Abstract
While designing poly(ethylene glycol) hydrogels with high moduli suitable for in situ placement is attractive for cartilage regeneration, the impact of a tighter crosslinked structure on the organization and deposition of matrix is not fully understood. The objectives for this study were to characterize the composition and spatial organization of neo-matrix as a function of gel crosslinking and study its impact on chondrocytes via anabolic and catabolic gene expressions and catabolic activity. Bovine articular chondrocytes were encapsulated in hydrogels of three crosslinking densities (compressive moduli were 60, 320 and 590 kPa) and cultured for 25 days. Glycosaminoglycan production increased with culture time and was greatest in gels with lowest crosslinking. Collagens II and VI, aggrecan, link protein, and decorin were localized to pericellular regions in all gels, but their presence decreased with increases in gel crosslinking. Collagen II and aggrecan expressions were initially up-regulated in gels with higher crosslinking, but increased similarly up to day 15. Matrix metalloproteinases (MMP)-1 and -13 expressions were elevated (~25-fold) in gels with higher crosslinking throughout the study, while MMP-3 was not affected by gel crosslinking. The presence of aggecan and collagen degradation products confirmed MMP activity. These findings indicate that chondrocytes synthesize the major cartilage components within PEG hydrogels, however, gel structure strongly impacts the composition and spatial organization of the neo-tissue and impacts how chondrocytes respond to their environment, particularly with respect to their catabolic expressions.
Keywords: hydrogels, cartilage, chondrocytes, extracellular matrix, pericellular matrix
Introduction
The complex structure and organization of cartilage gives the tissue its unique ability to withstand large forces. Articular cartilage is a complex fibrillar mesh of interacting collagens, proteoglycan aggregates, and other non-collagenous proteins, which all reside in a highly aqueous environment. Proteoglycans contain large amounts of negatively charged, sulfated glycosaminoglycans (sGAG), predominantly that of chondroitin sulfate, resulting in an osmotic environment with high swelling pressures that are counteracted by the collagen fiber network [1, 2]. These aggregates, along with the collagen type II mesh, form the bulk of the extracellular matrix (ECM) [3].
The pericellular matrix (PCM), which has a distinctly different composition from the ECM, serves to protect the chondrocyte from mechanical stresses and is thought to regulate both biochemical and biophysical cues presented to the cells, thus influencing their biological function [4, 5]. The PCM is characterized by the presence of a collagen VI mesh network, although aggrecan and collagen II are also present in the PCM. Collagen VI fibrils, found exclusively in the PCM [6, 7], contain multiple globular units with binding motifs that can assemble with smaller proteoglycans, such as decorin and biglycan [8–10]. These assemblies, which are unique to the PCM, help to bridge the surrounding ECM with the cells, and may also participate in the assembly of aggregates prior to their release into the ECM [11, 12]. The composition and structure of the PCM also serves to regulate the passage of soluble factors that can interact with the cell (i.e. cytokines, growth factors, matrix catabolites) [13]. Therefore, extracellular cues that affect the development and/or maintenance of the PCM may in turn directly impact the signals perceived by the cells and subsequently affect the long-term formation and/or maintenance of the ECM.
One of the major goals in designing scaffolds for cartilage tissue engineering is to provide cells with an environment in which they can synthesize and deposit a neotissue that recapitulates the native tissue’s composition, structure, and mechanics towards restoring joint function. A variety of natural and synthetic hydrogels have been examined as chondrocyte carriers due to their ability to maintain the chondrocyte phenotype and the highly swollen network that supports nutrient and cell waste diffusion [14–16]. For example, isolated chondrocytes encapsulated in agarose have been shown to support the development of a distinct PCM region [17], rich in collagen VI, followed by the maturation of a cartilage-like ECM composed of collagen II and full-length aggrecan [18]. In some cases, these constructs have resulted in physiologic concentrations of glycosaminoglycans and have approached the compressive moduli of cartilage [19–22] even though collagen content and dynamic mechanical properties remained inferior to that of native articular cartilage [23]. Chondrocytes cultured in alginate gels developed a mechanically stiff PCM, composed of collagen and proteoglycans, as early as day 7 of culture [24]. Recent developments of peptides that self-assemble into hydrogels have also shown to support the deposition of neotissue by encapsulated chondrocytes, exhibiting an ECM rich in collagen type II and glycosaminoglycans [25, 26]. However, one of the limitations with many of the aforementioned hydrogel systems is that their mechanical properties are often much lower than that of the native tissue limiting their ability to withstand the high stresses seen in the native environment, at least initially, until sufficient matrix has been deposited.
In an effort to design synthetic hydrogels capable of matching the high compressive moduli of articular cartilage, photopolymerizable poly(ethylene glycol) (PEG) based hydrogels have been studied [27–31]. The mild polymerization allows for in situ formation and the encapsulation of cells, while the synthetic chemistry may be modified to introduce degradable linkages [32–35] and/or biomimetic moieties [36–39]. The mechanical properties of the hydrogel may be finely tuned through manipulations in gel crosslinking density (ρx), which can be controlled through simple changes in the gel formulation. High moduli PEG hydrogels (~900 kPa) have been used to successfully encapsulate chondrocytes, maintaining cell viability and matrix synthesis [27, 40]. However, changes in ρx will impact other properties such as water content and diffusion of molecules [41], including newly deposited matrix molecules, throughout the network [27, 42]. These properties will have a significant impact on how the encapsulated chondrocytes sense their environment, which can in turn affect their proliferation, metabolism, and the ECM synthesis and deposition [28, 30, 40, 43–45].
Gross examination of neotissue development within PEG hydrogels has demonstrated that the ECM matrix is composed of sGAGs and collagen type II [27, 46]. Short-term studies have indicated that a PCM develops, based on deposition of chondroitin sulfate, within a few days in the PEG hydrogel constructs [44], followed by the deposition of sGAGs into the ECM. The extent of PCM and ECM development has been shown to be dependent on the gel ρx, illustrating that a higher ρx resulted in a thinner, denser PCM with decreased GAG deposition in the extracellular regions of the hydrogel. These previous studies illustrate that the crosslinked structure impacts the developing tissue, at least by gross examination of GAGs and collagen. However, the structure and composition of cartilage, which gives rise to its unique functional properties, is much more complex. Therefore, in designing hydrogels with high moduli suitable for in situ placement, it is equally important to understand how the crosslinked structure impacts the structural organization of the developing tissue.
Therefore, the objectives for this study were two-fold. First, we assessed the role of gel crosslinked structure on the composition and distribution of newly deposited cartilage matrix molecules, particularly matrix molecules which give rise to the PCM (e.g., collagen VI), play an important role in the organization of the tissue (e.g., link protein, and decorin), and contribute to the PCM, but also more importantly make up most of the native ECM (i.e., chondroitin sulfate a building block of aggrecan, aggrecan and collagen type II). Because the PCM is thought to be a critical mechanism by which cells receive external cues, any differences in the PCM composition and structure may impact the function of the chondrocyte. Therefore, in the second part of this study, we assessed the role of the crosslinked structure in mediating anabolic and catabolic functions of chondrocytes through gene expression analysis and by assaying for catabolic activity through the detection of degraded matrix. Overall, our findings illustrate that PEG hydrogels are supportive of cartilage-matrix molecule deposition, but that the crosslinked structure largely impacts the type of tissue deposited, the spatial deposition of the tissue, and the anabolic and catabolic activity by the chondrocytes. This information should aid in the design of high moduli hydrogels for cartilage tissue engineering applications.
Materials and Methods
Materials
Collagenase type II and papain were from Worthington Biochemical (Lakeshore, NJ). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), 100× penicillin-streptomycin (P/S), fungizone, HEPES-buffer, gentamicin, and MEM-nonessential amino acids (NEAA), goat anti-rabbit IgG Alexa Fluor 488, goat anti-mouse IgG Alexa Fluor 546, DAPI and all D-LUX PCR primers were from Invitrogen (Carlsbad, CA). L-proline, ascorbic acid, bovine serum albumin (BSA), 1,9-dimethylmethylene blue (DMMB), protease-free chondroitinase ABC, hyaluronidase, methylene chloride, methacryloyl chloride, ethyl ether, triethylamine, dithiothreiotol (DTT), and iodoacetamide were from Sigma-Aldrich (St. Louis, MO). Irgacure 2959 was from Ciba Specialty Chemicals (Newport, DE). E.Z.N.A.-Total RNA Mini-kit was from Omega-Biotek (Norcross, GA). High Capacity cDNA kit and Taqman® Fast Universal PCR Master Mix were from Applied Biosystems (Foster City, CA).
The aggrecan (A1059-53F) and collagen II (C5710-20F) antibodies were from US Biologicals (Swampscott, MA). Chondroitin-6-sulfate antibody (MAB2035) was from Chemicon (Billerica, MA). The anti-collagen VI antibody (ab6588) was from Abcam (Cambridge, UK). Decorin (7b1), and link protein (9/30/8-A-4) antibodies were from the University of Iowa Developmental Studies Hybridoma Bank (Iowa City, IA). The C1,2C ELISA kit was from IBEX Pharmaceuticals (Quebec, Canada). BCA Protein Assay kit was from Thermo Scientific Pierce (Rockford, IL). For western blot analysis, primary antibodies for mouse monoclonal to aggrecan and N- terminal neoepitope FFG4 were acquired from MD Biosciences (St. Paul, MN). Western blot gels, membranes, buffers, Tween-20, and blot equipment were from Bio-Rad Labs (Hercules, CA).
Chondrocyte Isolation
Full depth articular cartilage was harvested from the patellar-femoral groove of 1–3 week old calves (n=2, Research 87, Marlborough, MA) within 24 hours of slaughter and digested in 500 units/mL collagenase II in DMEM supplemented with 5% FBS for 16 hours at 37°C on an orbital shaker (40 rpm). The digest was passed through a 100 μm cell-strainer, pelleted and rinsed 3× with PBS containing 1% P/S, 0.5 μg/mL fungizone, and 20 μg/mL gentamicin (PBS-Antis). Isolated cells were counted using trypan blue exclusion assay and resuspended in chondrocyte medium (DMEM supplemented with 10% FBS (v/v), 0.04 mM L-proline, 50 mg/L L-ascorbic acid, 10 mM HEPES, 0.1 M MEM-NEAA, 1% P/S, 0.5 μg/mL fungizone, and 20 μg/mL gentamicin) prior to encapsulation.
Hydrogel Formation
Poly(ethylene glycol) dimethacrylate (PEGDM) was synthesized by reacting PEG (3 kDa) with methacryloyl chloride in the presence of triethylamine for 24 hrs at 4°C. PEGDM was purified using a series of precipitations in chilled ethyl ether and analyzed by 1H NMR (Varian YVR-500S), which indicated 90% methacrylation. PEGDM was dissolved in chondrocyte medium at 10, 15, or 20% (w/w) and mixed with 0.05, 0.022, or 0.0125 (w/w) photoinitiator Irgacure 2959, respectively. Sterile macromer solution was added to pelleted chondrocytes at 50 million cells/mL, mixed thoroughly, and immediately photopolymerized using 365 nm light (6 mW/cm2) for 10 minutes. Hydrogel constructs (5 mm diameter × 5mm height cylinders) were rinsed in PBS+Antis and individually placed into wells of a 24-well tissue culture plate. Gels were cultured at 37°C in 5% CO2 and allowed to equilibrate for 24 hrs prior to the start of the experiment. Medium was changed every 2–3 days and saved at −20°C for GAG analysis.
Hydrogel Characterization
At day 0, equilibrated acellular hydrogels (n=3) were weighed to obtain wet weight measurements. Hydrated hydrogels were subsequently compressed to 15% strain at 0.5 mm/min to obtain stress-strain curves (MTS Synergie 100, 10N). Hydrogels were then lyophilized for 48 hrs and their dry mass determined. Equilibrium volumetric swelling ratio (Q) was calculated from the mass swelling ratio (q). Crosslinking densities (ρx) and mesh sizes (ζ) were estimated from Q as described elsewhere [28].
Biochemical Analysis
At days 0, 5, 10, 15, and 20, hydrogels were removed from culture and cut in half. One half (n=3) was weighed to obtain wet weight, lyophilized for 48 hours and subsequently homogenized and enzymatically digested by papain for 16 hrs at 60°C. Gel samples and collected media samples were assessed for sulfated GAG content by the DMMB dye method [47]. GAG content within hydrogels were normalized to gel wet weights.
Histological Visualization
At 25 days of culture, constructs (n=3) were fixed for 24 hours in 4% paraformaldehyde, dehydrated, paraffin-embedded, and sectioned (10 μm). Negatively charged glycosaminoglycans (GAGs) were detected using Safranin-O/Fast Green while collagen deposition was analyzed by Masson’s Trichrome. Cell nuclei were counterstained by hematoxylin. For immunohistochemistry, sections were analyzed for collagen type II and VI, aggrecan, link protein, chondroitin-6-sulfate, and decorin. Samples were treated with chondroitinase ABC (10 mU) and hyaluronidase (200 U) for 1 hr at 37°C. For antigen retrieval of the hyaluronan binding region and link protein, samples were also reduced and alkylated. All samples were blocked using 1% BSA for 30 minutes. Sections were then incubated overnight at 4°C with primary antibodies for collagen II (1:100), collagen VI (1:100), aggrecan (1:10), chondroitin-6-sulfate (1:100), hyaluronan (1:5), link protein (1:5), or decorin (1:5). Fluorescent detection of each protein was achieved using either secondary goat anti-rabbit IgG Alexa Fluor 488 or goat anti-mouse IgG Alexa Fluor 546 antibodies (1:200) and counterstained using DAPI (1:1000). Sections were mounted and preserved using VectaMount, and a laser scanning confocal microscope (Zeiss LSM 5 Pascal) was used to acquire images. All antibodies were validated to ensure their specificity. Negative controls were performed on sections from cell-laden hydrogels that did not receive primary antibody showing no positive staining. Positive controls were performed on hyaline cartilage and the data are shown in Figures 2–4.
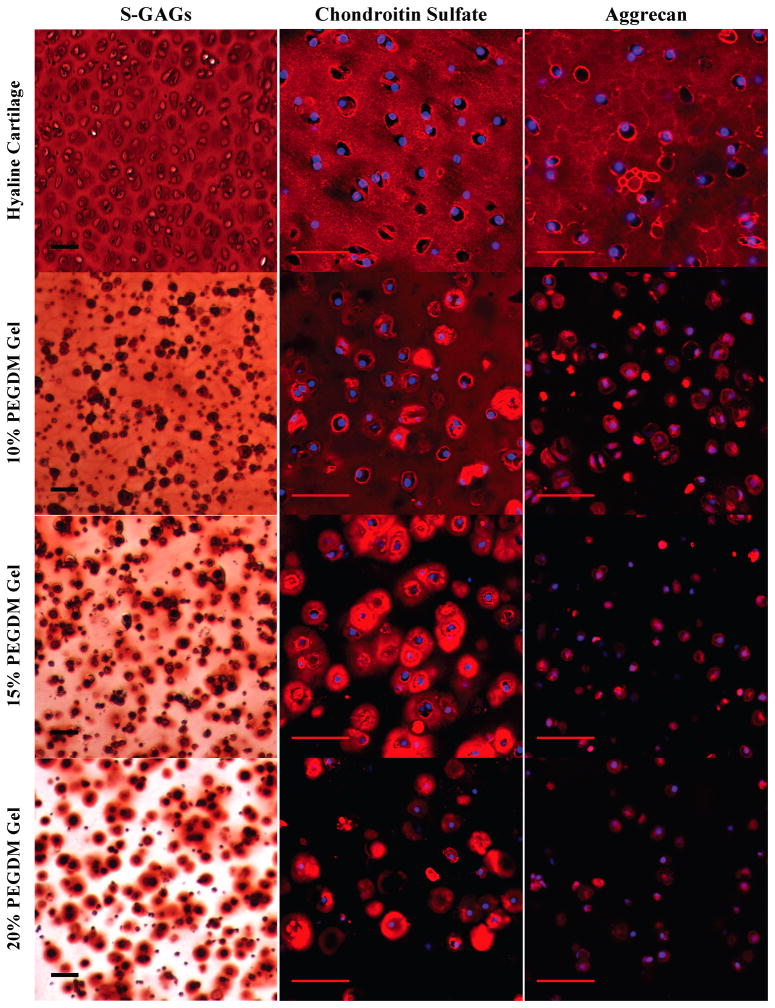
Figure 2.
Gross examination of proteoglycan matrix deposition by histological and immunohistochemical (IHC) evaluation for chondrocytes encapsulated in 10, 15, or 20% PEG gels and cultured for 4 weeks. Sections were stained for negatively charged glycosaminoglycans (S-GAGs) (red) using Safranin O/Fast green. Cell nuclei (dark purple) were counterstained using hematoxylin. For IHC, sections were using antibodies against chondroitin-6-sulfate (red), and aggrecan (red). Cell nuclei (blue) were counterstained using DAPI. Images were acquired by laser scanning confocal microscopy. Scale bars represent 50 μm.
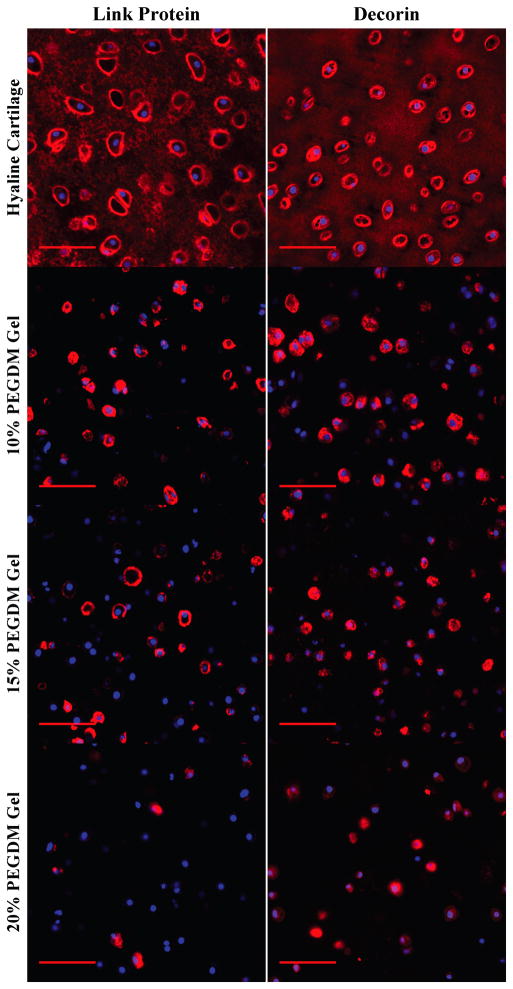
Figure 4.
Gross examination of matrix deposition of ECM connective proteins by immunohistochemical (IHC) evaluation for chondrocytes encapsulated in 10, 15, or 20% PEG gels and cultured for 4 weeks. Sections were stained using antibodies against hyaluronan (red), link protein (red), and decorin (red). Cell nuclei (blue) were counterstained using DAPI. Images were acquired by laser scanning confocal microscopy. Scale bars represent 50 μm.
Gene Expression
Construct halves at 0, 5, 10, 15, and 20 days (n=3) were immediately snap-frozen under liquid nitrogen and processed using E.Z.N.A-Total RNA Mini-Kit columns; total RNA was obtained with a A260/A280 ratio > 1.8 (Nanodrop, ND-1000, Thermo-Fisher). 100 ng of RNA was transcribed to cDNA using the High Capacity cDNA Kit. Real-time RT-PCR (ABI 7500 Fast) was performed for collagen type II (COL2), aggrecan (AGC) and matrix metalloproteinases (MMP) -1, -3, and -13 using the Taqman® Fast Universal PCR Master Mix [48]. Gene expression was measured relative to the expression of the mitochondrial ribosomal protein L30 (housekeeping gene) and normalized to 10% gels at each time point (calibrator) as described elsewhere [44].
Western Blot Analysis
Whole constructs at 0 and 20 days (n=2) were immediately snap-frozen in liquid nitrogen and stored at −80°C. These constructs were later thawed and homogenized in assay buffer (0.05 M Tris HCl (pH 7.5), 0.2 M NaCl, 0.01 M CaCl2, 0.02% NaN3, and 0.05% Triton-X). Constructs and conditioned medium samples (n=2) were assayed for aggrecan and its degradation products by Western blot analysis. Samples were measured for protein content using the BCA Protein kit, deglycosylated, mixed with reducing loading buffer, and loaded into wells of a 10% Criterion Tris-HCl gel (Bio-Rad). Each lane contained 10 μg of protein from the construct samples or 30 μg of protein from the media samples. After separation by electrophoresis (40 min, 200V), proteins were transferred to Immun-Blot PVDF membranes for 1 hour at 100V. The membranes were blocked with 5% BSA in phosphate buffered saline with 0.1% Tween-20 (PBS-T) for 2–4 hr, and probed with 1 μg/mL primary antibodies for epitopes FFGV (BC-14) and the linear IGD domain of aggrecan (bovine-EPEEPFTFVPEV, 6B4) overnight at 4°C. Secondary detection was performed using a goat anti-mouse Alexa Fluor 546 antibody (1:400) and imaged using a Bio-Rad Versadoc 4000 MP imaging system (3.5 AP/Exp. 30 s).
ELISA for Collagen Degradation Products
Constructs and conditioned medium samples (n=2) were assayed for the MMP-cleaved collagen degradation product. Equal volumes of media and of assay buffer from the homogenized constructs (described above for western blot analysis) were assayed for the carboxy terminus of the 3/4 peptide (C1,2C or Col 2 3/4C Short) generated by cleavage of types I and II collagens by collagenases using the competitive immunoassay C1,2C ELISA per the manufacturer.
Statistical Analysis
Data are represented as a mean ± standard deviation. Accumulated GAG release into the media is reported using the cumulative average ± standard deviation for each sampling (hydrogel and time point). Gene expression values as a function of culture time and gel crosslinking density were analyzed by two-way analysis of variance (ANOVA) and significant differences due to ρx at each time point were analyzed post-hoc using Tukey’s HSD with a statistical significance of α=0.05.
Results
Hydrogels of three different crosslinking densities were fabricated from 10, 15 and 20% PEGDM macromer, referred to as 10, 15, and 20% PEG gels, respectively, which spanned a range of macroscopic properties, described in Table 1. All hydrogels imbibed high equilibrium water contents, greater than 86%. The compressive moduli of the hydrogels ranged over an order of magnitude from 60 to 590 kPa with increasing crosslinking density. The mesh size or average distanced between crosslinks was estimated, decreasing from 195 to 65 Å with increasing crosslinking density.
Table 1.
Properties of PEGDM hydrogels
| PEGDMa (w/w) % | Equilibrium Volumetric Swelling Ratio (Q) | Crosslinking density (mol/L) | Water Content (%) | Compressive Modulus (kPa) | Mesh Size (ζ) (Å) |
|---|---|---|---|---|---|
| 10 | 11.6 ± 0.2 | 0.11 ± 0.01 | 91.6 ± 0.5 | 60 ± 10 | 148 ± 5 |
| 15 | 8.4 ± 0.1 | 0.22 ± 0.01 | 88.8 ± 0.2 | 320 ± 30 | 94 ± 2 |
| 20 | 6.5 ± 0.2 | 0.38 ± 0.02 | 85.9 ± 0.5 | 590 ± 60 | 65 ± 3 |
Poly(ethylene glycol) dimethacrylate (PEGDM) macromer concentration prior to photopolymerization
Matrix Deposition
Proteoglycan deposition and accumulation was measured by sGAG content (Fig. 1a, b). There was a significant increase in sGAG content with culture time for all three crosslinked hydrogels (p<0.0001). Crosslinking density was a significant factor affecting sGAG content (p<0.0001). After day 0, sGAG content was significantly higher in the 10% and 15% PEG gels compared to the 20% PEG gels. The mean sGAG content was generally higher in the 10% PEG gels when compared to the 15% PEG gels, which was significant at days 15 and 20, but by day 25 similar contents were reported. The presence of sGAGs in the culture medium (Fig. 1b) revealed significant loss from the construct and accumulation in the medium with culture time. The percentage of sGAG lost was 36%, 34% and 49% of the total sGAG deposited (i.e., sGAGs in the construct + sGAG in medium) after 25 days for the 10%, 15%, and 20% PEG gels, respectively.
Figure 1.
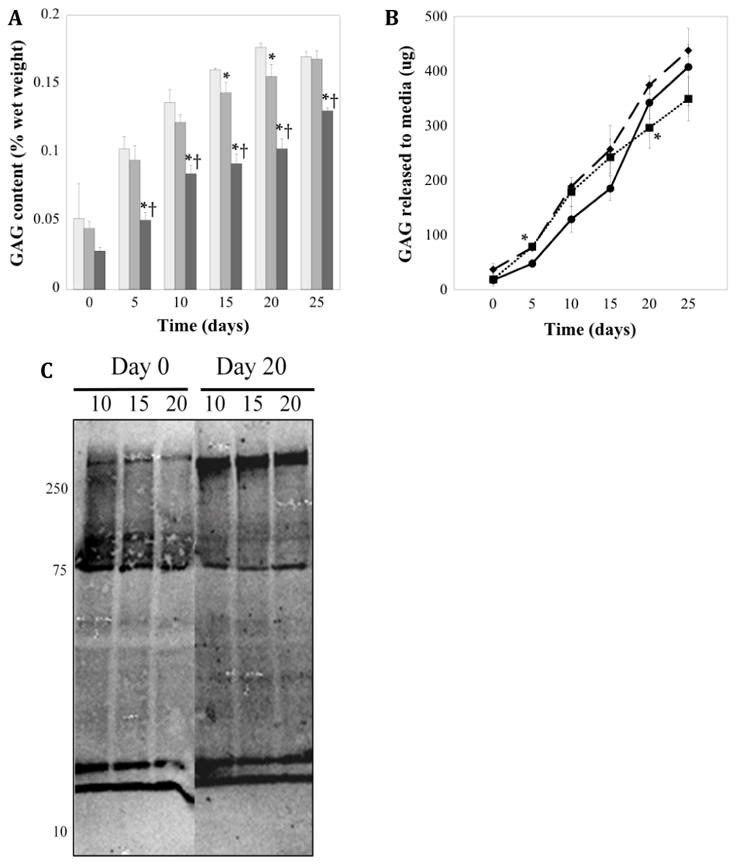
The total amount of sulfated GAG (a) accumulated in the hydrogel construct per wet weight hydrogel as a function of crosslinking density and culture time for 10% (□), 15% (■), and 20% (■) (w/w) PEG gels. Accumulated sGAG released from construct (b) into surrounding media during FS culture time for 10% (solid ●), 15% (dashed ◆), and 20% (dotted ■) gels at each time point. * indicates significant difference from 10% gels (p<0.05), † indicates a significant difference from 15% gels (p<0.05). Aggrecan detection by western blot analysis (c) within 10, 15, and 20% PEG gels at days 0 and 20. Anti-IGD probes were used to detect the intact IGD region between G1 and G2 domains of aggrecan.
The relative size of the aggrecan molecules, after deglycosylation, present within the constructs was also probed at days 0 and 20 by western blot analysis (Fig. 1c). While the same amount of protein was loaded into each well for analysis, the total protein extracted from the hydrogels differed and was highest in the 10% PEG gels and lowest in the 20% PEG gels. The amounts were 265±9, 229±3, and 204±3 μg at day 0 and 304±20, 254±14 and 219±13 μg at day 20 for the 10%, 15% and 20% gels, respectively. Several sizes of aggrecan molecules were detected, which were consistent across crosslinking densities, but differed between days 0 and 20. At day 0, the majority of aggrecan was ~75 kDa and ~15–20 kDa. By day 20, aggrecan was much larger with sizes in the range of 275 kDa with few of the intermediate sizes, but a similar presence of the smaller molecules (~15–20kDa).
To determine the influence of crosslinked structure on matrix formation, deposition, and distribution, sulfated glycosaminoglycans were initially evaluated by Safranin-O staining and immunofluorescence (Fig. 2). After 25 days of culture, sGAGs distribution in the 10% PEG gels was present throughout the extracellular regions, mimicking to a certain degree the spatial distribution of sGAGs in native cartilage. However, a sharp contrast in sGAG deposition was observed in gels with higher crosslinking. The higher gel crosslinking resulted in decreased sGAG diffusion throughout the hydrogel, to the extent that sGAGs were solely localized to the immediate pericellular region in the 20% PEG gels. Detection of the major sGAG, chondroitin-6-sulfate, and major proteoglycan, aggrecan, also showed significant differences with gel crosslinking. The decreased presence of chondroitin sulfate deposition in the extracellular regions as crosslinking increased mirrored that of the general sGAG stain. Chondroitin sulfate staining in the ECM regions was greatest in the 10% PEG gels, but in the PCM region, it was greater in the 15% PEG gels compared to the 10% PEG gels. Further increases in gel crosslinking from 15% to 20% PEG gels resulted in decreased chondroitin sulfate presence where not all cells showed the same level of deposition. Aggrecan was detected only in the PCM for all three crosslinked gels. Staining for aggrecan also decreased with increasing crosslinking density, but did not match the observed chondroitin sulfate or general sGAG distributions. It should be noted that the interpretation of the PCM encompasses matrix excreted and deposited adjacent to the cell and matrix that has been synthesized by the cell, but which has not yet been excreted. In cartilage and to a certain extent in the 10% PEG gels, the PCM deposited extracellularly is more evident, while in the higher crosslinked gels the distinction between extracellular and intracellular matrix is less clear.
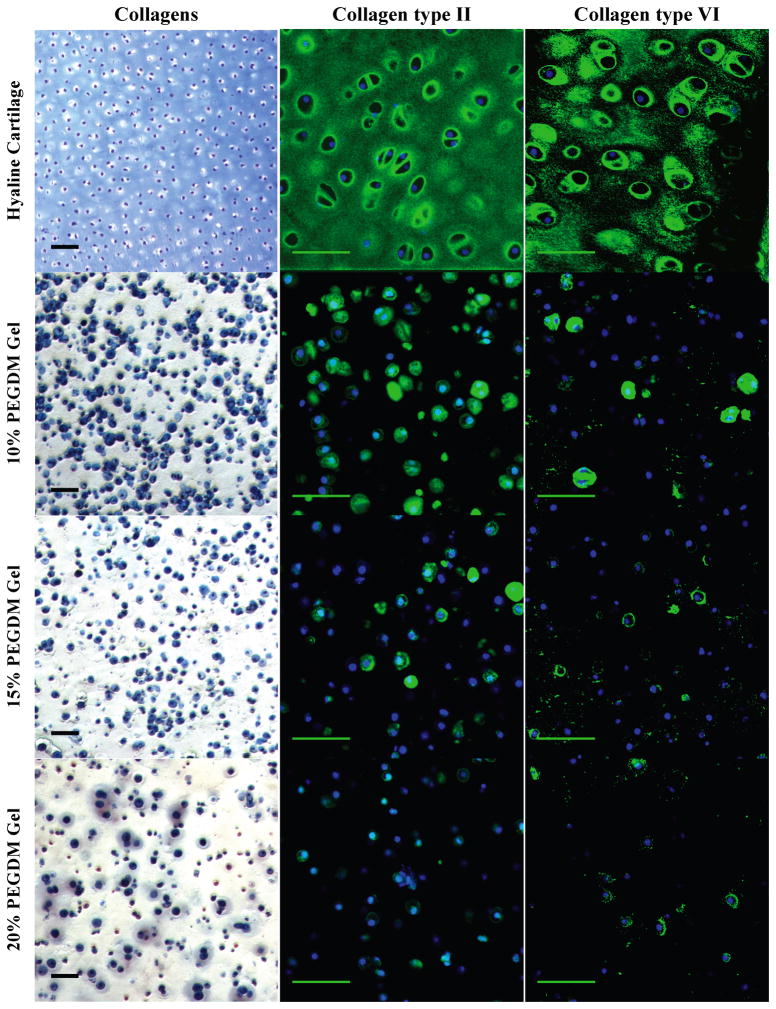
A decrease in the presence of other matrix proteins was also observed with higher ρx gels. Collagen II deposition was restricted to the pericellular regions, which correlated with the collagen staining seen with Masson’s Trichrome (Fig. 3). Pericellular staining for collagen VI revealed inhomogeneities in the staining where not all cells showed the same level of deposition regardless of crosslinking. Cells in the 10% PEG gels exhibiting collagen VI deposition showed thick, spherical staining, whereas higher crosslinked gels resulted in more diffuse, non-uniform collagen VI distribution. Similar trends were observed for link protein and decorin (Fig. 4). Link protein was densest in the pericellular region and showed little to no deposition in the 20% PEG gels. The pattern of decorin deposition followed closely with collagen type II distribution, showing uniform densities in the PCM and greatest accumulation in the 10% PEG gels.
Figure 3.
Gross examination of collagen matrix deposition by histological and immunohistochemical (IHC) evaluation for chondrocytes encapsulated in 10, 15, or 20% PEG gels and cultured for 4 weeks. Sections were stained for collagens (blue) using Masson’s Trichrome. Cell nuclei (dark purple) were counterstained using hematoxylin. For IHC, sections were stained using antibodies against collagen type II (green) and collagen type VI (green). Cell nuclei (blue) were counterstained using DAPI. Images were acquired by laser scanning confocal microscopy. Scale bars represent 50 μm.
Chondrocyte Matrix Expression
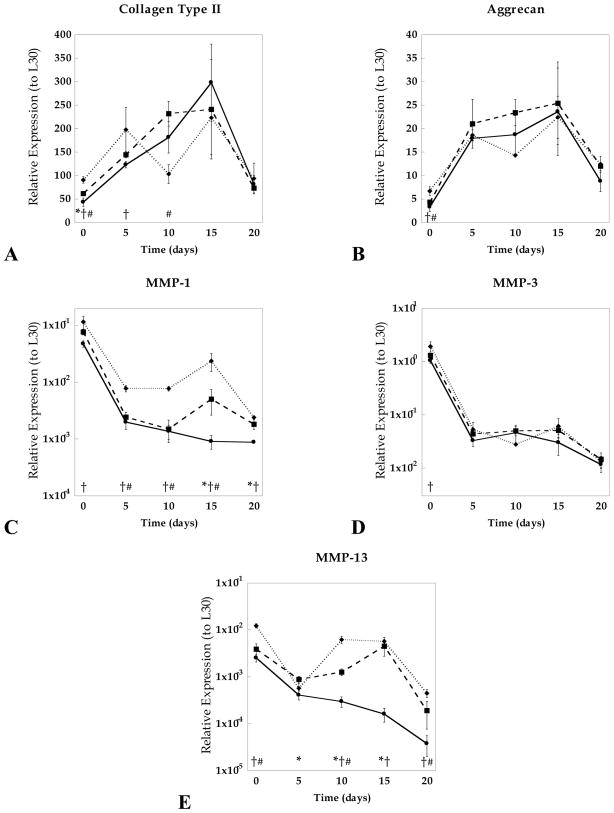
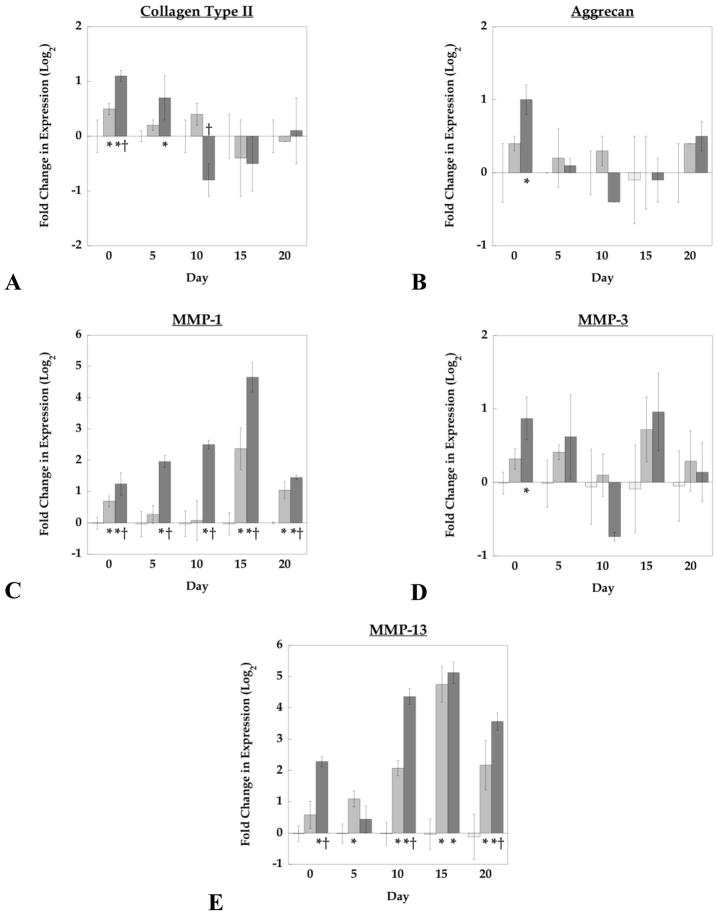
The effects of hydrogel crosslinking on the gene regulation of structural proteins, collagen II (COL2) and aggrecan (AGG), as well as catabolic enzymes, matrix metalloproteinases-1, -3, and -13, were assessed. Analysis by two-way ANOVA revealed that COL2 and AGC were significantly regulated by culture time (p<0.001), whereas culture time, gel crosslinking, and their interactions, affected all MMP expressions (p<0.001). Specifically, differences in gel crosslinking affected initial expression levels of COL2 and AGC but these differences diminished by day 20 (Fig. 5). Regardless of crosslinking and culture time, COL2 expression was approximately 10-fold higher than AGC expression, which is consistent with greater collagen II accumulation seen by immunohistochemistry. A strong positive correlation (r=0.86) was observed between COL2 and AGC expressions regardless of culture time and gel structure. Analysis of MMPs-1, 3, and 13 showed opposite expression profiles from the anabolic trends, exhibiting an overall decrease in expressions compared to day 0. MMP-3 was expressed at the highest levels of the three MMPs. Interestingly, 15% and 20% PEG gels exhibited sharp increases in MMP-1 and -13 expression between 5–15 days of culture, whereas 10% PEG gels exhibited a continual decrease in all MMP levels over the 20 day culture period. Following day 5 of culture, a positive correlation between MMP-3 and AGC expression was observed (r=0.73) as well as between MMP-1 and -13 (r=0.78).
Figure 5.
Relative gene expression for collagen II (a), aggrecan (b), matrix metalloproteinase (MMP) -1 (c), -3 (d), and -13 (e) compared to the housekeeping gene (HKG) L30 for 10% (solid ●), 15% (dashed ◆), and 20% (dotted ■) PEG gels conditioned up to 20 days in culture. Significant differences (p<0.05) between crosslinking densities at specific time points are represented as (*) for 10% vs. 15%, (†) 10% vs. 20%, and (#) 15% vs. 20%.
To investigate differences in gene expression levels due to hydrogel crosslinking, relative expression levels were normalized to the 10% PEG gel values at their respective time points (Fig. 6). Initially, increasing crosslinking from the 10% to 15% PEG gels promoted both COL2 and AGC expression (1.4×), which was further enhanced in the 20% PEG gels by 2×. However, by 20 days of culture, expression of these genes reached similar levels regardless of crosslinking. Analysis of MMP-1 revealed that 20% PEG gels expressed higher levels at all time points compared to 10% PEG gels (as high as 25× at day 15). MMP-13 expressions were highly dependent on ρx showing similar characteristics to MMP-1 for 20% PEG gels (as high as 35× at day 15 when compared to 10% PEG gels). Crosslinking density did not affect MMP-3 levels at any of the observed time points.
Figure 6.
The effects of crosslinking density on gene expression at specific time points for collagen type II (a), aggrecan (b), matrix metalloproteinase (MMP) -1 (c), -3 (d), and -13 (e) 10% (□), 15% (■), and 20% (■) PEG gels. Data are presented as normalized expression relative to 10% gels at each specified time point. * indicates a significant difference from 10% gels (p<0.05).
Presence of Matrix Degradation Products
The activity of catabolic enzymes MMP-1, MMP-13, and MMP-3 was assessed by assaying for their respective matrix degraded products using two different methods. First, the amount of C1,2C fragment, which results from MMP-1 and MMP-13 cleavage of collagen I or II, was measured by ELISA in the constructs (Fig. 7a) and in the media (Fig. 7b). There were no significant changes in content of C1,2C fragments in the gel as a function of culture time or as a function of gel crosslinking. Although, the mean levels dropped around days 10 and 15 for all crosslinked gels, but returned to levels that were similar to day 0. There were no significant changes in the amount of C1,2C fragment present in the culture medium as a function of culture time or gel crosslinking.
Figure 7.
The total amount of C1,2C accumulated in the hydrogel constructs (a) and total amount of C1,2C released to the media per day (b) as a function of gel crosslinking density and culture time for 10% (□), 15% (■), and 20% (■) PEG gels. Detection of MMP-3 cleaved aggecan degradation product, FFGV fragment, by western blot analysis for 10, 15, and 20% PEG hydrogels at days 0 and 20 within the construct (c) and in the culture medium (d).
Presence of the N-terminal FFGV fragment, which is generated by MMP-3 cleavage of aggrecan, was determined for the constructs (Fig. 7c) and for the media (Fig. 7d) using western blot analysis. In the constructs, FFGV fragments were detected at ~75 kDa, which appeared to be more abundant in the 10% gels at both days 0 and 20; although the total amount of protein extracted from the 10% gels was highest. Total protein amounts extracted from the culture medium were much higher due to the fact that the majority of the proteins were from the serum and were generally similar for all crosslinked gels. There were several FFGV fragments present in the media at day 0 with two distinct bands between 15 and 20 kDa and a band ~75 kDa along with several faint bands visible at higher MWs. By day 20, all of these same bands were visible but much fainter, with the most intense band occurring ~75 kDa in the 10% gels.
Discussion
The ability of the scaffold to promote deposition and organization of an engineered tissue is critical towards engineering functional cartilage. Initially, cells will ‘see’ cues provided by the scaffold, but as neotissue is deposited the biochemical cues perceived by the cells will change and be largely dictated by the matrix molecules comprising the neotissue. This interplay will likely impact the long-term growth and maturation of the engineered tissue. Overall, the PEG hydrogels supported the deposition of cartilage-specific matrix molecules comprised of the two main building blocks of cartilage ECM (aggrecan and collagen II), the primary matrix molecule found in the PCM of cartilage (collagen VI), and smaller matrix molecules which are thought to be important in matrix assembly (link protein and decorin). However, the newly deposited tissue was largely limited to the immediate pericellular regions within all of the three crosslinked gels. Increasing the gel crosslinking density resulted in decreased positive staining for collagens II and VI and aggrecan. Together, these findings illustrate the distinct differences in composition and organization of the neotissue as a function of the PEG crosslinked structure and that after 25 days the neotissue deposited remains in an immature state when compared to native cartilage.
Examining both the building blocks of the ECM, such as sGAGs, the large ECM molecules including collagen type II and aggrecan, and one of the molecules associated with the aggregation of aggrecan (i.e. link protein) revealed large spatial discrepancies as a result of the crosslinked structure. For all crosslinked gels, there was limited diffusion observed for collagen II, which is not surprising as it has characteristic fibril dimensions ranging from 40–300 nm in length and 1–2 nm in diameter [49]. The average mesh size of the PEG hydrogels used in this study ranged between 5 and 20 nm. The major proteoglycan in cartilage, aggrecan, reaches molecular weights between 1–4 MDa depending on the amount of glycosylation [50]. The N-terminal G1 domain of aggrecan interacts solely with long chains of hyaluronan [51], which are stabilized by the 45-kDa link protein [52]. The synthesis of both aggrecan and link protein occurs through the same intracellular pathways whereas hyaluronan is synthesized on the plasma membrane and is translocated directly into the extracellular space [53]. Thus both aggrecan and link protein assemble with hyaluronan through extracellular mechanisms, and can result in aggregates reaching several hundred million Daltons on the order of 1–2 μm in length [3]. Although proteins upwards of 65 kDa have been observed to diffuse through similar gels [42], diffusion of aggrecan and the larger proteoglycan aggregates are hindered by the gels’ smaller mesh sizes. Therefore, it is not surprising that aggrecan is localized to the PCM region in all crosslinked gels. The localization of link protein in the PCM region suggests that it is likely binding to aggrecan and beginning to form an organized matrix. Interestingly, there was a range of aggrecan sizes (which did not stain positive as a FFGV fragment or to the same degree) detected in the constructs suggesting that this molecule is in different stages of organization throughout the culture period. The smaller molecules that are present at both early and late cultures may indicate that the cells are continuing to produce new, smaller aggregates, which are being assembled at the cell membrane. In addition, there was positive staining for sGAG and chondroitin sulfate in the extracellular regions of the hydrogel but only for the 10% PEG gels, which may suggest the presence of smaller proteoglycans, such as the small leucine-rich proteoglycans associated with matrix binding or of degraded aggrecan fragments that are capable of diffusing through the hydrogel (discussed below). Overall, the restricted deposition of collagen II and aggrecan suggests that the mesh size of the PEG hydrogels is not sufficient to permit diffusion of these large macromolecules into the extracellular space of the hydrogel.
Cells receive insoluble biochemical signals by interacting with their surrounding matrix, and in the case of cartilage by interacting directly with the PCM [4]. Here, we demonstrate that the pericellular matrix produced by chondrocytes within PEG hydrogels was distinctly different from the native PCM environments and was drastically affected by changes in crosslinking density. Findings reported here indicate that the gel structure hinders the deposition of collagen VI in the PCM. In the native tissue, collagen type VI forms an organized, multimeric fibrillar network specific to the PCM, which interacts with collagen II present in the PCM [8, 10]. Even though a majority of cells exhibited collagen II and decorin in their PCM region, a lower fraction (~40%) of chondrocytes stained positive with collagen VI deposition. Increases in crosslinking caused a decrease in the density and thickness of collagen VI in the PCM even though the fraction of cells staining positive for collagen VI remained the same. Chondrocytes cultured in agarose gels also exhibited collagen VI within their PCM, however, significant structural differences in the orientation and distribution of the fibrillar mesh were observed compared to native cartilage [17]. This study suggested that the agarose environment did not provide adequate spatial requirements for the unique structural organization of collagen VI, which may explain the decreased staining observed in our hydrogels with higher crosslinking. In addition, the heterogeneity observed in the collagen VI staining may be due to the fact that chondrocytes encapsulated in the PEG hydrogels were isolated from full-depth cartilage, where the degree of staining for collagen VI was noted to vary with depth in our cartilage explants (data not shown). Similarly, the density and thickness of collagen type II and aggrecan in the PCM regions also decreased with increasing gel crosslinking. These overall differences in the PCM composition and structure as a result of gel crosslinking will likely affect how cells interact with their surrounding environment, respond to external stimuli, and ultimately form tissue.
In native cartilage, matrix molecules are secreted into the pericellular space, where they are able to interact and assemble to form large macromolecules. In the PEG hydrogel systems, we observe that the major cartilage-matrix components are in fact being produced by the cells and localized to the PCM region. It is thought that enzymatic activity within the PCM may be necessary to release these molecules from the PCM [18, 54], permitting their diffusion into the surrounding extracellular spaces and forming the ECM. In addition, chondrocytes are known to maintain cartilage through a balance of anabolic and catabolic mechanisms, where, for example there is a normal turnover of aggrecan through cell-secreted proteolytic enzymes [55–57]. Therefore, anabolic and catabolic events may be important to initiate a remodeling phase for regenerating functional cartilage. For example, when MMP-inhibitors were delivered to chondrocyte-laden agarose constructs, GAG content and mechanical properties were negatively impacted, strongly suggesting an important role of MMPs in matrix formation [18]. Other studies have similarly suggested the importance of proteolytic activities in tissue remodeling phases [26, 48, 54, 58, 59].
Here, we observed a significant increase in anabolic gene expression for AGC and COL2 during early culture times for all three PEG hydrogels, corresponding with synthesis and deposition of new matrix. Interestingly, the anabolic response was initially higher in gels with higher crosslinking. This observation may be partly attributed to differences in gel stiffness whereby the cells may ‘sense’ higher resistance during matrix deposition, and hence stress, which may have impacted their function. By day 20, however, AGC and COL2 expression levels decreased significantly suggesting that after the development of a collagen and GAG-rich PCM, cells may reach homeostatic levels. Additional studies are necessary to confirm these hypotheses.
MMP-3 catabolic activity was confirmed by the presence of the N-terminal FFGV fragment in the constructs and in the culture medium. The predominate FFGV fragment appears to be ~75 kDa in the constructs and a second smaller fragment between 15–20 kDa which was mainly found in the culture medium (both are confirmed to be aggrecan, Fig 1). While MMP-3 expression decreased with culture time, there was evidence of the FFGV fragment in the constructs at early and late culture times. Since degradation products can be metabolized by cells, we cannot confirm whether the FFGV fragments were cleaved early during the culture period (when expression levels were high) and retained or whether downstream processes differentially regulated translation of MMP-3 and/or its activation. More interesting is the decreased presence of two FFGV fragments in the culture medium after longer culture periods, which more closely agrees with the MMP-3 expression data and is more representative of temporal changes in catabolic activity. However, some discrepancies are noted for the low crosslinked gel. While MMP-3 expression was similar among all gels, there was a greater presence of the FFGV fragment in the low crosslinked gel. Interestingly, in the low crosslinked gels there was evidence of chondroitin sulfate, but not aggrecan in the extracellular space of the hydrogels, which may be indicative of an aggrecan degradation product. In addition, the sGAGs released into the culture medium will include any degraded aggrecan. However, the amount of sGAG released was similar among the crosslinked hydrogels, yet there was little detected at least for the higher crosslinked gels. Since the FFGV fragment encompasses the G1 domain of the aggrecan molecule, and the other MMP-3 cleaved fragment (not tested) will encompass the glycosylated region of the aggrecan, it is possible that the other fragment more readily diffuses into the culture medium and was detected by the sGAG assay and not by western blot. Therefore, it is likely that some of the sGAGs released are degraded aggrecan, but we cannot confirm to what degree, all or some. In addition, other enzymes involved in matrix breakdown may be responsible for sGAG release, specifically that of the aggrecanases (ADAMTS-4/-5). Nonetheless, the positive correlation between aggrecan and MMP-3 expression supports the involvements of MMPs in matrix synthesis and remodeling. Additional studies, however, are needed to confirm this hypothesis.
Interestingly, increases in crosslinking density resulted in higher expression levels of MMP-1 and -13, most notably after 5 days of culture, indicating that the structure of the hydrogel significantly impacted catabolic gene expression. This up-regulation may be in part due to the fact that gels with higher crosslinking tended to be more restrictive to PCM formation whereby the cells would need to breakdown this nearby matrix to create space for new matrix and/or to allow these matrix molecules to diffuse and deposit into the ECM. The presence of C1,2C fragments indicates that MMP’s were indeed synthesized and activated within the PEG hydrogels. While there were no significant differences in the total amount of C1,2C fragments in the construct or the culture medium as a function of gel crosslinking, the lower collagen content observed in the histology images suggests that there may be a higher fraction of C1,2C fagment generated in the gels with higher crosslinking. This observation is supported by the increased expressions in MMP-1 and 13 observed in the higher crosslinked gels. However, it is important to note that C1,C2 fragments are also generated from MMP cleavage of type I collagen, which was not evaluated in this study.
While we have limited the scope of our discussion to the role that gel crosslinking has on the diffusion of matrix molecules and its subsequent effect on cells, changes in the crosslinking density will alter the mechanical properties of the hydrogel. Therefore, differences in how chondrocyte’s response to changes in crosslinking density may be a result of not only the disparate PCM, but also due to mechanotransduction events as the cells ‘sense’ differences in substrate stiffness [60]. Since hydrogel structure and chemistry dictate the mesh size and the mechanical properties of the hydrogel [61], it is not possible to de-couple the physical from the mechanical effects that the hydrogel is having on chondrocyte response. However, by manipulating the hydrogel chemistry, it would be possible to de-couple these two effects and gain a better understand of their individual contributions to mediating chondrocyte response, but is beyond the scope of the current study.
While these findings indicate that encapsulated chondrocytes synthesize the major cartilage components (aggrecan, collagen II and VI) within PEG hydrogels, the restricted location of these molecules to the regions of the PCM strongly indicates the need for engineering degradation into the hydrogels. Previous studies have shown that hydrolytically degradable PEG hydrogels permit the evolution of a macroscopic cartilage-like tissue composed of sGAGs and collagen II, which was not possible in non-degrading PEG hydrogels [32, 33]. We are currently incorporating degradable linkages, based on poly(lactic acid), into the PEG hydrogels and our initial unpublished findings confirm the ability for these large macromolecules of aggrecan and collagen II to diffuse into the extracellular space. One of the attractive features of designing synthetic biodegradable hydrogels is the ability to tailor the degradation to control changes in the gel properties with time [35, 62] and to match degradation with tissue evolution.
Conclusions
The ability to design in situ forming scaffolds with high moduli is attractive for creating a material suitable to withstand the large stresses in vivo. However, our findings illustrate that the relatively tight mesh network provided by the crosslinked PEG hydrogels dramatically impacts the type of tissue deposited and the spatial evolution of the tissue. While hydrogels with high moduli are supportive of cartilage-specific matrix deposition, the use of these high moduli gels must be coordinated with hydrogel degradation. Our long-term goal is to design synthetic degradable hydrogels by which the gel itself provides initial stiffness to support the loads in situ and as tissue is deposited and the hydrogel matrix erodes, the load is then transferred to the developing tissue.
Acknowledgments
This research was supported by a grant from the National Institute of Health (grant number K22DE016608), a Department of Education’s Graduate Assistantships in Areas of National Need (GAANN) fellowship to GN, and a Chancellor’s Fellowship to SCS. The authors would also like to thank Eric Greenwald for his technical assistance in immunohistochemistry. The antibodies developed by B. Caterson (link protein) and G.A. Pringle (decorin) used here were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G.D. Nicodemus, Email: nicodemu@colorado.edu.
S.C. Skaalure, Email: stacey.skaalure@colorado.edu.
S.J. Bryant, Email: stephanie.bryant@colorado.edu.
References
- 1.LAI WM, Hou JS, Mow VC. A Triphasic Theory for the Swelling and Deformation Behaviors of Articular-Cartilage. Journal of Biomechanical Engineering-Transactions of the Asme. 1991;113:245–58. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 2.Maroudas A. Balance between Swelling Pressure and Collagen Tension in Normal and Degenerate Cartilage. Nature. 1976;260:808–9. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 3.Hardingham TE. Proteoglycans and Glycosaminoglycans. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. 2. Burlington: Elsevier; 2006. pp. 85–98. [Google Scholar]
- 4.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Annals of the New York Academy of Sciences: Skeletal Development and Remodeling in Health, Disease, and Aging. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 5.Poole CA. Articular cartilage chondrons: Form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engvall E, Hessle H, Klier G. Molecular Assembly, Secretion, and Matrix Deposition of Type-Vi Collagen. J Cell Biol. 1986;102:703–10. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole CA, Flint MH, Beaumont BW. Chondrons in Cartilage - Ultrastructural Analysis of the Pericellular Microenvironment in Adult Human Articular Cartilages. Journal of Orthopaedic Research. 1987;5:509–22. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 8.Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the Proteoglycan Decorin to Collagen Type-Vi. Journal of Biological Chemistry. 1992;267:5250–6. [PubMed] [Google Scholar]
- 9.Guidetti GF, Bartolini B, Bernardi B, Tira ME, Berndt MC, Balduini C, et al. Binding of von Willebrand factor to the small proteoglycan decorin. Febs Letters. 2004;574:95–100. doi: 10.1016/j.febslet.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. Journal of Biological Chemistry. 2003;278:37698–704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 11.Larson CM, Kelley SS, Blackwood AD, Banes AJ, Lee GM. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002;21:349–59. doi: 10.1016/s0945-053x(02)00026-4. [DOI] [PubMed] [Google Scholar]
- 12.Sandy JD, O’Neill JR, Ratzlaff LC. Acquisition of hyaluronate-binding affinity in vivo by newly synthesized cartilage proteoglycans. Biochem J. 1989;258:875–80. doi: 10.1042/bj2580875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruoslahti E, Yamaguchi Y. Proteoglycans as Modulators of Growth-Factor Activities. Cell. 1991;64:867–9. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 14.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28:134–46. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Elisseeff JH, Lee A, Kleinman HK, Yamada Y. In: Biological response of chondrocytes to hydrogels. Sipe JD, Kelley CA, McNicol LA, editors. 2002. pp. 118–22. [DOI] [PubMed] [Google Scholar]
- 16.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering Part B-Reviews. 2008;14:149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimicco Ma, Kisiday JD, Gong H, Grodzinsky AJ. Structure of pericellular matrix around agarose-embedded chondrocytes. Osteoarthritis and Cartilage. 2007;15:1207–16. doi: 10.1016/j.joca.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Connelly JT, Wilson CG, Levenston ME. Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthritis and Cartilage. 2008;16:1092–100. doi: 10.1016/j.joca.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte agarose culture. Journal of Cell Science. 1995;108:1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 20.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. Journal of Orthopaedic Research. 2002;20:842–8. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 21.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Engineering. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 22.Mauck RL, Wang CCB, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis and Cartilage. 2003;11:879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Hung CT, Mauck RL, Wang CCB, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Annals of Biomedical Engineering. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 24.Ng L, Hung HH, Sprunt A, Chubinskaya S, Ortiz C, Grodzinsky A. Nanomechanical properties of individual chondrocytes and their developing growth factor-stimulated pericellular matrix. Journal of Biomechanics. 2007;40:1011–23. doi: 10.1016/j.jbiomech.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisiday JD, Jin MS, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. Journal of Biomechanics. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. Journal Of Biomedical Materials Research. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 28.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Annals of Biomedical Engineering. 2004;32:407–17. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 29.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. Journal of Biomedical Materials Research Part A. 2003;67A:1430–6. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 30.Villanueva I, Klement BJ, von Deutsch D, Bryant SJ. Cross-Linking Density Alters Early Metabolic Activities in Chondrocytes Encapsulated in Poly(Ethylene Glycol) Hydrogels and Cultured in the Rotating Wall Vessel. Biotechnology and Bioengineering. 2009;102:1242–50. doi: 10.1002/bit.22134. [DOI] [PubMed] [Google Scholar]
- 31.Appelman TP, Mizrahi J, Elisseeff JH, Seliktar D. The differential effect of scaffold composition and architecture on chondrocyte response to mechanical stimulation. Biomaterials. 2009;30:518–25. doi: 10.1016/j.biomaterials.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 32.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. Journal Of Biomedical Materials Research Part A. 2003;64A:70–9. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 33.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating Chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnology And Bioengineering. 2004;86:747–55. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 34.Rice MA, Anseth KS. Encapsulating chondrocytes in copolymer gels: Bimodal degradation kinetics influence cell phenotype and extracellular matrix development. Journal Of Biomedical Materials Research Part A. 2004;70A:560–8. doi: 10.1002/jbm.a.30106. [DOI] [PubMed] [Google Scholar]
- 35.Rice MA, Anseth KS. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Engineering. 2007;13:683–91. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–8. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomaterialia. 2005;1:243–52. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–76. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt O, Mizrahi J, Elisseeff J, Seliktar D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnology and Bioengineering. 2006;95:1061–9. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- 40.Villanueva I, Hauschulz DS, Mejic D, Bryant SJ. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis and Cartilage. 2008 doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins AW, Anseth KS. Investigation of molecular transport and distributions in poly(ethylene glycol) hydrogels with confocal laser scanning microscopy. Macromolecules. 2005;38:1326–34. [Google Scholar]
- 42.Weber LM, Lopez CG, Anseth KS. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. Journal of Biomedical Materials Research Part A. 2009;90A:720–9. doi: 10.1002/jbm.a.32134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. Journal of Orthopaedic Research. 2004;22:1143–9. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Nicodemus GD, Bryant SJ. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. Journal of Biomechanics. 2008;41:1528–36. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant SJ, Nicodemus GD, Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharmaceutical Research. 2008;25:2379–86. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- 46.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. Journal Of Biomedical Materials Research. 2000;51:164–71. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Farndale RW, Buttle DJ, Barretta J. Improved Quantitation and Discrimination of Sulfated Glycosaminoglycans by Use of Dimethylmethylene Blue. Biochimica Et Biophysica Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 48.Nicodemus GD, Bryant SJ. Mechanical Loading Regimes Affect the Anabolic and Catabolic Activities by Chondrocytes Encapsulated in PEG Hydrogels. Osteoarthritis and Cartilage. 2009 doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Mark KVD. Structure, Biosynthesis, and Gene Regulation of Collagens in Cartilage and Bones. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. 2. Burlington: Elsevier; 2006. pp. 3–40. [Google Scholar]
- 50.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–70. [PubMed] [Google Scholar]
- 51.Hardingham TE, Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972;279:401–5. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- 52.Hardingham TE. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979;177:237–47. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273:1923–32. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 54.De Croos JNA, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biology. 2006;25:323–31. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Hardingham TE, Fosang AJ. The structure of aggrecan and its turnover in cartilage. J Rheumatol Suppl. 1995;43:86–90. [PubMed] [Google Scholar]
- 56.Murphy G, Hembry RM, Hughes CE, Fosang AJ, Hardingham TE. Role and regulation of metalloproteinases in connective tissue turnover. Biochem Soc Trans. 1990;18:812–5. doi: 10.1042/bst0180812. [DOI] [PubMed] [Google Scholar]
- 57.Sandy JD. Editorial - A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis and Cartilage. 2006;14:95–100. doi: 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Blain EJ. Mechanical regulation of matrix metalloproteinases. Frontiers in Bioscience. 2007;12:507–27. doi: 10.2741/2078. [DOI] [PubMed] [Google Scholar]
- 59.Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Archives of Biochemistry and Biophysics. 2001;396:49–55. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- 60.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater (Weinheim, Ger) 2006;18:1345–60. [Google Scholar]
- 62.Rice MA, Sanchez-Adams J, Anseth KS. Exogenously triggered, enzymatic degradation of photopolymerized hydrogels with polycaprolactone subunits: Experimental observation and modeling of mass loss behavior. Biomacromolecules. 2006;7:1968–75. doi: 10.1021/bm060086+. [DOI] [PMC free article] [PubMed] [Google Scholar]