Abstract
Cancer cells exist in a constantly evolving tissue microenvironment of diverse cell types within a proteinaceous extracellular matrix. As tumors evolve, the physical forces within this complex microenvironment change, with pleiotropic effects on both cell- and tissue-level behaviors. Recent work suggests that these biomechanical factors direct tissue development and modulate tissue homeostasis, and, when altered, critically influence tumor evolution. In this review, we discuss the biomechanical regulation of cell and tissue homeostasis from the molecular, cellular and tissue levels, including how modifications of this physical dialogue could contribute to cancer etiology. Because of the broad impact of biomechanical factors on cell and tissue functions, an understanding of tumor evolution from the biomechanical perspective should improve risk assessment, clinical diagnosis and the efficacy of cancer treatment.
1. TUMOR EVOLUTION WITHIN A BIOMECHANICAL CONTEXT
Tumors are composed of a heterogeneous collection of cells surrounded by various soluble factors and an evolving extracellular matrix (ECM). In addition to the roles of genetic and biochemical events in tumor development, recent studies support the notion that biomechanical factors also critically direct tissue development, sculpt tissue organization and maintain tissue homeostasis 1. Central to this assumption is the concept that every tissue component (e.g. cells, proteins) is a biomaterial possessing unique mechanical properties that respond specifically to various physical forces. Mechanical inputs, such as tumor expansion leading to tissue compression and increased interstitial pressure, can increase both cell and tissue tension within the confined stroma, leading to the release, concentration and activation of various growth factors, ultimately assisting in tumor progression 2,3. Additionally, within the tumor, oncogene-mediated alterations in cellular actomyosin contractility and RhoGTPase activity can compromise cell-cell junction integrity to destroy tissue polarity and promote cell invasion while ECM remodeling and stiffening drive integrin clustering and actin remodeling to re-enforce focal adhesions. Taken together, these alterations enhance intracellular growth factor receptor signaling within the increased extracellular pool of activated growth factors, drive tumor cell growth and survival, and confer tumor drug resistance 4–6.
In this review, the effects of cell and tissue level forces on tissue behavior are discussed, with recent studies on the role of mechanics in tumor development and evolution highlighted. Because the composition and organization of tumors is continuously evolving, the influence of cell and tissue architecture on the material properties and physical behavior at the molecular, cellular and tissue levels are discussed. Finally, the clinical implications of current research on tumor mechanics, as well as future research directions, are discussed.
2. TISSUE MECHANICS AND MECHANOTRANSDUCTION
Cells and tissues experience various physical forces, which can be classified as externally applied or cell-generated. These physical forces can directly and indirectly affect many fundamental biological processes and, in turn, contribute to normal physiological and pathological phenomena. The direct impact of these forces on the cells and tissues subjected to these cues include displacement, deformation, and an alteration of tissue morphology and organization. For example, externally applied compression force can deform the ECM and decrease the interstitial space, which alters the transport and distribution of soluble factors within the ECM thereby modifying cell behavior 7. Indirect effects of mechanical force on tissues include changes in levels and/or activity of various growth, differentiation and motility regulators, as well as ECM remodeling. The specificity of these force-induced effects can depend on the direction of the force (e.g. tension, compression and shear forces, see Glossary) as well as its magnitude and duration. For instance, transient tensile forces up-regulate TGF- 1 expression in smooth muscle cells whereas constant tensile forces up-regulate both TGF- 1 and collagen I expression 8. Dynamic loading (Glossary) increases MMP-9 (Matrix metalloproteinase-9) in fibroblasts whereas static loading up-regulates MMP-2 9. Hydrostatic pressure decreases cell proliferation and increases hyaluronan production 10.
Importantly, cells can actively generate forces through multiple mechanisms including Rho-dependent actin-myosin contraction and actin assembly, and transmit these forces through cell-cell and cell-ECM interactions. These cell-generated forces contribute to the branching morphogenesis of both embryonic lungs and cultured epithelial cell cysts, facilitate blood vessel "sprouting" (angiogenesis), and influence convergent extension during embryonic development 11–13. Accumulating data demonstrate that cell-generated forces play broad roles in regulating cell survival, growth, migration and differentiation, as well as cell-cell and cell-ECM communication and the spatial organization of cells and tissues 14. Externally applied and cell-generated forces do not operate independently within normal tissues and instead are typically balanced through multiple mechanisms. This orchestrated behavior helps to maintain cell and tissue structure and homeostasis. To facilitate our understanding of how externally applied and cell-generated forces are transmitted in both normal physiological and pathological conditions, the basic concepts of the common mechanical properties of cells and tissues are reviewed below.
Mechanical properties of tissues, cells and ECM
Tissues are composed of multiple cell types, various ECM proteins and other constituents, each with unique mechanical characteristics such as elasticity, plasticity, viscosity, tensile strength and stiffness (Glossary). These physical properties collectively define the material properties of the tissue and dictate how the tissue responds to mechanical cues and how it will sense and transmit force. Specifically, the elasticity and stiffness of cells and tissues have been implicated in cancer biology. Methods such as tissue level tensile, compression and shear testing, and cellular and subcellular level atomic force microscopy (AFM) have been employed to measure these properties. These techniques have yielded valuable information that demonstrates the unique mechanical properties of each cell type that are reflected in its function, cellular origin and microenvironment (Table 1). These parameters allow the monitoring of differentiation or activation status of cells, as well as a method of assessing the state of disease progression.
Table 1.
Elastic Moduli of Tissues and Cells Involved in Cancer
| Normal or rest state | Pathological or activated state | ||
|---|---|---|---|
| Tissue | Breast 20,115,116 | 0.4 ~ 2 kPa | 4~12 kPa |
| Lung 117 | 10 kPa | 25 ~ 35 kPa | |
| Brain 48 | 0.26 ~ 0.49 kPa | 7 kPa | |
| Bone 116,118,119 | 2 ~ 14 G Pa | > 689 M Pa | |
| Liver 120,121 | 0.3 ~ 0.6 kPa | 1.6 ~20 kPa | |
| Cells | Epithelial Cells 76,122 | ~ 2 kPa | ~ 0.4 kPa |
| Fibroblasts 78 | ~ 0.4 kPa | ~1 kPa | |
| Mesenchymal stem cells 123 | 0.25 ~ 0.9 kPa | N/A | |
| Macrophages 79 | 1.5 kPa | 0.5 kPa | |
| Myeloid 124 | N/A | 0.17 ~ 1.5 kPa (HL60 cells) | |
| T lymphocyte 124 | 0.013 ~ 0.083 kPa (Jurkat) | N/A | |
| Neutrophils 124 | 0.07 ~ 0.24 kPa | N/A | |
The viscoelasticity of a tissue is dictated by its ECM and individual cellular constituents. Differences in the elastic moduli of various cell types or cellular states are partly due to structural variations in the cytoskeletal elements including filamentous actin, intermediate filaments and microtubules, and their organization 15. In response to biochemical and biomechanical cues from their local environment (e.g. ECM and adjacent interacting cells), cells can tune their elastic moduli by altering their transmembrane receptors, intracellular cytoskeletal organization, actomyosin contraction and cytoskeletal tension, and remodel the local microenvironment to achieve mechanical equilibrium (Figure 1). This active adaptation to the rigidity of the environment also contributes to the elasticity of a cell 16,17. Cellular elasticity and cell-generated forces are therefore closely related; the intracellular and extracellular mechano-responsive elements are linked by these dynamic and reciprocal conversations (Figure 1). Together, the extracellular and intracellular forces, the mechano-responsive elements, their mechanical properties and the cross-talk with intracellular signaling pathways maintain mechanical equilibrium and regulate diverse cellular behaviors such as adhesion, spreading, receptor signaling, gene expression and extracellular microenvironment remodeling 7. This mechanical regulatory paradigm is essential for normal tissue structure and function, as demonstrated by the fact that tissue-specific cells often prefer mechanical microenvironments that closely mimic those of their native tissues such as the improved growth of CNS neurites in neurite and glial cell co-cultures and the morphogenesis of normal mammary epithelial cells on soft matrices 18–20.
Figure 1. Dynamic and reciprocal conversation between matrix stiffness and cell tension.
Non-malignant cells can respond to exogenous mechanical forces and matrix stiffness by enhancing focal complex maturation, resulting in Rho and ROCK activation, MLC phosphorylation and actomyosin contraction. Cell-generated forces from increased myosin contraction feed into focal adhesion maturation to adjust focal adhesion size and contract the ECM until the exogenous forces are balanced and the elastic modulus of cells are “tuned” to reflect the ECM stiffness. This mechano-signaling circuit is crucial in the dynamic and reciprocal conversation between exogenous and cell-generated mechanical factors. (ROCK, Rho-associated kinase; MLC, myosin light chain.) (Adapted with permission from [20].)
Mechano-sensing and transduction
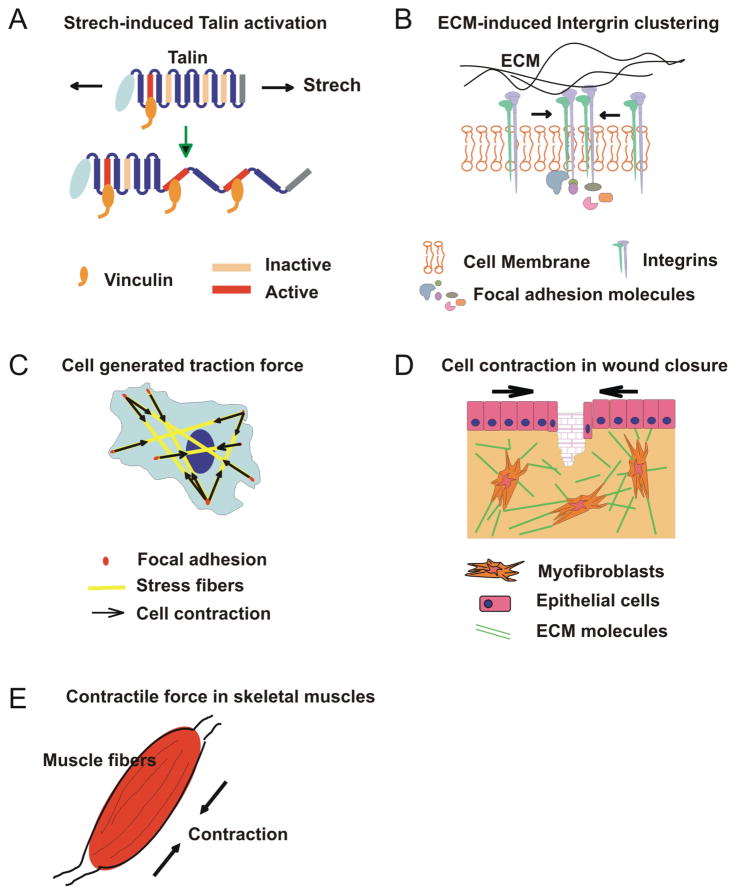
To respond to a mechanical stimulus, the cell must possess elements capable of responding to the applied force and translating this mechanical information into a biochemical signal. These cellular mechano-signaling pathways often overlap, feed into, and are themselves regulated by biochemical cascades. Several mechano-sensors that respond to different magnitudes and types of force have been identified, including highly specialized structures such as the mechano-sensory apparatus of the auditory hair bundle and the primary cilia in tubular epithelial cells 21,22. Biochemical signaling can be initiated by force-induced conformational changes and exposure of functional sites of signaling proteins (e.g. tension-induced conformation changes in fibronectin, intracellular talin and membrane ion channels) (Figure 2A) 23–25. Alternatively, intracellular signaling cascades can be activated by force-mediated alterations in membrane curvature, tension, and the distribution of membrane signaling molecules as seen with the transactivation of Ephrin receptors 26 and with integrin clustering 27 (Figure 2B). Cells can also sense force via cell-cell junctions deformed by mechanical forces, leading to a global remodeling of their cytoskeletal filaments (Figure 2C) 28,29. Many of these mechano-sensitive mechanisms operate concurrently, overlapping and interacting with simultaneously occurring biochemical pathways. When these mechano-sensing and transudation events at cellular and subcellular level are coordinated at the multicellular and organ levels, they contribute to tissue and organ functions, such as wound closure and muscle contraction (Figure 2, D and E).
Figure 2. Examples of mechanical behaviors at different levels of biological systems.
A) At the single-molecule level, mechanical stretching of talin rods exposes the cryptic binding sites for vinculin, which then activates downstream biochemical signaling pathways important in cell signaling, adhesion, and migration. B) At the multimolecule-level, increased matrix stiffness enhances integrin clustering, promotes large focal adhesions and activates downstream signaling cascades. C) At the single-cell level, cells can generate traction forces via actin polymerization (e.g. stress fibers) and actomyosin contraction between focal adhesions. D) At the tissue level, myofibroblasts differentiated from fibroblasts at wound sites exert contractile forces on the surrounding ECM and rearrange the ECM to close wounds. E) At the organ level, a muscle that contains multiple muscle fibers surrounded by connective tissues and sheaths can contract synchronously, generating tension and muscle motion upon the reception of signals from motor neurons.
3. DYNAMIC DIALOGUE BETWEEN BIOMECHANICAL AND BIOLOGICAL SIGNALING DURING TUMOR EVOLUTION
Normal tissue structure is disrupted during tumor initiation and progression. The microenvironment becomes both mechanically and biologically active, highlighted by continuous and progressive remodeling of the tumor mass and the stromal compartments. Within the tumor mass, the transformation of tumor cells is accompanied by increased cell division, reduced apoptosis, loss of tissue polarity, and alterations in the composition and organization of extracellular matrix components. Adjacent to the tumor mass, the tumor stroma assists tumor development via multiple stromal cells, ECM molecules, soluble factors and the circulatory systems (blood and lymphatic vessels). Tumor-associated stromal cells, including fibroblasts, myofibroblasts, endothelial cells, mesenchymal stem cells, inflammatory cells and immune cells are often recruited, locally differentiated or activated during different tumor development stages. These cells actively participate in ECM remodeling and tumor angiogenesis, providing growth factors and chemokines that promote tumor growth and metastasis 30,31. The non-cellular components of the tumor stroma, such as collagens, fibronectin, tenascin and proteoglycans, can be abnormally expressed and remodeled, resulting in new biochemical and mechanical signals 32. Soluble signals, produced by tumor or stromal cells, released from cleaved ECM molecules and delivered from the circulatory system, can re-distribute within this tumor stromal compartment and regulate cell functions and ECM composition during tumor progression 32.
Different from the well-maintained tissue homeostasis and mechanical equilibrium in normal physiological conditions, the loss of growth control and the disrupted tissue structure and organization during tumor evolution lead to unbalanced physical forces and altered material properties of each tumor component. As the behavior, structure and organization of tumors are continuously changing as the cancer evolves, the mechanical state in a tumor also evolves as the tumor develops (Figure 3). In breast cancer, the mechanical characteristics in a hyperplasia, carcinoma in situ, invasive lesion and metastasis lesion can be very different. A hyperplastic lesion typically involves the loss of normal cell polarization and organization, cell-cell contacts and cell-ECM interactions, which result in altered cellular tension and mechano-sensing and transduction 33,34. Increased matrix deposition, cell proliferation and altered cell tension in hyperplastic lesions also result in the thickening and remodeling of the basement membrane (BM) architecture (Figure 3A) 35. Carcinoma in situ is characterized by active cell growth within an intact BM and interstitial ECM. This uncontrolled cell growth confined by an intact BM leads to a restricted tumor volume expansion and corresponding reaction forces within the various BM and stromal components 36–38. In turn, the resistance of the BM and ECM to the expanding tumor mass leads to compression of the tumor mass 39. Simultaneously, ECM remodeling, as a consequence of tumor compression and stromal reaction, results in altered mechanical properties of the ECM that can further increase cell-generated forces and cell tension (Figure 3B) 40. In advanced carcinoma in situ lesions, intra-tumor pressure can be further elevated due to hypoxia and necrosis. Tumor and stromal cells secrete soluble factors, facilitating matrix remodeling and angiogenesis 41. Inefficient transport and dense ECM networks result in further increases in interstitial fluid pressure within the tumor 42. Increased interstitial flow due to blood vessel and BM permeability in the tumor microenvironment can promote TGF- dependent myofibroblast differentiation 43. In invasive tumors, cell-cell interactions further decrease and intracellular contractility increases, leading to the dissemination of tumor cells from the tumor mass and invasion through the BM and interstitial ECM. Invading cells are accompanied by non-transformed stromal cells (e.g. fibroblasts, macophages) and migrate through a progressively stiffened ECM and bio-chemical gradients towards the circulatory system 44–47. Various mechanical forces, such as interstitial compression and shear, interstitial fluidic pressure and ECM stiffness can critically influence the rate and direction of tumor cell migration (Figure 3C) 48. During intravasation, transportation (in the bloodstream or lymphatic flow) and extravasation, tumor cells are exposed to shear forces from adjacent cells and hydrodynamic flow. These shear forces assist tumor cell transport and facilitate interactions with leukocytes and endothelial cells to permit extravasation (Figure 3D) 49. Cancer cells display organ-specific metastasis which can depend on the intrinsic genetics of the tumor cells, the affinity of tissues to host a metastatic lesion and the pattern of circulation within the tissue 50–52. Additionally, different organs exhibit very different mechanical properties (e.g. lung is soft whereas bone is very stiff). Since cells can selectively grow on and within specific substrates according to their mechanical properties, organ-specific mechanical properties may contribute to the preferential migration, attachment, survival and proliferation of cancer cells in specific organs (Figure 3E and 3F) (Table 1) 48,53–55.
Figure 3. Tumor progression is associated with continuous alterations in tissue and cell mechanics.
A) In hyperplastic lesions, cells gradually lose polarity and grow into the luminal space. The myoepithelial cell layer and the basement membrane (BM) remain intact and the surrounding ECM is compliant. B) In carcinoma in situ lesions, cell polarity is lost and the lumen is filled by proliferating cells. This volume expansion and resistance from the BM and interstitial ECM lead to increased forces between tumor cells and the stromal matrix. Simultaneously, ECM components are abnormally deposited and remodeled, which results in increased ECM and tissue stiffness, and in turn, cell-generated tension. C) In invasive lesions, tumor cells break down the BM and invade into the interstitial ECM. The reciprocal forces between tumor cells and the ECM continuously increase. Abnormal deposition and remodeling of ECM collagen further increase ECM and tissue stiffness. Tumor cells generate greater tension in response to this increased mechanical stimulation. As tumor cells invade through the BM and ECM, they experience a range of different forces from the dense ECM network. These external forces together with genetic and epigenetic events can change the contractility and viscoelasticity of tumor cells. D) During intravasation and extravasation, tumor cells experience various forces including shear forces exerted by the ECM, blood flow and neighboring cells, which facilitate their transport and attachment to the endothelium. E) and F) Cancer cells often metastasize to different organs, which can have very different microenvironmental and mechanical properties (e.g. E. soft tissue lung, F. stiff tissue bone). The mechanics of remote tissues and cancer cells may regulate cell dormancy, proliferation and differentiation in these organs.
Influence of the ECM and tumor cell biomechanics on tumor progression
ECM mechanics broadly impact cell transcription, cell cycle control, cell-cell interactions, cell differentiation and migration. The mechanical influences of the ECM in tumor progression directly depend on the ECM composition, structure and organization, as well as the mechanical dialogue with intracellular mechano-responsive elements and cell generated forces (Figure 1) 32,56,57. Recent work demonstrates that breast tumor progression is often accompanied by increased deposition, cross-linking and de-regulated cleavage of type I collagen 58. This collagen remodeling is largely due to the increased expression and activity of various enzymes (lysyl oxidase, transglutaminase and MMPs) in the active tumor stroma 40,59–62. Increased collagen cross-linking and resulting tissue stiffening intensify the biomechanical feedback in breast tissue and promote breast tumor progression 40. Disrupting this feedback by targeting the cross-linking of fibrillar collagen through inhibition of the enzyme lysyl oxidase could delay both malignant transformation and tumor progression (Figure 4) 40. In addition, cell migration can be guided by the gradient of ECM stiffness (“durotaxis”) 63,64, indicating such a gradient may serve as an important cue leading the directional migration of cancer cells in the interstitial ECM toward the intravasation sites. Other ECM proteins, such as fibronectin, tenasin, decorin, fibromodulin, lumican, and osteopontin, are also involved in tumor development and modify the mechanical properties of the ECM; however, their roles in tumor mechanics and tumor development have yet to be clarified 32,65–67.
Figure 4. Increased collagen cross-linking and matrix stiffness modify the context of signaling and promote invasion of oncogene-transformed pre-malignant mammary cells.
A) MCF10A cells (non-malignant mammary epithelial cells) expressing either a drug-activated ErbB2/NGFR (NGFR, neural growth factor receptor) chimera or a tetracycline-inducible ErbB2 construct form polarized, growth-arrested colonies in soft collagen/rBM gels (bottom left). Stiffening the collagen gel (by adding ribose to cross-link the collagen) or activating ErbB2 signaling increases cell proliferation but fails to drive cell invasion (bottom right and top left). MCF10A colonies start invading into collagen gels only when the collagen gels are stiffened and ErbB2 signaling is activated. (top right) (Bar 20 m;-catenin, green; 4 integrin, red; DAPI, blue.) Second harmonic generation images show the collagen alignment and bundling around the colonies (insert). B) Schematic presentation of the cooperation between matrix stiffening and ErbB2 signaling in driving the invasive phenotype. Increased collagen cross-linking stiffens the ECM, which drives integrin clustering and promotes focal adhesion assembly, thereby activating PI3K and potentiating ErbB2/PI3K/Akt signaling. (Adapted with permission from [40].)
Growth factors bound by the ECM can be released and activated by mechanical perturbation. For example, mechanical stretch or contraction of the ECM can release ECM-bound TGF- into the extracellular space, increasing the availability of active TGF- 3,68. TGF- plays a key role during tumor progression; it is a strong chemoattractant for both monocytes and macrophages, a stimulant for pro-angiogenic factors such as bFGF (basic Fibroblast Growth Factor), MCP-1 (Monocyte Chemotactic Protein-1), TNF- (Tumor necrosis factor ) and IL-1 (Interleukin-1 ), an activator of ECM remodeling enzymes, a potent activating and differentiating signal for stromal fibroblasts, and a key regulator of the modes of cancer cell motility 69,70. Fibroblasts differentiated into myofibroblasts are able to generate stronger adhesions and greater cellular contractility, which, in turn, increase ECM tension, remodeling, and deposition that potentiate the further release of TGF- from the ECM 68. Indeed, TGF- signaling and myofibroblast differentiation associate with invasive human breast cancers, implying that the initiation of mechanical signaling by TGF- may be very important in tumor development. Similarly, the expression and release of other factors, such as PDGF (platelet-derived growth factor), VEGF (vascular endothelial growth factor) and bFGF, which are also involved in tumor cell growth, angiogenesis, cell invasion, and matrix deposition, can also be regulated by mechanical loading and mechanical properties of substrates 71–74.
Tumor cells exhibit very different mechanical properties than their normal counterparts (Table 1). Studies with isolated cancer cells suggest that they become increasingly compliant as they transform, such that highly metastatic tumor cells are less stiff than normal cells 75,76. However, this point is still under contention as all of these measurements were conducted in culture and the apparent viscoelasticity of living cells in situ and in isolation can be very different. Indeed, the viscoelasticity of living cells can be substantially modified by many factors present in the context of a three-dimensional tissue including heterotypic cell-cell interactions, localized effects of the vasculature and the ECM. In this respect, isolated cancer cells are hypersensitive to substrate stiffness 20,54,77 and exhibit elevated actomyosin-generated contractility when compared to matched normal cells 20. The increased cell contractility exhibited by tumor cells is mediated by increased activation of MLCK (myosin light chain kinase) and acto-myosin contraction through elevated Rho GTPase activity and EGFR signaling (Figure 1). Pharmacologically or genetically inhibiting these pathways is sufficient to reduce cell tension and normalize tumor tissue phenotype 20. These results suggest that the intrinsic adhesion and cytoskeletal behavior of cancer cells that participate in their tension behavior contribute to their tumor phenotype. This means that enhanced mechano-responsiveness coupled with increased stiffening of the tissue ECM could contribute to the progressive and incremental stiffening of tumor cells in situ (Lopez et al., unpublished). Conversely, inhibiting cell or ECM tension may inhibit tumor progression 40. Notably, ECM stiffness varies quite dramatically within the same tumor and ECM organization is non-uniform, providing a provocative explanation for some of the variability noted in tumor cell behavior within a cancerous tissue in vivo (Table 1) 40 (Lopez et al., unpublished). The discrepancy between in situ analysis and those studies using isolated tumor cells underscores the influences of the tissue microenvironment on cellular mechanical properties and the intrinsic differences of the mechanical properties between transformed and normal cells.
Importantly, the stromal cells associated with tumors also exhibit changes in their viscoelastic properties as tumors progress. Activated, highly contractile myofibroblasts, which frequently appear quite early during tumor progression, are stiffer than their non-malignant counterparts 78. Additionally, activated tumor-associated macrophages are more compliant than resting macrophages 79 and tumor-derived endothelial cells exhibit enhanced mechanosensing 80 (Table 1). These stromal cells, as well as infiltrating lymphocytes, monocytes and mesenchymal stem cells, frequently participate in the remodeling of interstitial collagen and produce a wide array of growth factors, cytokines and chemokines which help to establish the chemical and rigidity gradients for the growth, transformation and directional metastasis of tumor cells 46,63,81–84. Indeed, intravital imaging has shown that cancer cells and leukocytes migrate rapidly in collagen-rich regions 47,85 and that paracrine signaling between cancer cells and leukocytes facilitate the directional migration of cancer cells 82. Considering the many genetic and epigenetic modifications that occur in tumor-associated stromal cells 86 and the functional diversity of these cells, it is clear that it will be important to study the mechano-sensitivity of the vast array of tumor-related cells and to understand how ECM remodeling, mechanical regulation and stromal cell activities contribute to tumor progression.
Tissue mechanics and oncogenic transformation
Tumor cells encounter various ECM environments and physical forces during tumor initiation, progression, and metastasis (Figure 3). Concurrently, tumor cells undergo malignant transformation, adopting a series of genetic and epigenetic changes, including genetic mutations and expression changes of different ECM adhesion receptors, cell adhesion receptors, growth factor receptors and intra-cellular signaling molecules. These changes modify the ability of tumor cells to sense and respond to external and internal forces, as well as to the mechanical properties of other cells and the ECM. One widely studied family of mechano-sensors is the cell surface integrin family 87. Integrins can mediate the sensing of mechanical properties of the ECM by changing their avidity, conformation, clustering, and recruitment, and transducing these signals downstream to focal adhesion kinase (FAK), which then leads to the stabilization of focal adhesions and the activation of downstream intracellular signaling cascades. Stiff ECM substrates increase integrin clustering, and induce focal adhesion formation and FAK activation, which intensifies the oncogene ErbB2-mediated PI3K (Phosphoinositide 3-kinase) and ERK (extracellular signal-regulated kinase) signaling pathways and promotes tumor cell malignant transformation in both 3D culture and mouse models for breast cancer (Figure 4) 20,40. Inhibiting Rho GTPase-induced contractility normalizes tumor cell behavior and the inhibition of ECM stiffening delays oncogene-induced tumor progression. Other studies have demonstrated that elevated Rho signaling via oncogene Ras-driven ERK activation or ErbB2-driven PI3K induce cytoskeletal contractility, cell growth, and destabilize tissue architecture 88. These small GTPases and their effectors are key regulators of cytoskeleton dynamics, cell polarity and migration, and are often found over-expressed in different types of human tumors 89–93. Thus, knocking out specific small GTPase effectors including Tiam (a Rac activation factor) and GEP100 (an Arf6 activation factor) in mice reduced tumor incidences and metastasis 94,95. Taken together, these data suggest that the crosstalk between mechanical and oncogene signaling pathways is essential for oncogene-initiated tumor progression. Accordingly, targeting these abnormal mechanical stimuli and mechanotransduction pathways with pharmacological reagents could potentially be of benefit to clinical cancer therapies.
4. CONCLUSION AND FUTURE DIRECTIONS
Tumors are composed of heterogeneous tumor cell populations. This heterogeneity originates from genetic instability and/or the differentiation spectrum of cancer stem cells (CSCs), potentially leading to drug resistance in cancer therapies 96. Therefore, an understanding of the properties and regulation of tumor heterogeneity might improve clinical cancer treatment. As discussed in this review, the tumor microenvironment is also heterogeneous and can induce a series of non-uniform biological and biomechanical modifications in tumor cells and the surrounding ECM. Particularly, the biomechanical changes in a tumor microenvironment caused by multiple variable factors including tumor growth and expansion, increased interstitial pressure, cell contraction and ECM deposition and remodeling can modify the biochemical and biomechanical properties of both the tumor stromal microenvironment and tumor cells. This biomechanically modified tumor microenvironment exerts a higher resistance to drug delivery and penetration 97, enhancing the survival of tumor cells due to mechano-chemical coupling in the three-dimensional context and rendering the cells further resistant to drug-induced cell death 98. Drugs targeting certain oncogenes or signaling pathways may be less effective when these oncogenes and signaling pathways are connected to mechanical signaling at multiple levels (e.g. integrins, ERK, PI3K, Rho GTPase) (Figure 1, Figure 4).
Current work suggests CSCs contribute to drug resistance because of their special properties, including the ability to remain quiescent during tumor progression as well as their increased resistance to DNA damage and external environmental insults 99. What remains unknown is whether the tumor microenvironment also regulates these special properties of CSCs. CSCs have similar properties as stem cells, such as self-renewal, lineage differentiation and residence within specific niches. Studies on embryonic and adult stem cells revealed that self-renewal and differentiation can be regulated by various mechanical cues 100, suggesting that CSCs may also be similarly regulated by tissue mechanics. Indeed, the frequency of cancer stem cells is very sensitive to different microenvironments 101. CSCs express abundant adhesion molecules including integrins and CD44 102–104 and require specialized niches composed of soluble factors (e.g. Wnt, Notch, hedgehog, and TGF- ) 105–107, stromal cells 108,109, and tension-regulated ECM proteins (e.g. tenascin, fibronectin and laminin) for their self-renewal and differentiation 110,111. Many of these niche components can modulate and be modulated by cell- and tissue-level tension to regulate cell growth, survival and migration 112–114. Therefore, the properties of CSCs may be directly and indirectly regulated by the tumor microenvironment. If so, the drug resistance of CSCs may be potentially addressed by targeting the mechanical links in the CSC niches (ECM, stromal cells, and soluble signals). Clearly, elucidating the composition and structure of tumor microenvironment and their local and global mechanical influence on tumor phenotype and pathogenesis will be important for the understanding of cancer biology and for the treatment of multiple cancer types.
Both biochemical and biomechanical factors contribute crucial information to tumor development and evolution. Integral to this dialogue is the complex interplay between soluble factors, cell-cell and cell-ECM interactions and the mechanical environment, which cooperatively drive tumor progression. Indeed, we and others have demonstrated that genetic and epigenetic changes in cells combine with alterations in matrix architecture and material properties, propelling tumor evolution. However, many questions linking biomechanics and tumor progression still require resolution. Traditional cell biology approaches may need supplementation with techniques from materials science, engineering and physics. It will be critical to clarify the molecular basis of mechanotransduction in the development and progression of tumors to identify novel anticancer therapeutic targets.
Acknowledgments
We acknowledge Christian Frantz for contributing valuable figures and discussions. We apologize to the authors whose work is not cited due to space limitation. This work was supported by DOD- W81XWH-05-1-0330, NCI- U54CA143836-01 (to V.M. Weaver) and DOD BC062562 (to J.K. Mouw).
GLOSSARY
- Tension
a load that acts in the direction perpendicular to a surface and tends to pull an object apart
- Compression
a load that acts in the direction perpendicular to a surface and tends to push an object
- Tensile strength
the maximum amount of tensile stress that a material can be subjected to before failure. (Unit:force per unit area)
- Stress
describes the internal resistance of a material to distortion by an external force (average force per unit area). There are three basic stresses:tensile, compression and shear stress. Tensile and compression stress are the stresses normal to the cross-sectional area of a body; Shear stress is the stress tangential to the cross-sectional area of a body. (Unit:forces per unit area)
- Strain
the ratio of the change in length to the original length of a material in the loading direction (Dimensionless)
- Stiffness
describes the elasticity of a material or the property of restoration to its original shape after deformation. The unit of stiffness is force per unit length, which can be determined by the slope of the load-displacement curve in the linear region of loading. (Unit:force per unit length). Compliance is inversely related to stiffness
- Elasticity
describes the ability of the tissue to return to its original shape after a load is removed. Mathematically, elasticity is described by the Modulus of elasticity, which is defined as the ratio of stress to strain. For example, Young's modulus (E) describes the elasticity of a material subjected to tensile or compression loading: Shear modulus (G) describes the shear elasticity of a material subjected to shear loading. E and G can be related by:E= 2G (1+ ) where is the Poisson’s ratio (Ratio of lateral strain to axial strain in an axial-loaded material)
- Viscoelasticity
is the property of materials that exhibit both elastic and viscous properties when undergoing deformation. Most biological materials are viscoelastic. The strain of viscous materials is time-dependent, whereas that of elastic materials is time-independent. A dynamic test of viscoelastic materials is often performed to determine the frequency-dependent complex shear modulus, which contains the elastic storage modulus and the viscous loss modulus
- Dynamic tests
refers to material tests with periodic deformation or frequency-dependent loading, such as the shear rheometric test. Static tests refer to those tests with gradually increasing force at a slow speed, such as the classic tensile test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 2.Yang JH, Sakamoto H, Xu EC, Lee RT. Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol. 2000;156(5):1797–1804. doi: 10.1016/S0002-9440(10)65051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1(10):pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egeblad M, Littlepage LE, Werb Z. The fibroblastic coconspirator in cancer progression. Cold Spring Harb Symp Quant Biol. 2005;70:383–388. doi: 10.1101/sqb.2005.70.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454 (7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-beta1 and alpha1(I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol. 1999;277(5):G1074–1080. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- 9.Prajapati RT, Chavally-Mis B, Herbage D, Eastwood M, Brown RA. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000;8(3):226–237. doi: 10.1046/j.1524-475x.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 10.Toda S, Watanabe K, Yokoi F, Matsumura S, Suzuki K, Ootani A, Aoki S, Koike N, Sugihara H. A new organotypic culture of thyroid tissue maintains three-dimensional follicles with C cells for a long term. Biochem Biophys Res Commun. 2002;294(4):906–911. doi: 10.1016/S0006-291X(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 11.Moore KA, Huang S, Kong Y, Sunday ME, Ingber DE. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J Surg Res. 2002;104 (2):95–100. doi: 10.1006/jsre.2002.6418. [DOI] [PubMed] [Google Scholar]
- 12.Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J Cell Biology. 2003;162(7):1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11(7):859–864. doi: 10.1038/ncb1894. [DOI] [PubMed] [Google Scholar]
- 14.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463 (7280):485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Tolic'-Nørrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic' D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282(3):C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 18.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22(10):1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 19.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90(8):3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7(1):19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 22.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 23.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 24.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323 (5914):638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sbrana F, Sassoli C, Meacci E, Nosi D, Squecco R, Paternostro F, Tiribilli B, Zecchi-Orlandini S, Francini F, Formigli L. Role for stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am J Physiol Cell Physiol. 2008;295(1):C160–172. doi: 10.1152/ajpcell.00014.2008. [DOI] [PubMed] [Google Scholar]
- 26.Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327 (5971):1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5(12):e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167(6):1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao M, Sotomayor M, Villa E, Lee EH, Schulten K. Molecular mechanisms of cellular mechanics. Phys Chem Chem Phys. 2006;8(32):3692–3706. doi: 10.1039/b606019f. [DOI] [PubMed] [Google Scholar]
- 30.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 31.Whiteside TL. The role of immune cells in the tumor microenvironment. Cancer Treat Res. 2006;130:103–124. doi: 10.1007/0-387-26283-0_5. [DOI] [PubMed] [Google Scholar]
- 32.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borghi N, Nelson JW. Intercellular adhesion in morphogenesis: molecular and biophysical considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20(5):551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18(5):356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15(8):778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 37.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427 (6976):695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 38.Volokh KY. Stresses in growing soft tissues. Acta Biomater. 2006;2(5):493–504. doi: 10.1016/j.actbio.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Sarntinoranont M, Rooney F, Ferrari M. Interstitial stress and fluid pressure within a growing tumor. Ann Biomed Eng. 2003;31(3):327–335. doi: 10.1114/1.1554923. [DOI] [PubMed] [Google Scholar]
- 40.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139 (5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tlsty TD, Coussens LM. Tumor Stroma and Regulation of Cancer Development. Annu Rev Pathol Mech Dis. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 42.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 43.Chee PN, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118(20):4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 44.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60(9):2504–2511. [PubMed] [Google Scholar]
- 45.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 46.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124 (2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Lohela M, Werb Z. Intravital imaging of stromal cell dynamics in tumors. Curr Opin Genet Dev. 2010;20(1):72–78. doi: 10.1016/j.gde.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28(1–2):113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng. 2008;36(4):661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 51.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2008 doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kostic A, Lynch CD, Sheetz MP. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One. 2009;4(7):e6361. doi: 10.1371/journal.pone.0006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam WA, Cao L, Umesh V, Keung AJ, Sen S, Kumar S. Extracellular matrix rigidity modulates neuroblastoma cell differentiation and N-myc expression. Mol Cancer. 2010;10(9):35. doi: 10.1186/1476-4598-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C. A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS One. 2010;5(1):e8849. doi: 10.1371/journal.pone.0008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vakonakis I, Campbell ID. Extracellular matrix: from atomic resolution to ultrastructure. Curr Opin Cell Biol. 2007;19:578–583. doi: 10.1016/j.ceb.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buehler MJ. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci U S A. 2006;103(33):12285–12290. doi: 10.1073/pnas.0603216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6(11) doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 62.Sabeh F, Li XY, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284(34):23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hadjipanayi E, Mudera V, Brown RA. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton. 2009;66(3):121–128. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 65.Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD, Brekken RA. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech. 2010;3(1–2):57–72. doi: 10.1242/dmm.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106(43):18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oldberg A, Kalamajski S, Salnikov AV, Stuhr L, Mörgelin M, Reed RK, Heldin NE, Rubin K. Collagen-binding proteoglycan fibromodulin can determine stroma matrix structure and fluid balance in experimental carcinoma. Proc Natl Acad Sci U S A. 2007;104(35):13966–13971. doi: 10.1073/pnas.0702014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179 (6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21(1):49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A. 2009;106(36):15279–15284. doi: 10.1073/pnas.0905891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium. 2001;8 (1):41–48. doi: 10.3109/10623320109063156. [DOI] [PubMed] [Google Scholar]
- 73.Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, McAllister DR. The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue Eng. 2007;13(11):2721–2731. doi: 10.1089/ten.2006.0434. [DOI] [PubMed] [Google Scholar]
- 74.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J Cell Physiol. 2010;225(1):115–122. doi: 10.1002/jcp.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3(4):413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cross SE, Jin YS, Tondre J, Wong R, Rao JY, Gimzewski JK. AFM-based analysis of human metastatic cancer cells. Nanotechnology. 2008;19 (38):384003–384011. doi: 10.1088/0957-4484/19/38/384003. [DOI] [PubMed] [Google Scholar]
- 77.Baker EL, Bonnecaze RT, Zaman MH. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J. 2009;97(4):1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park S, Koch D, Cardenas R, Käs J, Shih CK. Cell motility and local viscoelasticity of fibroblasts. Biophys J. 2005;89(6):4330–4342. doi: 10.1529/biophysj.104.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leporatti S, Gerth A, Köhler G, Kohlstrunk B, Hauschildt S, Donath E. Elasticity and adhesion of resting and lipopolysaccharide-stimulated macrophages. FEBS Lett. 2006;580(2):450–454. doi: 10.1016/j.febslet.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105(32):11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 82.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69(24):9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Isenberg BC, Dimilla PA, Walker M, Kim S, Wong JY. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J. 2009;97(5):1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 85.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1(2–3):155–167. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer. 2008;44(18):2760–2765. doi: 10.1016/j.ejca.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker EL, Zaman MH. The biomechanical integrin. J Biomech. 2010;43(1):38–44. doi: 10.1016/j.jbiomech.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81(5):682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 90.Bellizzi A, Mangia A, Chiriatti A, Petroni S, Quaranta M, Schittulli F, Malfettone A, Cardone RA, Paradiso A, Reshkin SJ. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int J Mol Med. 2008;22(1):25–31. [PubMed] [Google Scholar]
- 91.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129 (5):865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 92.Engers R, Zwaka TP, Gohr L, Weber A, Gerharz CD, Gabbert HE. Tiam1 mutations in human renal-cell carcinomas. Int J Cancer. 2000;88(3):369–376. doi: 10.1002/1097-0215(20001101)88:3<369::aid-ijc8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 93.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2 (2):133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 94.Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, Mazaki Y, Kodama H, Nio Y, Manabe T, Wada H, Kobayashi H, Sabe H. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol. 2008;10(1):85–92. doi: 10.1038/ncb1672. [DOI] [PubMed] [Google Scholar]
- 95.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4(8):621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 96.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 97.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 98.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14(1):71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Baguley BC. Multidrug resistance in cancer. Methods Mol Biol. 2010;596:1–14. doi: 10.1007/978-1-60761-416-6_1. [DOI] [PubMed] [Google Scholar]
- 100.Keung AJ, Kumar S, Schaffer DV. Presentation Counts: Microenvironmental Regulation of Stem Cells by Biophysical and Material Cues. Annu Rev Cell Dev Biol. 2010 Jun 29; doi: 10.1146/annurev-cellbio-100109-104042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456 (7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fässler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10(6):716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirkland SC, Ying H. Alpha2beta1 integrin regulates lineage commitment in multipotent human colorectal cancer cells. J Biol Chem. 2008;283(41):27612–27619. doi: 10.1074/jbc.M802932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawaguchi-Ihara N, Murohashi I, Nara N, Tohda S. Promotion of the self-renewal capacity of human acute leukemia cells by Wnt3A. Anticancer Res. 2008;28(5A):2701–2704. [PubMed] [Google Scholar]
- 106.Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, Lockwood G, Gallinger S, Egan SE. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33(6):1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michael LE, Westerman BA, Ermilov AN, Wang A, Ferris J, Liu J, Blom M, Ellison DW, van Lohuizen M, Dlugosz AA. Bmi1 is required for hedgehog pathway-driven medulloblastoma expansion. Neoplasia. 2008;10(12):1343–1349. doi: 10.1593/neo.81078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322 (5909):1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 109.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7(10):733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 110.Liu JM, Mao BY, Hong S, Liu YH, Wang XJ. The postoperative brain tumour stem cell (BTSC) niche and cancer recurrence. Adv Ther. 2008;25(5):389–398. doi: 10.1007/s12325-008-0050-x. [DOI] [PubMed] [Google Scholar]
- 111.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1(6):607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whitehead J, Vignjevic D, Fütterer C, Beaurepaire E, Robine S, Farge E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2008;2(5):286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15(3):470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 114.Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16(20):1986–1997. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Samani A, Bishop J, Luginbuhl C, Plewes DB. Measuring the elastic modulus of ex vivo small tissue samples. Phys Med Biol. 2003;48(14):2183–2198. doi: 10.1088/0031-9155/48/14/310. [DOI] [PubMed] [Google Scholar]
- 116.Gefen A, Dilmoney B. Mechanics of the normal woman's breast. Technol Health Care. 2007;15(4):259–271. [PubMed] [Google Scholar]
- 117.Ebihara T, Venkatesan N, Tanaka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. Am J Respir Crit Care Med. 2000;152(4 pt1):1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- 118.Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech. 1999;32 (10):1005–1012. doi: 10.1016/s0021-9290(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 119.Odgaard A, Linde F. The underestimation of Young's modulus in compressive testing of cancellous bone specimens. J Biomech. 1991;24(8):691–698. doi: 10.1016/0021-9290(91)90333-i. [DOI] [PubMed] [Google Scholar]
- 120.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 121.Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28(4):467–474. doi: 10.1016/s0301-5629(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 122.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88(5):3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan SC, Pan WX, Ma G, Cai N, Leong KW, Liao K. Viscoelastic behaviour of human mesenchymal stem cells. BMC Cell Biol. 2008;9:40. doi: 10.1186/1471-2121-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenbluth MJ, Lam WA, Fletcher DA. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys J. 2006;90(8):2994–3003. doi: 10.1529/biophysj.105.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]