenger cAMP and subsequent alterations in NF-κB and JNK. However, other signaling pathways can be regulated by glucagon and cAMP that may also contribute to iNOS regulation. Cyclic AMP regulates mitogen activated protein (MAP) kinase activity and can either increase or decrease MAP kinase signaling depending on the different cell type. Cyclic AMP regulates Akt phosphorylation and activation in hepatocytes. In hepatocytes, camp decreased MAPK p44/42activation induced by epidermal growth factor. cAMP also decresed MAPK p44/42activity in primary hepatocytes independent of PI3K or Akt but has no effect on MAPK p44/42 in hepatoma cells. Both MAP kinase and Akt exert strong regulatory effects on hepatocyte gene expression and hepatocellular function but the role of Akt and MAP kinase, specifically MAPK p44/42, in mediating the effects of cAMP on hepatocyte iNOS expression is unknown. We therefore investigated the role of Akt in mediating the inhibition of iNOS expression produced by cAMP. Our data demonstrate that Akt regulates, in part, hepatocyte iNOS expression following exposure to cytokines and dbcAMP but does so independent of MAPK p44/42activation.
2. Materials and Methods
2.1 Materials
Williams Medium E, penicillin, streptomycin, L-glutamine and HEPES were all from Invitrogen Corporation (Carlsbad, CA). Insulin was from Lilly (Indianapolis, IN). Polyclonal antibodies to iNOS, NFκB and IkBα were purchased from BD Bioscience (Billerica, MA). Antibiodies to Raf1 (phosphorylated at serine 259 and total), Akt (total and phosphorylated at serine 473), MEK1/2 (total and phosphorylated at ser 217/ser 221), MAPK p44/42(total and phosphorylated at Thr 203/Thr 204), and actin were purchased from Cell Signaling Technology (Danvers, MA). Antibody to PCNA was purchased from Santa Cruz biotechnology (Santa Cruz, CA). LY294002 (10 mM liquid product in DMSO) and PD98059 (dissolved and re-suspended with DMSO to different concentration at 5, 10, 15 and 20 mM) were purchased from Calbiochem (San Diego, CA). Human recombinant interleukin 1β (IL-1β) was from Dupont (Boston, MA) and murine recombinant interferon γ (IFN) was from Invitrogen Life Technologies (Carlsbad, CA). The plasmid expressing dominant negative Akt was provided by Drs. Burgering and Triest from Utrecht University, Belguim. The plasmid expressing constitutively active MAPK P44/42 was provided by Dr. Wang from the University of Pittsburgh. FTI-276 (re-suspended to 10 mM with water) and all other reagents were from Sigma (St. Louis, MO).
2.2 Primary Hepatocyte Isolation and Culture
Primary hepatocytes were isolated from male Sprague Dawley rats (200–250g) using the modified collagenase perfusion technique as previously described. Purified hepatocytes (>98% pure with > 95% viability by trypan blue exclusion) were cultured onto collagen-coated 100mm dishes in Williams Medium E with L-arginine (0.5 mM), L- glutamine (2 mM), HEPES (15 mM) penicillin, streptomycin and 10% low endotoxin calf serum (HyClone Laboratories, Logan, UT) for 4 hours, then cells were washed to remove non attached cells and media was replaced with insulin-free media with 5% calf serum. After 16 hours of further incubation, the experimental conditions were established. Conditions were performed in duplicate or triplicate and experiments were repeated three times to ensure reproducibility.
2.3 Preparation of Nuclear Extract
A modification of the method by Schreiber et al was used for preparation of primary hepatocyte nuclear extracts. Primary hepatocytes were washed with cold phosphate-buffered saline (PBS). After centrifugation, the cell pellets were frozen at −80 °C for at least 1 hour and resuspended in 500 μL buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5% NP40). After 10 minutes of incubation on ice, cells were centrifuged for 10 minutes at 6,000 rpm. Nuclei were then washed with 500 μL buffer A without NP40 and centrifuged for 10 minutes at 6,000 rpm. The supernatant was collected as cytosolic protein and the pellet was then resuspended and salt extracted in 200 μL buffer B (20 mM HEPES [pH 7.9], 25% glycerol [v/v], 1.5 mM MgCl2, 0.5 mM ethylenediaminetetraacetic acid [EDTA], 0.5 M NaCl) on ice for 30 minutes. All buffers included 0.5 mM DTT (dithiothreitol), 0.2 mM PMSF, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, and 1 μg/mL chymostatin. After centrifugation at 20,000g for 30 minutes, supernatants were collected, aliquoted, and stored at −80 °C. Protein concentration was measured using bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical Company, Rockford, IL).
2.4 Western Blot
Hepatocytes were washed with ice-cold PBS and then scraped from the plate in 500 μl of lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2-EDTA, 1mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1 μg/ml leupeptin, and 1mM PMSF). After 30 min at 4° C, the lysates were centrifuged (15,000 × g for 15 minutes) and stored at −80° C until use. Proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding was blocked with TBS-T (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% non-fat milk for 1 hour. Primary antibodies were diluted and incubated with membranes for 1–2 hours at room temperature or overnight at 4 °C with agitation. After washing three times with TBS-T, secondary antibodies were incubated at 1:10,000 dilution for 1 hour. After 5 additional washes with TBS-T, the bands were visualized with chemiluminescence according to the manufacturer’s instructions.
2.5 Plasmid Transfection
Hepatocytes were plated in 6-well plates (4 × 105cells/well) and transfected with plasmids as previously described. Briefly, plasmids were transfected using LipofectAMINE for 6 hours, allowed to recover overnight, and the experimental conditions were established. The transfection efficiency by this method is approximately 30% for primary hepatocytes.
2.6 NO measurement
Supernatent NO2− was measured as an index of NO production by the Griess reaction as described. Data are presented as the mean ± SD and analysis of variance (ANOVA) was used to determine statistical significance.
3. Results
3.1 cAMP increases Akt phosphorylation
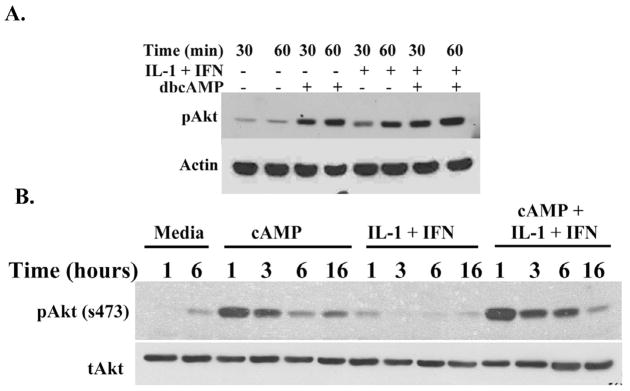
Cyclic AMP increased Akt phosphorylation in cultured hepatocytes but not in hepatoma cells. To investigate the role of Akt signaling in hepatocyte iNOS expression, we measured Akt phosphorylation in the presence and absence of dbcAMP in cultured hepatocytes exposed to proinflammatory cytokines (IL-1β + IFNγ). Both dbcAMP and IL-1β + IFNγ increased Akt phosphorylation but the greatest stimulation of Akt occurred in hepatocytes stimulated with IL-1β + IFNγ and dbcAMP (Figure 1). dbcAMP induced a prolonged Akt phosphorylation lasting up to 16 hours of culture, while the IL-1β + IFNγ-induced Akt phosphorylation was brief.
Figure 1.
IL-1β+IFNγ and dbcAMP induce Akt phosphorylation in hepatocytes. Hepatocytes were treated with IL-1β (200 U/ml) + IFNγ (100 U/ml) with or without dbcAMP (0.5 mM) and cells were harvested at the indicated time point for Western blot analysis. A: Early time point. B: Late time point. The blots shown are representative of three independent experiments.
3.2 Akt mediates the effect of cAMP on iNOS
Akt is phosphorylated by the activation of upstream kinases such as PI3K To test the hypothesis that cAMP-induced activation of Akt regulates hepatocyte iNOS expression, isolated hepatocytes were stimulated to induce NOS by IL-1β + IFNγ in the presence and absence of dbcAMP (0.5 mM). LY294002 (10μM) was used to inhibit Akt activation. IL-1β+IFN increased hepatocyte iNOS expression and this expression was inhibited by dbcAMP (Figure 2). LY294002 partially reversed the inhibitory effect of dbcAMP on iNOS expression (Figure 2A) and NO2− accumulation (Figure 2B), while significantly inhibiting Akt phosphorylation (Figure 2C). LY294002 was also associated with a slight increase in iNOS expression and NO2− accumulation in IL-1β+ IFN-treated hepatocytes (Figure 2). To confirm the results of this experiment, we transfected hepatocytes with a dominant negative Akt plasmid and then stimulated them with IL-1β+IFN in the presence and absence of dbcAMP. Overexpression of a dominant negative Akt decreased dbcAMP – induced Akt phosphorylation (Figure 2D) and partially reversed the inhibiting effect of dbcAMP on cytokine-stimulated hepatocyte NO−2 production (Figure 2E). The concentration of dbcAMP (0.5 mM) used in these experiments suppressed NO production as measured by the Griess reaction (data not shown) as previously demonstrated
Figure 2.
PI3K mediates the effects of cAMP on Akt phosphorylation and iNOS production. A: Hepatocytes were pre-incubated with 10 μM of the PI3K inhibitor, LY 294002, and then stimulated with IL-1β + IFNγ with or without 0.5 mM dbcAMP. After 24 hours, cell lysates were subjected to Western Blot analysis using a specific iNOS antibody. The blot shown is representative of three independent experiments. B: Hepatocytes were treated as in Figure 2A and superantants analyzed for nitrite using the Griess assay. C. Hepatocytes were pre-incubated with 10 μM P13K inhibitor (LY294002) for 30 minutes, and then stimulated with with IL-1β + IFNγ with or without 0.5 mM dbcAMP for 30 and 60 minutes. Cells were lysed for Western Blot analysis and probed with antibodies against phosphorylated Akt (s473) and total Akt. The blot shown is representative of three independent experiments. D. Hepatocytes were transfected with a dominant negative Akt expression plasmid (AktKD) for 24 hours and stimulated with 0.5 mM dbcAMP for 0, 15 or 30 minutes. The cell lysates were subjected to Western blot with Akt antibody. The blot shown is representative of three independent experiments. E: Hepatocytes were transfected with a plasmid that expresses a dominant negative Akt (Akt KD). The cells were stimulated with IL-1β +IFN with or without 0.5 mM dbcAMP. After 24 hours, the supernatant was analyzed for nitrite using the Griess assay. In B and E, the data represent the mean ± S.D. from three independent experiments. **p<0.05 versus vector transfection group, n=6.
3.3 Ras mediates the effect of cAMP on iNOS
Akt can also be phosphorylated by Ras. Akt phosphorylation induced by IL-1β+IFNγ and dbcAMP was reduced by culturing hepatocytes with a farnesyltransferase inhibitor (FTI-276) 10μM to inhibit Ras activity (Figure 3A). Inhibiting Ras with FTI (10μM) partially reversed the inhibitory effect of dbcAMP on IL-1β+IFNγ-induced hepatocyte iNOS expression (Figure 3B).
Figure 3.
Ras is involved in cAMP-induced Akt phosphorylation and its inhibitory effect on iNOS expression. A: Hepatocytes were pre-incubated with 10 μM farnesyltransferase inhibitor FTI-276 (dissolved in water accounting to manufacturers’ instruction) for 30 minutes, and then stimulated with IL-1β + IFNγ with or without 0.5 mM dbcAMP for 60 minutes. Cells were lysed for Western Blot analysis and probed with antibodies against phosphorylated Akt (s473) and total Akt. B: Hepatocytes were pre-incubated with 10 μM of the Ras inhibitor, FTI-276, and then stimulated with IL-1β + IFNγ with or without 0.5 mM dbcAMP. After 24 hours, cell lysates were subjected to Western Blot analysis using a specific iNOS antibody. The blots shown are representative of three independent experiments.
3.4 cAMP decreases Raf1 and MAPK p44/42activation
Akt activation can alter the activity of downstream signaling pathways such as NF-κB and MAPK P44/42. MAPK P44/42activation mediates the up regulation of iNOS expression by cytokines in macrophages, vascular smooth muscle cells, and cardiac myocytes. We therefore tested the hypothesis that Akt activation regulates hepatocyte iNOS expression through regulation of the Raf/MEK/MAPK P44/42pathway. Since Akt phosphorylates Raf1 at serine 259 (pRaf1) to inhibit Raf/MEK/MAPK P44/42signaling, we first measured pRaf1 phosphorylation at serine 259 in hepatocytes. Hepatocytes cultured alone or with IL-lβ+IFN had little pRaf-1 immunoreactivity (Figure 4A). Incubation of hepatocytes with dbcAMP (0.5 mM) increased pRaf1 which was increased further in hepatocytes cultured with dbcAMP and IL-lβ+IFN (Figure 4A). cAMP also decreased IL-1β+IFN – induced phosphorylation of MEK (Figure 4B) and MAPK p44/42(Figure 5A) which is consistent with the work of others
Figure 4.
dbcAMP increases hepatocyte Raf1 (s259) phosphorylation and decreases MEK1/2 phosphorylation. Hepatocytes were treated with IL-1β (200 U/ml) + IFN (100 U/ml) with or without dbcAMP (0.5 mM) for the indicated time and cell lysates were harvested for Westen blot analysis. The membranes were probed with antibodies specific for p-Raf1 (ser259) (A), p-MEK1/2 and total MEK1/2 (B). The blots shown are representative of three independent experiments.
Figure 5.
Decreased hepatocyte MAPK P44/42phosphorylation by cAMP does not regulate hepatocyte iNOS expression.
A: Hepatocytes were treated with IL-1β (200 U/ml) + IFN (100 U/ml) with or without dbcAMP (0.5 mM) for the indicated time and cell lysates were harvested for Westen blot analysis. The membranes were probed with antibodies specific for and pMAPK P44/42 and tMAPK P44/42. B. Hepatocytes were incubated with PD98059 at indicated concentration for 30 minutes and then stimulated with IL-1β (200 U/ml) + IFN (100 U/ml) for 24 hours for the Griess assay (left panel) and Western blot analysis to iNOS (right panel, upper) or 60 minutes for Western blot analysis with antibodies against phosphorylated MAPK P44/42 or total MAPK P44/42. C: Hepatocytes were transfected with a control plasmid (light gray) or plasmid that expresses a constitutively active MAPK P44/42 (dark gray) and then stimulated to produce NO in the presence or absence of dbcAMP (0.5 mM). Supernatants were collected after 24 hours and analyzed for nitrite (left panel) or harvest cells after 60 minutes for Western blot analysis (right panel). Data indicate mean ± S.D. from three independent experiments, n=6. The blots shown are representative of three independent experiments.
3.5 cAMP-inducedMAPK P44/42 inhibition does not inhibit hepatocyte iNOS
To directly test the role of MAPK P44/42 signaling on hepatocyte iNOS expression, we stimulated hepatocytes to produce iNOS with IL-1β+IFN in the presence and absence of the MEK inhibitor PD98059 to decrease MAPK P44/42activity. PD98059 decreased IL-lβ+IFN-induced MAPK P44/42 phosphorylation but did not alter IL-lβ+IFN-induced NO2− production and iNOS expression (Figure 5B). PD98059 did not enhance the effect of dbcAMP on iNOS expression and production. We confirmed the effect of PD98059 on iNOS regulation by transfecting hepatocytes with a plasmid that expresses constitutively active MAPK P44/42 (Figure 5C). Overexpressing MAPK P44/42 had no effect on cytokine-stimulated iNOS activation or on the inhibition of iNOS produced by dbcAMP (Figure 5C). These data demonstrate that the cytokine- and dbcAMP-induced activation of Akt decreases MAPK P44/42in cultured hepatocytes but that MAPK P44/42does not mediate the inhibition of iNOS expression produced by cAMP.
3.6 PI3K inhibitor affect NFκB nucleus translocation
We previously showed that cAMP decreased IL-lβ+IFNγ-induced NFκB DNA binding and p65 translocation. To test if cAMP/Akt signaling mediates the effect of cAMP on p65 translocation, hepatocytes were pre-incubated with the PI3K inhibitor to attenuate Akt activation and then treated with IL-lβ+IFNγ with or without dbcAMP. The nuclear extracts and cytosolic proteins were prepared after 60 minutes and subjected to Western blot analysis (Figure 6A). Similar to our previous work, IL-lβ+IFNγ increased p65 in nuclear extracts and this was reduced by dbcAMP (Figure 6A and 6B). Inhibition of PI3K and Akt signaling with LY294002 increased p65 in the nucleus in IL-lβ+IFNγ –stimulated hepatocytes and reversed the dbcAMP-induced suppression of nuclear p65 (Figure 6A and 6B).
Figure 6.
ΠI3K ινηιβιτoρ ρεγελατεσ NΦκB nuclear translocation. Hepatocytes were pre-incubated with 10 μM LY294002 for 30 minutes and then stimulated with IL-1β (200 U/ml) + IFNγ(100 U/ml) with or without 0.5 mM dbcAMP. The cells were collected after 60 minutes for nuclear extracts and cytosolic proteins. Proteins were subject to Western blot analysis by polyclonal antibodies against NFκB (p65) (A) and IκBα (B) antibodies. Figure 6B shows digitalized results from 3 blots of Figure 6A. The ratios of sum density of p65 bands to PCNA bands were calculated and then normalized by Media with DMSO group. The data represent the mean ± S.D. from three independent scans, * p<0.05 vs DMSO. The blots shown are representative of three independent experiments.
4. Discussion and Conclusion
Hepatocyte iNOS expression is regulated primarily by control of gene transcription. While several transcription factors that regulate iNOS expression have been identified, the intracellular signaling pathways leading to iNOS expression and their specific mechanisms are incompletely defined. We have shown that increasing cAMP suppresses cytokine-induced iNOS expression and NO synthesis in hepatocytes both in vitro and in vivo.
In hepatocytes, cAMP regulates gene expression and cell function in via several downstream signaling mechanisms. cAMP induced hepatic HO-1 gene transcription in a PKA-dependent manner and induced glucose output through Ca2+. PKA is the most thoroughly studied pathway in hepatocytes mediating cAMP-induced changes in gene expression, The effect of cAMP on iNOS in hepatocytes is primarily PKA-independent and involves inhibition of NF- κB DNA binding and an increase in JNK activation, but cAMP also regulates Akt and MAP kinase signaling. We therefore investigated the role of Akt and MAPK P44/42 in regulating the inhibitory effects of cAMP on hepatocyte iNOS expression. Our data demonstrate that dbcAMP and IL-1β+IFN induce a sustained increase in Akt phosphorylation and activation. Both cytokine- and cAMP-induced Akt phosphorylation appear to involve PI3K and Ras since inhibiting both proximal kinases decreases Akt phosphorylation. Inhibition of PI3K and Ras also reversed the camp-induced suppression of iNOS expression. MAP kinases regulate iNOS expression in other cell types and are themselves regulated by Akt. Despite demonstrating that cAMP-induced Akt activation regulates Raf/MEK/MAPK P44/42 signaling in hepatocytes, however, we could find no role for MAPK P44/42 activation in the regulation of iNOS expression by cytokines or dbcAMP. MAPK P44/42 can regulate the expression of a number of genes. It is possible that other hepatocyte genes are regulated by Raf/MEK/MAPK P44/42 signaling and are altered by cAMP through this pathway but identifying those genes will require further investigation.
The specific mechanism for the regulation of iNOS expression by Akt has not yet been defined. Akt can regulate the activation of other signaling pathways besides Raf/MEK/MAPK P44/42including NF-κB and JNK. Akt phosphorylation increases NF-κB activation and NF-κB binding is an important inducer of iNOS expression. In our experiments, LY294002 increased NF-kB nucleus translocation and may partially mediate cAMP’s effects on iNOS. This is consistent with our previous finding that dbcAMP produced a decrease in NF-κB DNA binding in cytokine-stimulated hepatocytes.
As discussed above, Akt also regulates JNK in some cells and can both downregulate and upregulate JNK activation. How Akt and JNK interact in hepatocytes and mediate inflammation-induced gene expression is unknown. Cytokines increase JNK phosphorylation and subsequent c-Jun-mediated gene regulation in cultured hepatocytes. Cyclic AMP also increases JNK signaling and this pathway mediates, in part, the inhibition of iNOS expression by dbcAMP. Whether cAMP regulates JNK activation through Akt is not yet known. There is evidence that cAMP activate PI3K/Akt signaling through cAMP-GEF pathway. Akt can also regulate the activity, either directly or indirectly, of several transcription factors such as CREB, C/EBP, and Foxo1 that could potentially contribute to the regulation of iNOS expression. Determining whether Akt regulates hepatocyte gene expression in general, and hepatocyte iNOS expression more specifically, through these transcription factors is currently being investigated.
Research Highlights.
Cytokine- and cAMP-induced Akt phosphorylation involve Ras and PI3K signaling.
cAMP regulates hepatocyte iNOS expression through an Akt-mediated signaling mechanism
Suppression of MAPK p44/42 signaling by cAMP do not affect on iNOS expression.
Akt decreased NF-kB translocation and may partially mediate cAMP’s effects on iNOS
Acknowledgments
We thank Drs. Burgering and Triest for providing the plasmids that express dominant negative Akt. This work was supported by National Institutes of Health grant DK055664 (BGH)
Footnotes
Disclosures
The authors declare that there are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harbrecht BG, Wu B, Watkins SC, Marshall HP, Jr, Peitzman AB, Billiar TR. Inhibition of nitric oxide synthase during hemorrhagic shock increases hepatic injury. Shock. 1995;4:332–337. doi: 10.1097/00024382-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. The Journal of experimental medicine. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, Tweardy DJ, Harbrecht BG. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol. 1999;277:G144–151. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 4.Vos TA, Gouw AS, Klok PA, Havinga R, van Goor H, Huitema S, Roelofsen H, Kuipers F, Jansen PL, Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997;113:1323–1333. doi: 10.1053/gast.1997.v113.pm9322528. [DOI] [PubMed] [Google Scholar]
- 5.Yao YM, Bahrami S, Leichtfried G, Redl H, Schlag G. Significance of NO in hemorrhage-induced hemodynamic alterations, organ injury, and mortality in rats. Am J Physiol. 1996;270:H1616–1623. doi: 10.1152/ajpheart.1996.270.5.H1616. [DOI] [PubMed] [Google Scholar]
- 6.Aranow JS, Zhuang J, Wang H, Larkin V, Smith M, Fink MP. A selective inhibitor of inducible in nitric oxide synthase prolongs survival in a rat model of bacterial peritonitis: comparison with two nonselective strategies. Shock. 1996;5:116–121. doi: 10.1097/00024382-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 8.Harbrecht BG, Perpetua M, Fulmer M, Zhang B. Glucagon regulates hepatic inducible nitric oxide synthesis in vivo. Shock. 2004;22:157–162. doi: 10.1097/01.shk.0000131579.22409.33. [DOI] [PubMed] [Google Scholar]
- 9.Harbrecht BG, Taylor BS, Xu Z, Ramalakshmi S, Ganster RW, Geller DA. cAMP inhibits inducible nitric oxide synthase expression and NF-kappaB-binding activity in cultured rat hepatocytes. J Surg Res. 2001;99:258–264. doi: 10.1006/jsre.2001.6200. [DOI] [PubMed] [Google Scholar]
- 10.Harbrecht BG, Wirant EM, Kim YM, Billiar TR. Glucagon inhibits hepatocyte nitric oxide synthesis. Arch Surg. 1996;131:1266–1272. doi: 10.1001/archsurg.1996.01430240020002. [DOI] [PubMed] [Google Scholar]
- 11.Christophe J. Glucagon receptors: from genetic structure and expression to effector coupling and biological responses. Biochim Biophys Acta. 1995;1241:45–57. doi: 10.1016/0304-4157(94)00015-6. [DOI] [PubMed] [Google Scholar]
- 12.Deutschman CS, De Maio A, Clemens MG. Sepsis-induced attenuation of glucagon and 8-BrcAMP modulation of the phosphoenolpyruvate carboxykinase gene. Am J Physiol. 1995;269:R584–591. doi: 10.1152/ajpregu.1995.269.3.R584. [DOI] [PubMed] [Google Scholar]
- 13.Lee KA. Transcriptional regulation by cAMP. Curr Opin Cell Biol. 1991;3:953–959. doi: 10.1016/0955-0674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 14.Roesler WJ, McFie PJ, Puttick DM. Evidence for the involvement of at least two distinct transcription factors, one of which is liver-enriched, for the activation of the phosphoenolpyruvate carboxykinase gene promoter by cAMP. J Biol Chem. 1993;268:3791–3796. [PubMed] [Google Scholar]
- 15.Zhang B, Perpetua M, Fulmer M, Harbrecht BG. JNK signaling involved in the effects of cyclic AMP on IL-1beta plus IFNgamma-induced inducible nitric oxide synthase expression in hepatocytes. Cell Signal. 2004;16:837–846. doi: 10.1016/j.cellsig.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal. 2004;16:565–575. doi: 10.1016/j.cellsig.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 18.Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G334–343. doi: 10.1152/ajpgi.00517.2003. [DOI] [PubMed] [Google Scholar]
- 19.Webster CR, Anwer MS. Cyclic adenosine monophosphate-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology. 1998;27:1324–1331. doi: 10.1002/hep.510270519. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. The Journal of biological chemistry. 2000;275:13026–13034. doi: 10.1074/jbc.275.17.13026. [DOI] [PubMed] [Google Scholar]
- 21.Westwick JK, Fleckenstein J, Yin M, Yang SQ, Bradham CA, Brenner DA, Diehl AM. Differential regulation of hepatocyte DNA synthesis by cAMP in vitro in vivo. Am J Physiol. 1996;271:G780–790. doi: 10.1152/ajpgi.1996.271.5.G780. [DOI] [PubMed] [Google Scholar]
- 22.Thoresen GH, Johasen EJ, Christoffersen T. Effects of cAMP on ERK mitogen-activated protein kinase activity in hepatocytes do not parallel the bidirectional regulation of DNA synthesis. Cell Biol Int. 1999;23:13–20. doi: 10.1006/cbir.1998.0314. [DOI] [PubMed] [Google Scholar]
- 23.Webster CR, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- 24.Kagawa T, Varticovski L, Sai Y, Arias IM. Mechanism by which cAMP activates PI3-kinase and increases bile acid secretion in WIF-B9 cells. Am J Physiol Cell Physiol. 2002;283:C1655–1666. doi: 10.1152/ajpcell.00041.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis through NK-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1357–1368. doi: 10.1152/ajpgi.2001.281.6.G1357. [DOI] [PubMed] [Google Scholar]
- 26.Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. The Journal of biological chemistry. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 27.Au WS, Kung HF, Lin MC. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 2003;52:1073–1080. doi: 10.2337/diabetes.52.5.1073. [DOI] [PubMed] [Google Scholar]
- 28.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Wang M, Carr BI. Hepatocyte growth factor enhances protein phosphatase Cdc25A inhibitor compound 5-induced hepatoma cell growth inhibition via Akt-mediated MAPK pathway. J Cell Physiol. 2005;203:510–519. doi: 10.1002/jcp.20243. [DOI] [PubMed] [Google Scholar]
- 30.Kietzmann T, Samoylenko A, Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J Biol Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Liu S, Perpetua MD, Walker WH, Harbrecht BG. Cytokines increase CRE binding but decrease CRE-mediated reporter activity in rat hepatocytes by increasing c-Jun. Hepatology. 2004;39:1343–1352. doi: 10.1002/hep.20200. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 34.Yan J, Roy S, Apolloni A, Lane A, Hancock JF. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J Biol Chem. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]
- 35.Park D, Pandey SK, Maksimova E, Kole S, Bernier M. Akt-dependent antiapoptotic action of insulin is sensitive to farnesyltransferase inhibitor. Biochemistry. 2000;39:12513–12521. doi: 10.1021/bi000995y. [DOI] [PubMed] [Google Scholar]
- 36.Webster CR, Anwer MS. Phosphoinositide 3-kinase, but not mitogen-activated protein kinase, pathway is involved in hepatocyte growth factor-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology. 2001;33:608–615. doi: 10.1053/jhep.2001.22756. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 38.Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. The Journal of biological chemistry. 2001;276:33630–33637. doi: 10.1074/jbc.M105322200. [DOI] [PubMed] [Google Scholar]
- 39.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi H, Kaibori M, Teshima S, Yoshida H, Kwon AH, Kamiyama Y, Nishizawa M, Ito S, Okumura T. Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1beta at a step of NF-kappaB DNA binding in hepatocytes. J Hepatol. 2004;41:730–736. doi: 10.1016/j.jhep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res. 2004;49:433–439. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Ginnan R, Guikema BJ, Singer HA, Jourd’heuil D. PKC-delta mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C1583–1591. doi: 10.1152/ajpcell.00390.2005. [DOI] [PubMed] [Google Scholar]
- 43.LaPointe MC, Isenovic E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p44/42 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 44.Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem. 2003;278:29819–29823. doi: 10.1074/jbc.C300182200. [DOI] [PubMed] [Google Scholar]
- 45.Hong G, Zhang B, Harbrecht BG. Cyclic AMP Inhibits IL-1beta Plus IFNgamma-Induced NF-kappaB Translocation in Hepatocytes by a PKA Independent Mechanism. J Surg Res. 2009 doi: 10.1016/j.jss.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. The Journal of biological chemistry. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 47.Marks-Konczalik J, Chu SC, Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 48.Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. The Journal of biological chemistry. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 49.Rensing H, Bauer I, Kubulus D, Wolf B, Winning J, Ziegeler S, Bauer M. Heme oxygenase-1 gene expression in pericentral hepatocytes through beta1-adrenoceptor stimulation. Shock. 2004;21:376–387. doi: 10.1097/00024382-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat heme oxygenase-1 gene is transcriptionally induced via the protein kinase A signaling pathway in rat hepatocyte cultures. Mol Pharmacol. 1998;53:483–491. doi: 10.1124/mol.53.3.483. [DOI] [PubMed] [Google Scholar]
- 51.Yamatani K, Saito K, Ikezawa Y, Ohnuma H, Sugiyama K, Manaka H, Takahashi K, Sasaki H. Relative contribution of Ca2+-dependent mechanism in glucagon-induced glucose output from the liver. Arch Biochem Biophys. 1998;355:175–180. doi: 10.1006/abbi.1998.0710. [DOI] [PubMed] [Google Scholar]
- 52.Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J Biol Chem. 2003;278:47922–47927. doi: 10.1074/jbc.M307357200. [DOI] [PubMed] [Google Scholar]
- 53.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145:4522–4531. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 54.Zhang QG, Wang XT, Han D, Yin XH, Zhang GY, Xu TL. Akt inhibits MLK3/JNK3 signaling by inactivating Rac1: a protective mechanism against ischemic brain injury. J Neurochem. 2006;98:1886–1898. doi: 10.1111/j.1471-4159.2006.04020.x. [DOI] [PubMed] [Google Scholar]
- 55.Funakoshi-Tago M, Tago K, Sonoda Y, Tominaga S, Kasahara T. TRAF6 and C-SRC induce synergistic AP-1 activation via PI3-kinase-AKT-JNK pathway. Eur J Biochem. 2003;270:1257–1268. doi: 10.1046/j.1432-1033.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- 56.Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem Biophys Res Commun. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 57.Piwien-Pilipuk G, Van Mater D, Ross SE, MacDougald OA, Schwartz J. Growth hormone regulates phosphorylation and function of CCAAT/enhancer-binding protein beta by modulating Akt and glycogen synthase kinase-3. J Biol Chem. 2001;276:19664–19671. doi: 10.1074/jbc.M010193200. [DOI] [PubMed] [Google Scholar]