Abstract
There is increasing evidence that traumatic brain injury (TBI) induces hypofunction of the striatal dopaminergic system, the mechanisms of which are unknown. In this study, we analyzed the activity of striatal tyrosine hydroxylase (TH) in rats at 1 day, 1 week, and 4 weeks after TBI using the controlled cortical impact model. There were no changes in the level of TH phosphorylated at serine 40 site (pser40TH) at 1 day or 4 weeks. At 1 week, injured animals showed decreased pser40TH to 73.9±7.3% (p≤0.05) of sham injured rats. The in vivo TH activity assay showed no significant difference between injured and sham rats at 1 day. However, there was a decreased activity in injured rats to 62.1±8.2% (p≤0.05) and 68.8±6.2% (p≤0.05) of sham injured rats at 1 and 4 weeks, respectively. Also, the activity of protein kinase A, which activates TH, decreased at 1 week (injured: 87.8±2.8%, sham: 100.0± 4.2%, p≤0.05). To study the release activity of dopamine after injury, potassium (80 mM)-evoked dopamine release was measured by microdialysis in awake, freely moving rats. Dialysates were collected and analyzed by high-performance liquid chromatography. There were no significant differences in dopamine release at 1 day and 4 weeks between sham and injured groups. At 1 week, there was a significant decrease (injured: 0.067±0.015 μM, sham: 0.127 ± 0.027 μM, p ≤ 0.05). These results suggest that TBI-induced dopamine neurotransmission deficits are, at least in part, attributable to deficits in TH activity.
Keywords: Dopamine, Traumatic, Microdialysis, Tyrosine, Hydroxylase, Phosphorylation
1. Introduction
The effects of traumatic brain injury (TBI) on the dopamine system have been shown in various studies in the past (Bales et al., 2009). Several pharmacological treatments aimed at the dopamine system have been reported to be beneficial in improving cognitive functions in animals and humans. Catecholamine agonist therapy shows motor and cognitive improvement in both humans and animals (Phillips et al., 2003). Methylphenidate (Kline et al., 1994, 2000) and D-amphetamine (Feeney et al., 1981; Hornstein et al., 1996), which increase synaptic dopamine levels by inhibiting dopamine transporter (DAT) function, have shown to enhance functional outcome after experimental TBI. Also, L-DOPA (Kraus and Maki, 1997; Koeda and Takeshita, 1998; Lal et al., 1988), which increases dopamine synthesis, and amantadine (Sawyer et al., 2008; Dixon et al., 1999), which increases extracellular dopamine concentrations by inhibition of reuptake and facilitation of dopamine synthesis (Von Voightlander and Moore, 1971; Bak et al., 1972; Gianutsos et al., 1985), both facilitate functional recovery after TBI.
Neurochemical studies also suggest that there are alterations in the levels of striatal dopamine and proteins that synthesize and transport dopamine after injury. Dopamine levels increase acutely in the striatum (Massucci et al., 2004; McIntosh et al., 1994), and electrically evoked dopamine release as measured by fast scanning cyclic voltammetry is later decreased in the striatum following TBI (Wagner et al., 2005, 2009). Protein levels of tyrosine hydroxylase (TH) increase chronically (Yan et al., 2007), and DAT decreases chronically after TBI (Wagner et al., 2005), possibly reflecting compensatory changes for recovery of the dopaminergic system.
Tyrosine hydroxylase is a rate-limiting synthesis enzyme for dopamine and norepinephrine. However, striatal tissue contains very small level of norepinephrine and TH represents the presence of dopaminergic fibers (Hokfelt et al., 1977; Anden et al., 1964). The activity level of striatal TH after TBI has not been previously reported. We provide evidence here for the first time that striatal dopamine neurotransmission deficits after TBI may be attributable to deficits in tyrosine hydroxylase activity. Increased activity of TH has previously been associated with phosphorylation of TH at serine 40 site (pser40TH), but not serine 19 site (pser19TH) (Lindgren et al., 2000; Harada et al., 1996). Thus, we monitored pser40TH and pser19TH levels by Western blotting at 1 day, 1 week, and 4 weeks after injury. Activity levels of striatal cAMP-dependent protein kinase A (PKA), a major kinase for TH, were also measured at these time points. In vivo activity level of TH and potassium stimulus-evoked dopamine release, which are parameters that could be affected by dopamine synthesis, were also monitored at 1 day, 1 week, and 4 weeks following injury.
2. Results
2.1. Western blots: tyrosine hydroxylase phosphorylation after TBI
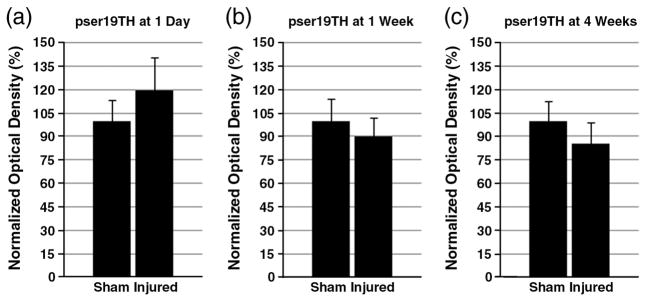
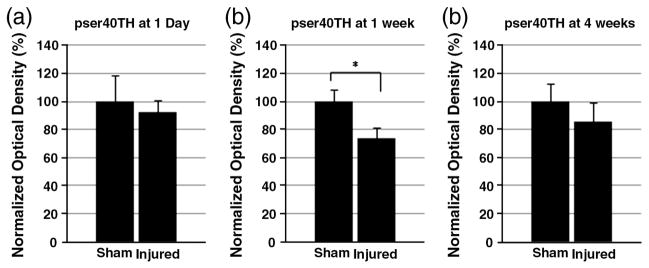
To characterize the changes in dopamine synthesis, Western blot assay was used to detect phosphorylated TH in the striatum. The levels of pser19TH and pser40TH at 1 day, 1 week, and 4 weeks after CCI or sham injury were analyzed. For pser19TH levels, no statistically significant difference was found at any time point (Fig. 1). For pser40TH levels, there were no alterations at 1 day. At 1 week, pser40TH of CCI injured striatum showed a significant decrease (sham: 100±8.5%, injured: 73.9±7.3%, p≤0.05) (Fig. 2). By 4 weeks, this difference was no longer significant. In addition, dopamine decarboxylase (DDC) protein levels were monitored to assess the possibility of its alteration affecting dopamine synthesis downstream to TH after TBI. There were no significant alterations for DDC levels in the striatum at 1 day, 1 week, and 4 weeks after injury [data not shown].
Fig. 1.
Western blot of striatal pser19TH. The levels of striatal pser19TH after CCI or sham injury were analyzed by Western blotting. Optical densities of each phosphorylated protein were normalized by β-actin, and each group had n=6. The levels of TH at 1 day (a), 1 week (b), and 4 weeks after injury are summarized here. Each normalized optical density±SEM is displayed. There was no alteration of pser19TH at any of the time points.
Fig. 2.
Western blot of striatal pser40TH. Optical densities of striatal pser40TH at 1 day (a), 1 week (b), and 4 weeks (c) after injury are normalized by β-actin and displayed as optical density±SEM. Although there was no significant change of pser40TH levels between sham and CCI injured rats at 1 day, there was a significant decrease in injured rats at 1 week (p≤0.05). At 4 weeks, there is no significant difference between the injured and sham groups. *p≤0.05.
2.2. PKA activity assay
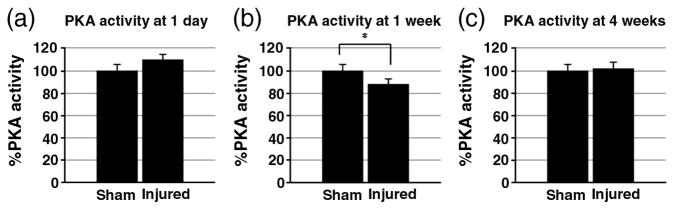
The activity levels of PKA in striatum after TBI was determined at 1 day, 1 week, and 4 weeks (Fig. 3). There was no significant difference between sham and injured striatum at 1 day (sham: 100.0±5.9%, injured: 110.0 ± 5.2%). At 1 week, there is a decrease of PKA activity in injured rats (sham: 100.0±4.2%, injured: 87.8± 2.8%). This decrease was reversed by 4 weeks, and no significant changes were shown in injured rats compared to sham rats (sham: 100.0±3.2%, injured: 102.3±6.0%).
Fig. 3.
PKA activity assay. PKA activity levels at 1 day (a), 1 week (b), and 4 weeks (c) after injury are normalized by sham striatal PKA activity. There were no significant differences between injured and sham rats at 1 day and 4 weeks. However, 1-week time point showed significant decrease in injured striatal PKA activity levels compared to shams. *p≤0.05.
2.3. In vivo tyrosine hydroxylase activity
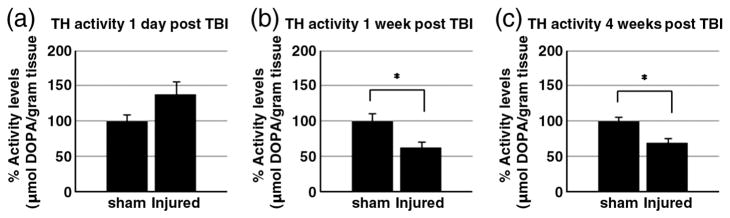
After Inhibition of DDC activity by NSD-1015 injection, L-DOPA accumulation was detected by HPLC in striatal tissue. The accumulation of L-DOPA was used to assess TH activity level. At 1 day after CCI (Fig. 4a), there is a no change TH activity (sham: 100.0±9.7%, injured: 138.0±17.9%). At 1 week after CCI (Fig. 4b), TH activity was decreased significantly in the CCI injured animals compared to sham animals (sham: 100.0±10.6%, injured: 62.1± 8.2%, p≤0.05). TH activity remains decreased up to 4 weeks (sham: 100.0±5.2%, injured: 68.8±6.2%, p≤0.05) (Fig. 4c).
Fig. 4.
TH activity assay. The results of in vivo TH activity assay at 1 day (a), 1 week (b), and 4 weeks (c) after injury are shown. Each group’s striatal DOPA levels are normalized by β-actin content of tissue homogenates and displayed±SEM (n=6). At 1 day after injury, there is a trend but no statistically significant increase in TH activity of CCI injured rats compared to sham rats. At 1 week and 4 weeks after injury, there is a decrease in TH activity in CCI injured rats compared to sham rats (p≤0.05). *p≤0.05.
2.4. Microdialysis for dopamine release and dopamine metabolites
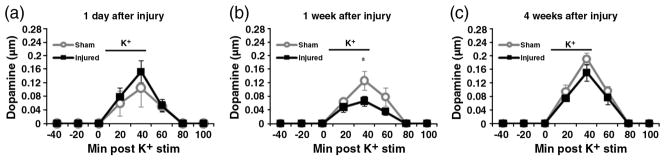
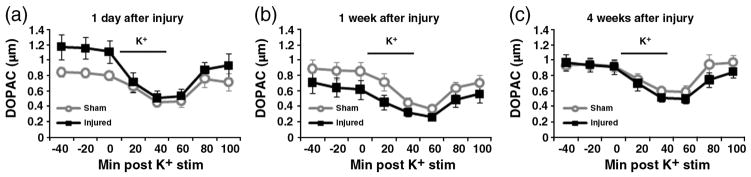
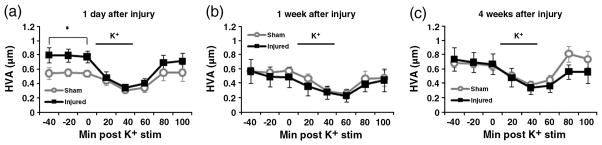
To assess the changes in striatal extracellular dopamine and dopamine metabolites, microdialysis was used in conjunction with high-performance liquid chromatography (HPLC). At 1 day after CCI (Fig. 5a), the peak levels of injured striatal dopamine after 80 mM potassium stimulus was not statistically different from peak sham striatal dopamine (sham: 0.105±0.032 μM, injured: 0.153±0.056 μM). The basal levels of DOPAC (Fig. 6a) were also not significantly different between the sham and injured animals (sham: 0.829 ± 0.043 μM, injured: 1.148± 0.130 μM). However, baseline HVA levels of injured animals were significantly higher compared to the levels of sham animals (sham: 0.547±0.046, injured: 0.789±0.079, p≤0.05) (Fig. 7a).
Fig. 5.
Potassium-stimulated dopamine release. The levels of dopamine in microdialysates are quantified by HPLC at 1 day (a), 1 week (b), and 4 weeks (c). Potassium stimulus duration is labeled as a line between 4th and 5th collection times. There is no significant alteration at 1 day between CCI injured rats (n=8) and sham injured rats (n=8). At 1 week, there is a statistically significant decrease in peak dopamine levels of CCI injured rats (n=10) compared to sham injured rats (n=10). By 4 weeks, there is no significant difference between CCI injured (n=7) and sham rats (n=7). *p≤0.05.
Fig. 6.
DOPAC measured by microdialysis. The levels of dopamine metabolite DOPAC are quantified by HPLC at 1 day (a), 1 week (b), and 4 weeks (c) after injury. There were no significant differences between CCI injured rats and sham-injured rats at any time point.
Fig. 7.
HVA measured by microdialysis. The levels of dopamine metabolite HVA are quantified by HPLC at 1 day (a), 1 week (b), and 4 weeks (c) after injury. There is a significantly higher average baseline HVA levels in CCI injured rats (n=8) compared to sham injured rats at 1 day (n=8). There were no significant differences between CCI injured rats (n=10) and sham injured rats (n=10) at 1 week. Similarly, there was no significant difference between CCI injured rats (n=7) and sham injured rats at 4 weeks. *p≤0.05.
At 1 week after CCI (Fig. 5b), the peak levels of potassium-evoked striatal dopamine release showed a statistically significant decrease (sham: 0.127±0.027 μM, injured: 0.067± 0.015 μM, p≤0.05). There was no difference between DOPAC levels of sham and injured animals (sham: 0.872±0.114 μ injured: 0.661±0.126 μM) (Fig. 6b). HVA levels were also not significantly different between the sham and injured animals (sham: 0.569±0.074 μM, injured: 0.520±0.112 μM) (Fig. 7b). By 4 weeks after CCI (Figs. 5c, 6c, 7c), there are no significant differences between the sham and injured groups for potassium stimulated dopamine (sham: 0.191±0.017 μM, injured: 0.151 ± 0.024 μM). Also, the baseline values of dopamine metabolites showed no significant differences: DOPAC (sham: 0.937±0.061 μM, injured: 0.943±0.078 μM), and HVA (sham: 0.672±0.061 μM, injured: 0.703±0.097 μM).
3. Discussion
In this study, we demonstrated for the first time a deficit in striatal TH activity 1 week (subacute) and 4 weeks (chronic) after TBI in rats as indicated by decreased tissue levels of L-DOPA. There was decreased pser40TH in injured striatum at 1 week but not at 4 weeks. Since TH is a rate-limiting enzyme in dopamine synthesis, the decrease in its activity suggests a dopamine synthesis deficit. However, a previous report of striatal dopamine content after TBI demonstrates no significant change at 1 week or 4 weeks after injury (Massucci et al., 2004). Dopamine levels depend on both synthesis and degradation. Therefore, activities of monoamine oxidase and catechol-O-methyl transferase may be decreased at these time points, reducing the degradation of dopamine. Future studies are needed to elucidate TBI induced alterations of various synthesis and metabolizing enzymes for dopamine.
The Western blot results showing decreased level of pser40TH suggest decreased activity of TH, since the level of pser40TH correlates with enzymatic TH activity (Lindgren et al., 2000; Harada et al., 1996; Waymire et al., 1988). However, the level of pser19TH has no significant decrease. Since the level of pser19TH does not correlate with TH activity (Jedynak et al., 2002), a specific decrease in pser40TH level is consistent with decreased activity of TH. Previously reported TH levels (Yan et al., 2007) showed TH increase at 4 weeks post injury. The chronic increase in striatal TH may be a compensatory action of the striatum. After the TH activity decreases at 1 week after injury, total TH levels may be upregulated in the striatum at a chronic time point in order to increase the efficiency of dopamine neurotransmission during recovery. Also, present data on decreased striatal TH activity after injury contrast with previous study showing increased TH activity in the medial prefrontal cortex (Kobori et al., 2006). This implicates that TH activity levels may be altered differently in a region specific manner.
In this study, decreased activity of PKA is demonstrated at 1 week after TBI. However at 1 day and 4 weeks, there are no significant changes. Since PKA is a major regulator of the serine 40 site of TH, the activity level of PKA is in agreement with pser40TH levels at 1 day, 1 week, and 4 weeks after injury. Deficits in PKA activity at 1 week after injury may cause decreased TH phosphorylation, which could then reduce TH activity. It should also be noted that other kinases such as protein kinase C and protein kinase G may also regulate pser40TH levels (Dunkley et al., 2004), although less extensively characterized than PKA’s effect is on pser40TH. At 4 weeks, there is a recovery of PKA activity and pser40TH levels in injured striatum, but TH activity deficits are still present (Fig. 4c). This suggests that while TH activity may partially depend on phosphorylation of pser40TH by PKA, other factors in the setting of injury could contribute to activity deficits.
A decrease in PKA activity has been previously reported in the parietal cortex and hippocampus at acute time points (15 min to 48 h) using a fluid percussion injury model (Atkins et al., 2007). In contrast, pser40TH level and PKA activity in our study do not decrease at 1 day after injury. However, this study and our current study are not directly comparable due to the differences in brain regions studied, injury models, and time points after injury. The directionality of PKA activity change may depend strongly on each of these factors.
Dopamine release induced by potassium stimulation has been used to compare differences of exocytic release in young and aged rats (Shui et al., 1998; Stanford et al., 2000) and different dietary treatments (Agut et al., 2000; Wang et al., 2005). Consistent with these previous studies, potassium stimulation induces dopamine release and decreases extracellular DOPAC and HVA concentrations in our current data. Dopamine release is not significantly altered at 1 day after injury but shows a decrease at 1 week after CCI injury compared to sham injured animals. These data are in agreement with a previous study showing reduction in medial forebrain bundle-stimulated evoked dopamine release 2 weeks after CCI injury detected by fast scanning cyclic voltammetry (Wagner et al., 2005). By 4 weeks, there is a recovery of dopamine release in injured animals.
Potassium-stimulated dopamine release depends on newly synthesized dopamine, since depletion of vesicular stores of dopamine using reserpine does not alter the potassium evoked dopamine release (Fairbrother et al., 1990). Thus, our microdialysis data showing decrease of dopamine release associated with a dopamine synthesis deficit at 1 week are consistent with a decrease in TH activity shown by in vivo activity assay and pser40TH Western blot results.
The fact that evoked dopamine levels show a decrease in injured animals at 1 week whereas dopamine metabolites show no change may be due to a combination of release and reuptake. It should be noted that the extracellular level of dopamine is dependent on activity levels of dopamine transporters, which rapidly uptake it to presynaptic terminals. However, DOPAC and HVA do not utilize such rapid reuptake mechanism. Previous study from our group has shown that dopamine transporter function is not reduced at 2 weeks after TBI (Wilson et al., 2005). If there is no functional deficit in dopamine transporter also at 1 week, this may explain the discrepancy between extracellular levels of dopamine and dopamine metabolites for injured animals.
At 1 day after TBI, there is a significant increase in baseline HVA levels but not DOPAC levels. Since DOPAC is formed by monoamine oxidase and aldehyde dehydrogenase whereas HVA is formed by catechol-O-methyltransferase, differential changes in activities of these enzymes after TBI may explain this discrepancy.
The results of this study indicate decreased striatal dopamine synthesis and release at a subacute time point (1 week) after TBI. Deficits in striatal dopaminergic function after TBI has been implicated by the effectiveness of pharmacological agents that stimulate the dopaminergic system after TBI. Methylphenidate, D-amphetamine, L-DOPA, bromocriptine, and amantadine, which increase dopamine levels at the synapse or activate dopamine receptors, have been effective in improving cognitive outcome after injury (Barbay and Nudo, 2009; Napolitano et al., 2005). Treatments such as methylphenidate, which inhibits DAT to increase synaptic dopamine levels, have been shown to improve electrically evoked dopamine release (Wagner et al., 2009) and behavioral performance (Kline et al., 2000) in the setting of TBI. These agents lead to functional recovery after TBI by possibly compensating for the decrease in dopamine neurotransmission after injury.
3. Conclusions
In summary, the results of this study suggest that there is a decreased synthesis of dopamine at a subacute time point following TBI, and that this may lead to decreased dopamine release. This alteration in dopamine regulation may be a possible contributor to post TBI dysfunction of striatal dopamine system.
4. Experimental procedures
4.1. Animals
One hundred and three male Sprague-Dawley rats (Harlan Laboratories) weighing 280–350 g were used for this study. All experiments were in line with the guidelines for Care and Use of Laboratory Animals set by University of Pittsburgh. The Institutional Animal Care and Use Committee has approved all the experiments in this study. Animals were housed in 12-h light/dark cycle with food and water given ad libitum.
4.2. Surgery
Animals were injured by the controlled cortical impact (CCI) device previously described (Dixon et al., 1991). After intubation, animals were mounted on a stereotaxic frame secured by incisor bar and ear bars. Mechanical ventilation maintained anesthesia with 2% isoflurane in 2:1 N2O/O2, while parasagittal craniectomy was made with the center of craniectomy at (AP: +4.0 mm, L: +2.8 mm). For injured groups, 2.6–3.2 mm deformation depth (severe injury) at 4 m/s was given while sham groups received only craniectomy.
4.3. Western blot of phosphorylated TH and dopa decarboxylase
Anesthetized rats were sacrificed at 1 day, 1 week, or 4 weeks after surgery (n=6 for each group). Brains were dissected on a chilled ice plate, and striata ipsilateral to the injury were frozen in liquid nitrogen and stored in −70 °C until preparation. Tissues were prepared by sonicating in a lysis buffer (0.1 M NaCl, 0.01 M Tris-HCl (pH 7.6), 0.001 M EDTA (pH 8.0), 1 μg/ml phenylmethylsulfonyl fluoride, Phosphatase Inhibitor Cocktails 1 and 2 (1:100, Sigma, St. Louis, MO), Protease Inhibitor Cocktail (Complete Mini, Roche Applied Science, Mannheim, Germany)). The sonicated tissues were centrifuged at 13,000×g for 30 min and supernatants were used for Western blot. Using a BCA protein assay kit (Pierce, Rockford, IL), samples containing 40 μg of protein were electrophoresed on 10% SDS-poly-acrylamide gels, transferred to polyvinylidene fluoride membranes, and blocked by 5% bovine serum albumin (BSA) (Sigma, St. Louis, MO) in 0.05 M TBS with 0.05% Tween-20 (TBST) for 1 h. The membranes were incubated with anti-pser19TH (1:1000, Millipore), anti-pser40TH (1:1000, Millipore), or anti-DDC (1:1000, Sigma, St. Louis, MO) overnight, then washed with TBST and incubated for 1 h at room temperature with anti-mouse or anti-rabbit immunoglobulin G-conjugated to peroxidase (1:20,000, Pierce). Membranes were exposed to chemiluminescence (Western Lighting, Perkins Elmer, Boston, MA) and pser19TH, pser40TH, and DDC were visualized by exposing the membranes to autoradiographic X-ray film from 10 s to 1 min. Afterwards, membranes were stripped using 100 mM glycine pH 2.3 at 55 °C for 1 h, incubated with anti-β-actin monoclonal antibody (1:10,000, Sigma-Aldrich) for 1 h, then incubated with anti-mouse immunoglobulin G-conjugated to peroxidase. Same steps were taken as described to visualize β-actin blots. To measure the optical density of Western blots, Scion Image PC software (Frederick, MD) was used. Optical densities of pser19TH, pser40TH, and DDC were normalized by β-actin of each blot, and the values displayed are given as a percentage change compared to sham tissue levels.
4.4. PKA activity assay
The activity level of PKA was found using a commercially available kit (Promega, Madison, WI). Briefly, the tissue homogenates used for Western blots were suspended in PepTag PKA 5× reaction buffer, Peptag A1 peptide, and PKA activator 5× solutions. These mixtures were incubated at room temperature for 30 min. The reaction was stopped by placing the mixture in boiling water for 10 min. Glycerol (30%, 1 μl) was added to the mixture, and samples were loaded onto 0.8% agarose/Tris–HCl gel. The gels were run at 100 V until separation of phosphorylated and nonphosphorylated samples became apparent. The gels were then scanned using Kodak Image Station 440CF. The optical densities of the phosphorylated product from PKA reaction were then normalized by total protein level in each sample using NIH Image J software. PKA activity levels were reported with respect to sham striatal PKA activity levels.
4.5. In vivo TH activity assay
As described in Urbanavicius et al. (2007), in vivo TH activity was assessed by quantifying L-DOPA accumulation after inhibiting DDC with 3-hydroxybenzylhydrazine (NSD-1015) (Sigma, St. Louis, MO). Thirty minutes before sacrifice, rats were intraperitoneally injected with NSD-1015 (100 mg/kg, suspended in 0.9% saline). Striata were dissected out, immediately frozen in liquid nitrogen, and stored in −70 °C until neurochemical analysis. On the day of analysis, the tissues were weighed and sonicated in 0.2 M HClO2. The samples were then centrifuged at 13,000×g for 30 min and supernatants were used to quantify L-DOPA levels using HPLC.
4.6. Microdialysis
Artificial cerebrospinal fluid (ACSF) with 126.5 mM NaCl, 2.4 mM KCl, 1.1 mM CaCl2, 0.83 mM MgCl2, 27.5 mM NaHCO3, and 0.5 mM KH2PO4 was used for this experiment. In the high-potassium ACSF, KCl concentration was adjusted to 80 mM and NaCl concentration was adjusted to 48.9 mM to maintain osmolarity. One day before micro-dialysis experiment, a microdialysis probe (SciPro, Sanborn, NY) with the following specifications were used: membrane length: 3 mm, diameter: 0.6 mm, permeability cutoff: 35 kDa. The probe was implanted into the striatum (AP: +0.0 mm, L: +2.8 mm, DV: −4.0 mm) and secured with dental cement. The animals were then disconnected from the anesthesia apparatus and placed in a Plexiglas chamber as previously described (Dixon et al., 1997). Microdialysates were collected in awake, freely moving animals. Overnight, ACSF was continuously perfused at 0.2 μl/min. On the day of the experiment, flow rate was adjusted to 2 μl/min for 1 h then samples were collected every 20 min into a tube containing 5 μl of 0.3 M HClO2. Samples were immediately analyzed by HPLC. At 60 min (4th collection time), ACSF was switched to a high-potassium ACSF solution. Forty minutes after beginning of stimulus (6th collection time), potassium challenge was stopped and ACSF was infused. After microdialysis, the rats were sacrificed and the locations of the probes were verified. For data analysis, micromolar concentrations of dopamine and dopamine metabolites are reported.
4.7. Neurochemical analysis
Neurochemical measurements were made by HPLC with CoulArray Detector using two four-channel analytical cells (ESA, Chelmsford, MA, USA). Eight coulometric electrodes with potentials from −120 to +300 mV in 60 mV increments were used, and C18 column was used to separate the analytes. Dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were monitored in microdialysates. From striatal homogenates for TH activity assay, L-DOPA was monitored. Baseline values of dopamine below the linear range of detection were assigned values of 0 μM.
4.8. Statistical analysis
The normalized TH activity levels and optical densities of DDC, pser19TH, and pser40TH were analyzed using unpaired Student’s T test. Baseline values of DOPAC and HVA were reported as their average level in first three microdialysates detected by HPLC. For potassium-evoked dopamine release, the peak values were used to compare the sham injured and CCI injured animals. Statistical analysis for microdialysis data was performed using a nonparametric Mann-Whitney U Test. All statistical calculations were performed by using PASW Statistics 19 (SPSS Inc., Chicago, IL) software.
Acknowledgments
We would like to thank Ruth Perez and Teresa Hastings for insightful comments and suggestions. This project is supported by NIH grant 1F30NS067731-01, NIH grant 5R01NS060672-02, and the Copeland Fund of the Pittsburgh Foundation.
References
- Agut J, Ortiz JA, Wurtman RJ. Cytidine (5′)diphosphocholine modulates dopamine K(+)-evoked release in striatum measured by microdialysis. Ann NY Acad Sci. 2000;920:332–335. doi: 10.1111/j.1749-6632.2000.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K. Demonstration and mapping out of nigrostriatal dopamine neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich DD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak IJ, Hassler R, Kim JS, Kataoka K. Amantadine actions on acetylcholine and GABA in striatum and substantia nigra of rat in relation to behavioral changes. J Neural Transm. 1972;33:45–61. doi: 10.1007/BF01244727. [DOI] [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. Epub 2009 Apr 1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbay S, Nudo RJ. The effects of amphetamine on recovery of function in animal models of cerebral injury: a critical appraisal. NeuroRehabilitation. 2009;25:5–17. doi: 10.3233/NRE-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Ma X, Marion DW. Reduced evoked release of acetylcholine in the rodent neocortex following traumatic brain injury. Brain Res. 1997;21 (749):127–130. doi: 10.1016/s0006-8993(96)01310-8. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14:285–294. [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. Review. [DOI] [PubMed] [Google Scholar]
- Fairbrother IS, Arbuthnott GW, Kelly JS, Butcher SP. In vivo mechanisms underlying dopamine release from rat nigrostriatal terminals: II. Studies using potassium and tyramine. J Neurochem. 1990;54:1844–1851. doi: 10.1111/j.1471-4159.1990.tb04881.x. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzales A, Law WA. Amphetamine restores locomotor function after motor cortex injury in the rat. Proc West Pharmacol Soc. 1981;24:15–17. [PubMed] [Google Scholar]
- Gianutsos G, Chute S, Dunn JP. Pharmacological changes in dopaminergic systems induced by long-term administration of amantadine. Eur J Pharmacol. 1985;110:357–361. doi: 10.1016/0014-2999(85)90564-3. [DOI] [PubMed] [Google Scholar]
- Harada L, Wu J, Haycock JW, Goldstein M. Regulation of L-DOPA biosynthesis by site-specific phosphorylation of tyrosine hydroxylase in AtT-20 cells expressing wild-type and serine 40-substituted enzyme. J Neurochem. 1996;67:629–635. doi: 10.1046/j.1471-4159.1996.67020629.x. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol. 1977;55:21–40. [PubMed] [Google Scholar]
- Hornstein A, Lennihan L, Seliger G, Lichtman S, Schroeder K. Amphetamine in recovery from brain injury. Brain Inj. 1996;10:145–148. doi: 10.1080/026990596124647. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Ali SF, Haycock JW, Hope BT. Acute administration of cocaine regulates the phosphorylation of serine-19, -31 and -40 in tyrosine hydroxylase. J Neurochem. 2002;82:382–388. doi: 10.1046/j.1471-4159.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Behav. 1994;48:773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Koeda T, Takeshita K. A case report of remarkable improvement of motor disturbances with L-DOPA in a patient with post-diffuse axonal injury. Brain Dev. 1998;20:124–126. doi: 10.1016/s0387-7604(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Maki P. The combined use of amantadine and L-DOPA/carbidopa in the treatment of chronic brain injury. Brain Inj. 1997;11:455–460. doi: 10.1080/026990597123430. [DOI] [PubMed] [Google Scholar]
- Lal S, Merbtiz CP, Grip JC. Modification of function in head-injured patients with Sinemet. Brain Inj. 1988;2:225–233. doi: 10.3109/02699058809150946. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hokfelt T, Fisone G. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser(19) and Ser(40) via activation of glutamate NMDA receptors in rat striatum. J Neurochem. 2000;74:2470–2477. doi: 10.1046/j.1471-4159.2000.0742470.x. [DOI] [PubMed] [Google Scholar]
- Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after traumatic brain injury in rats. Neurosci Lett. 2004;372:127–131. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- Napolitano E, Elovic EP, Qureshi AI. Pharmacological stimulant treatment of neurocognitive and function deficits after traumatic and non-traumatic brain injury. Med Sci Monit. 2005;11:RA212–RA220. [PubMed] [Google Scholar]
- Phillips JP, Devier DJ, Feeney DM. Rehabilitation pharmacology: bridging laboratory work to clinical application. J Head Trauma Rehabil. 2003;18:342–356. [PubMed] [Google Scholar]
- Sawyer E, Mauro LS, Ohlinger MJ. Amantadine enhancement of arousal and cognition after traumatic brain injury. Ann Pharmacother. 2008;42:247–252. doi: 10.1345/aph.1K284. [DOI] [PubMed] [Google Scholar]
- Shui HA, Peng YI, Tsai YF. Recovery of high potassium-evoked dopamine release after depolazation challenge in the striatum of young and old male rats. Neurosci Lett. 1998;257:1–4. doi: 10.1016/s0304-3940(98)00801-5. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Giardina K, Gerhardt GA. In vivo microdialysis studies of age-related alterations in potassium-evoked overflow of dopamine in the dorsal striatum of Fischer 344 rats. Int J Dev Neurosci. 2000;18:411–416. doi: 10.1016/s0736-5748(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Urbanavicius J, Ferreira M, Costa G, Abin-Carriquiry JA, Wonnacott S, Dajas F. Nicotine induces tyrosine hydroxylase plasticity in the neurodegenerating striatum. J Neurochem. 2007;102:723–730. doi: 10.1111/j.1471-4159.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- Von Voightlander PF, Moore KE. Dopamine: release from the brain in vivo by amantadine. Science. 1971;174:408–410. doi: 10.1126/science.174.4007.408. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Chen X, Harun R, Clossin DP, Khan AS, Andres-Koback M, Michael AC, Dixon CE. Controlled cortical impact injury influences methylphenidate-induced changes in striatal dopamine neurotransmission. J Neurochem. 2009;110:801–810. doi: 10.1111/j.1471-4159.2009.06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pooler AM, Albrecht MA, Wurtman RJ. Dietary uridine-5′-monophosphate supplementation increases potassium-evoked dopamine release and promotes neurite outgrowth in aged rats. J Mol Neurosci. 2005;27:137–145. doi: 10.1385/JMN:27:1:137. [DOI] [PubMed] [Google Scholar]
- Waymire JC, Johnston JP, Hummer-Lickteig K, Lloyd A, Vigny A, Craviso GL. Phosphorylation of bovine adrenal chromaffin cell tyrosine hydroxylase. Temporal correlation of acetylcholine’s effect on site phosphorylation, enzyme activation, and catecholamine synthesis. J Biol Chem. 1988;263:12439–12447. [PubMed] [Google Scholar]
- Wilson MS, Chen X, Ma X, Ren D, Wagner AK, Reynolds IJ, Dixon CE. Synaptosomal dopamine uptake in rat striatum following controlled cortical impact. J Neurosci Res. 2005;80:85–91. doi: 10.1002/jnr.20419. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase in tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2007;1134:171–179. doi: 10.1016/j.brainres.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]