Abstract
Cocaine/heroin combinations (speedball) induce a synergistic elevation in extracellular dopamine concentrations ([DA]e) in the nucleus accumbens (NAc) that can explain the increased abuse liability of speedball. To further delineate the mechanism of this neurochemical synergism, in vivo fast-scan cyclic voltammetry (FSCV) was used to compare NAc DA release and reuptake kinetic parameters following acute administration of cocaine, heroin and speedball in drug-naïve rats. These parameters were extracted from accumbal DA overflow induced by electrical stimulation of the ventral tegmental area. Evoked DA efflux was increased following both cocaine and speedball delivery, whereas heroin did not significantly change evoked DA release from baseline. DA efflux was significantly greater following cocaine compared to speedball. However, DA transporter (DAT) apparent affinity (Km) values were similarly elevated following cocaine and speedball administration, but unaffected by heroin. Neither drug induced substantial changes in the maximal reuptake rate (Vmax). These data, combined with published microdialysis and electrophysiological results, indicate that the combination of cocaine-induced competitive inhibition of DAT and the increase in the DA release elicited by heroin is responsible for the synergistic increase in ([DA]e) induced by speedball.
Keywords: cocaine, heroin, speedball, dopamine, uptake, release, voltammetry
Introduction
The co-use of cocaine and opiates, termed speedball (Leri et al., 2003a), has been on the rise since the 1970s and represents a growing subpopulation of drug abusers (Craddock et al., 1997; Greberman and Wada, 1994; Kosten et al., 1987). The prevalence of cocaine use among heroin addicts ranges from 30% to 80% (Leri et al., 2003a; Schutz et al., 1994). Negative health and social consequences of such combinations are severe, particularly when cocaine is used intravenously (Schutz et al., 1994). Specifically, the likelihood of positive treatment outcomes for opiate addicts decreases severely when high amounts of cocaine are co-abused (Downey et al., 2000). Several hypotheses have been proposed to explain the combined use of cocaine and heroin in humans, including an enhancement of the positive effects of either drug, reduction in the magnitude or duration of undesired side effects, induction of euphoria beyond what either drug alone provides, or independent, non-additive effects even though the drugs are administered concurrently (Foltin and Fischman, 1992; Hemby et al., 1996; Kosten et al., 1987). The hypothesis of enhanced euphorigenic effects in humans is paralleled in preclinical studies, which demonstrate that cocaine and heroin potentiate the reinforcing effects of one another in the self-administration paradigm (Mattox et al., 1997; Rowlett and Woolverton, 1997); however, the specific biological contributors which mediate the amplified reinforcing effects of speedball have not been confirmed.
The potentiated euphorigenic effects in humans and the corresponding enhancement of reinforcing effects in animal models may be based on an augmented neurochemical response in brain pathways underlying reinforcement processes. The mesolimbic dopamine (DA) system is recognized as a critical substrate for the reinforcing effects of drugs of abuse. In general, drugs of abuse activate this pathway and stimulate DA neurotransmission in the nucleus accumbens (NAc) in humans, nonhuman primates and rodents (Lyons et al., 1996; Porrino, 1993; Volkow et al., 1997), which is an effect associated with the abuse liability of such substances. Previous studies have shown that extracellular DA concentrations ([DA]e) in the NAc are significantly elevated (300–400% baseline) during cocaine self-administration (Hemby et al., 1997; Pettit and Justice, 1989; Wise et al., 1995b) and moderately increased (120–150% baseline) by heroin self-administration (Leri et al., 2003b; Wise et al., 1995a) in rats as measured by in vivo microdialysis (but see (Hemby et al., 1999; Hemby et al., 1995; Smith et al., 2006) where heroin self-administration did not significantly alter NAc [DA]e). Interestingly, we have demonstrated that intravenous speedball self-administration resulted in synergistic elevations in NAc [DA]e (~1000% of baseline) compared with cocaine (400%) or heroin alone (no change) (Hemby et al., 1999). Subsequent studies examining self-administration of lower dose combinations, as well as experimenter-administered speedball combinations, have reported similar synergistic elevations (Hemby et al., 1999; Smith et al., 2006; Zernig et al., 1997). While in vivo microdialysis studies provide information on the extent to which NAc [DA]e is increased during speedball self-administration, the mechanisms by which heroin potentiates the effects of cocaine on NAc [DA]e are not fully understood.
In vivo voltammetry is an electrochemical technique that is commonly used in conjunction with in vivo microdialysis to further characterize DA dynamics (Mateo et al., 2004; Ng et al., 1991; Oleson et al., 2009a; Oleson et al., 2009b; Stamford et al., 1989; Wu et al., 2001a). Voltammetry facilitates analysis of DA uptake kinetics aside from DA release or metabolism contributions (Wu et al., 2001b; Zimmerman and Wightman, 1991). Importantly also, the higher temporal resolution of this technique allows the time course of DA uptake inhibition to be determined more precisely (Budygin and Jones, 2008; Budygin et al., 2000). This is principally beneficial when drugs are administered intravenously and therefore have a fast onset neurochemical response (Mateo et al., 2004). In the present study, we utilized in vivo fast-scan cyclic voltammetry (FSCV) to assess electrically evoked DA release and DAT kinetic parameters in rat NAc in pursuit of unveiling the mechanisms of neurochemical synergy of speedball administration.
2. Methods
2.1 Animals
Male Fisher F-344 rats (120–150 days; 270–320 g; Charles River, Wilmington, MA) were housed in acrylic cages in a temperature-controlled vivarium on a 12 h reversed light/dark cycle (lights on at 6:00 PM). Rats were group-housed before surgery and singly housed after catheterization. Food was restricted to maintain starting body weight and water was available ad libitum, except during experimental sessions which were conducted during the dark phase. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996.
2.2 Intravenous catheterization
Drug-naïve rats (n = 15) were anesthetized with 1.5 g/kg urethane (i.p.) and implanted with chronic indwelling venous catheters as described previously (Hemby et al., 1999; Hemby et al., 1997; Hemby et al., 1996). Catheters were inserted into the right jugular vein, terminating just outside the right atrium and anchored to muscle near the point of entry into the vein. The distal end of the catheter was attached to a syringe containing heparinized saline. Immediately following catheterization, while still under urethane anesthesia, in vivo fast-scan cyclic voltammetry experiments were performed.
2.3 In vivo fast-scan cyclic voltammetry
While under urethane anesthesia, rats were secured in a stereotaxic frame in a flat skull position. Holes were drilled into the skull above the NAc and ventral tegmental area (VTA) (AP: +1.3, L: +1.3 and AP: −5.2, L: +1.0 in mm relative to bregma, respectively). An additional hole was drilled into the skull of the contralateral hemisphere into which an Ag/AgCl reference electrode was implanted just below the surface of the skull and connected to a voltammetric amplifier. A carbon fiber microelectrode (approximately 80 to 200 μm in length beyond the glass capillary in which it was contained) was secured to the stereotaxic frame arm and also connected to the voltage amplifier. The carbon fiber electrode was placed into the hole above the NAc, and lowered approximately 5 mm from the surface into the striatum. A bipolar stimulating electrode was connected to a voltage output box and lowered into the hole above the VTA approximately 7.5 mm. Voltammetric recordings were made at the recording electrode every 100 ms for a 15 s duration by applying a triangular waveform (−0.4 to +1.2 V, 400 V/s). The biphasic stimulation applied by the stimulating electrode consisted of 60 rectangular pulses at 60 Hz, 300 μA and was activated at 5 s into each recording. Recorded signals showed an oxidation peak at +0.6 V and a reduction peak at −0.2 V (vs. Ag/AgCl reference), ensuring that the released chemical was indeed DA. The depths of the carbon fiber microelectrode and bipolar stimulating electrode were adjusted from −6.2 to −7.2 and −7.5 to 8.3 mm, respectively, in order to optimize electrically evoked DA release in the NAc core.
Once evoked DA recordings were optimized, stable readings were collected every 5 min for at least 50 min. When baseline recordings were within 10% of each other for at least 5 measurements, drug (1 mg/kg cocaine, 0.03 mg/kg heroin or 1 mg/kg cocaine/0.03 mg/kg heroin combination; n = 5 per drug) was injected i.v. over a 6 sec period, immediately followed by 0.2 ml of heparinized saline i.v. over another 6 sec. Importantly, selected doses of the drugs resulted in reliable synergistic elevations in extracellular DA levels measured by microdialysis in rat NAc during speedball self-administration (Hemby et al., 1999). Stimulated recordings were then made at 1, 5 and 10 min and thereafter every 10 min up to 120 min following drug injection.
Carbon fiber microelectrodes were post-calibrated in vitro with known concentrations of DA (2–5 μM). Calibrations were performed in triplicate and the average value for the current at the peak oxidation potential was used to normalize recorded in vivo current signals to DA concentration. The kinetic parameters of DAT (DAT apparent affinity or the Michaelis-Menten constant, Km, and maximal velocity of uptake rate, Vmax) were calculated using LVIT software (UNC, Chapel Hill, NC). DA reuptake by DAT was assumed to follow Michaelis-Menten kinetics and the change in DA concentration during and after stimulated release was fit using the equation:
where f is the stimulation frequency (Hz), and [DA]p is the concentration of DA released per stimulus pulse. Vmax is the Michaelis-Menten parameter for maximal uptake rate of a first-order enzymatic reaction, such as DA reuptake by DAT. The Michaelis-Menten constant, or DAT reuptake apparent affinity, Km, is the [DA]e required for DAT-mediated reuptake rate to reach one half of Vmax. Baseline Km value was previously determined for DAT in rat brain synaptosomes to be 0.16 μM (Near et al., 1988). The integral form of this equation was used to model DA response for individual rat at all time points before and after drug injections (Wu et al., 2001b). Under current condition, there are two substrates competing for DAT binding, which are cocaine and endogenous DA. Therefore, Km is actually apparent Km under this circumstance, but for convenience will be referred to as just Km.
To perform an alternative analysis of DA uptake changes induced by cocaine and speedball administration, we fit a decaying exponential to the descending part of the electrically evoked DA efflux. The numbers reported in this study were obtained by fitting an exponential function of the form
to the DA concentration versus time before and 1 min after cocaine and heroin, when the maximal effects of drugs on DA efflux were observed. Here y represents the DA concentration, × represents the time, and y0, x0, A, and t are fitting parameters. The quantity y0 is the background level of the DA concentration in the absence of any electrical stimulation, x0 is the time at which the DA concentration begins to decline after an electrical stimulation, A is the amplitude (peak) of the DA concentration at time x0, and t represents the decay time for the decrease of DA concentration due to the reuptake mechanism. The portion of the curves that are fit are the points corresponding to about 70% of the peak to where the concentration is less than 5% above the background concentration value. The fitting was carried out in OriginLab’s OriginPro8 data analysis and fitting program.
2.4 Data analysis
Statistical analyses were performed using SigmaStat 3.1 software. One-way repeated-measures analyses of variance (ANOVA) were used to compare effects after acute i.v. drug administration to predrug baseline values for evoked peak DA, DAT apparent affinity (Km), and maximal uptake rate (Vmax) values within each drug group. These data were also analyzed using two-way ANOVA with drug group (cocaine, heroin or speedball) and time as the factors. Evoked DA values were analyzed as a percent of baseline values (baseline defined as the average of five measurements taken at 25, 20, 15, 10 and 5 min before drug injection). All post hoc analyses were performed using Bonferroni t-tests to compare postdrug injection values to baseline (for one-way repeated-measures ANOVAs) and for pairwise comparisons between drugs.
3. Results
3.1 Electrically evoked peak DA
The amplitude of current as detected by FSCV in rat NAc markedly increased after cocaine (1.0 mg/kg, i.v.) and speedball (1.0 mg/kg cocaine + 0.03 mg/kg heroin, i.v.), but not after heroin (0.03 mg/kg, i.v.) injection (Fig. 1). As determined by one-way repeated-measures ANOVA, cocaine significantly increased electrically evoked peak DA values from baseline (p < 0.001) at 1, 5, 10, 20, 30, 40 and 50 min after injection (p < 0.01), and speedball also significantly increased baseline electrically evoked DA levels (p < 0.001) at 1, 5, 10, 20, and 30 min (p < 0.005) after drug injection, as determined by Bonferroni post hoc analyses. Acute i.v. heroin did not significantly change evoked DA from baseline in drug-naïve animals (p = 0.529) at any time after injection. Two-way ANOVA revealed significant differences in evoked peak DA values between drug groups (F(2, 204) = 64.24; p < 0.001). Cocaine elicited an overall greater increase (p < 0.001) in electrically evoked DA levels than speedball in drug-naïve animals, significantly at 1 min after drug injections (p < 0.05; Fig. 2). Both speedball and cocaine provoked significant increases in evoked peak DA compared to heroin from the time of injection until 30 and 40 min after drug injections, respectively, as determined by post hoc analyses. A significant group × time interaction was also observed (F(16, 204) = 16.92; p < 0.001)
Fig. 1.

Representative traces of electrically evoked DA signals detected by FSCV in rat NAc before (solid lines) and 1 min after (dashed lines) cocaine (1.0 mg/kg, i.v.), heroin (0.03 mg/kg, i.v.) and speedball (1.0 mg/kg cocaine + 0.03 mg/kg heroin, i.v.) injection in drug-naïve animals (upper panel). These signals had an oxidation peak at +0.6 V and a reduction peak at −0.2 V vs. Ag/AgCl reference, identifying the released species as DA. Representative color plots topographically depict the voltammetric data before drug administration, with time on the x-axis, applied scan potential on the y-axis and background-subtracted faradaic current shown on the z-axis in pseudo-color (lower panel).
Fig. 2.
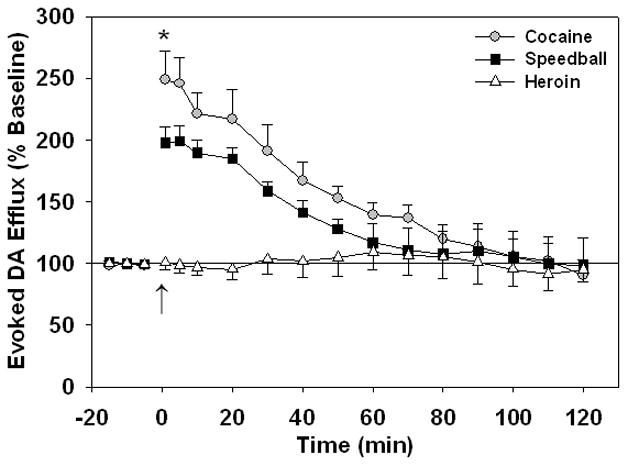
Electrically evoked DA efflux in the NAc of anesthetized drug-naïve rats following a single i.v. injection of cocaine (1.0 mg/kg), heroin (0.03 mg/kg) and speedball (1.0 mg/kg cocaine + 0.03 mg/kg heroin). Injections were given at time = 0 min (↑). Evoked DA efflux values are represented as a percent of the pre-drug baseline DA concentration. All data are means ± SEM of five rats per group. *p < 0.05 for cocaine versus speedball (two-way ANOVA followed by Bonferroni tests). Values significantly different from baseline readings and from the heroin group are not indicated on plots (see 3.1).
3.2 DAT apparent affinity
Cocaine induced a significant increase in Km from baseline ( p < 0.001) with post hoc analyses showing a significant effect (p < 0.05) at time points 1, 5, 10, 20, 30, 40, 50, 60 and 70 min following injection. Speedball resulted in a similar increase in Km values (p < 0.001) after drug injection, with significant increases from baseline at time points from 1 to 90 min after drug injection (p < 0.05). Heroin had no effect on baseline Km values (p = 0.841). Two-way ANOVA showed no difference in Km between cocaine and speedball following drug injection (p = 1.000) (Fig. 3). Both speedball and cocaine significantly increased Km compared to heroin (F(2, 204)= 277.0; p < 0.001). A significant group × time interaction was also observed (F(16, 204) = 64.88; p < 0.001).
Fig. 3.

DAT apparent affinity (Km, or Michaelis-Menten rate constant) in the NAc of anesthetized drug-naïve rats following a single i.v. injection of cocaine (1.0 mg/kg), heroin (0.03 mg/kg) and speedball (1.0 mg/kg cocaine + 0.03 mg/kg heroin). Injections were given at time = 0 min (↑). All data are means ± SEM of five rats per group. Values significantly different from baseline readings and from the heroin group are not indicated on plots (see 3.2).
3.3 DAT-mediated reuptake rate
No significant changes in Vmax were seen from baseline values after cocaine ( p = 0.880). Heroin also had no effect on baseline Vmax values (p = 1.000). Speedball injection did produce a modest (~ 10%) but significant increase (p < 0.05) in Vmax (Fig. 4). Post hoc analyses revealed that the increase in Vmax from baseline (1816.4 ± 478.6 nM/s) was significant at time points from 1 to 100 min (2023.6 ± 732.7 nM/s) (p < 0.005). However, two-way ANOVA comparing average Vmax values between drugs showed no statistical difference between the average Vmax following cocaine (1817.4 ± 662.4 nM/s) and speedball or heroin (F(2, 204) = 3.883; p > 0.05). To avoid any confusion regarding drug-induced uptake changes, we performed alternative analysis fitting a single exponential decay function to the descending phase of electrically evoked DA signal. No significant difference (t = 1.749, df = 8; p = 0.1184, unpaired t test) was revealed between effects of cocaine and speedball on the decay time (t) for the decrease of DA concentration due to reuptake (1.28 ± 0.28 vs 1.85 ± 0.18 s). The same analysis demonstrated a significant increase in the t after cocaine (0.95 ± 0.13 s for baseline value vs 1.85 ± 0.18 s after cocaine, t = 5.621, df= 4; p < 0.005; paired t test) and speedball (0.68 ± 0.10 s for baseline value vs 1.28 ± 0.28 s after speedball, t = 3.263, df = 4; p < 0.05; paired t test). Taken together, these results indicate the marked decrease in the accumbal DA reuptake following acute cocaine and speedball administration.
Fig. 4.

DAT-mediated maximal reuptake rate (Vmax) in the NAc of anesthetized drug-naïve rats following a single i.v. injection of cocaine (1.0 mg/kg), heroin (0.03 mg/kg) and speedball (1.0 mg/kg cocaine + 0.03 mg/kg heroin). Injections were given at time = 0 min (↑). All data are means ± SEM of five rats per group. Values significantly different from baseline readings and from the heroin group are not indicated on plots (see 3.3).
4. Discussion
The proposed mechanism of speedball’s actions on the mesolimbic DA system is a result of combinatory effects of cocaine and heroin on NAc [DA]e. Cocaine blocks DAT on dopaminergic terminals as well as cell bodies to increase extracellular DA concentrations (Koob and Bloom, 1988; Pettit and Justice, 1989; Ritz et al., 1987; Volkow et al., 1997). The DA uptake inhibition leads to several different pharmacological consequences. The inhibition of soma firing rate due to the activation of DA autoreceptors is the one of well described outcomes that should diminish basal DA release in the NAc. However, the DA release induced by electrical stimulation and measured by FSCV is increased, since cocaine inhibits DA reuptake between multiple electrical pulses (Mateo et al., 2004; Oleson et al., 2009a; Oleson et al., 2009b). Heroin binds μ-opioid receptors to inhibit VTA GABAergic interneurons and remove a tonic inhibition of DA neurons, thus upregulating cell firing rates and consequently DA release at NAc terminals (Johnson and North, 1992; Leone et al., 1991; Self et al., 1995). We evaluated the changes in kinetic parameters of DA release and uptake in order to better understand the neurobiological substrates involved in the potentiating effects of speedball on NAc [DA]e.
A significant increase in the magnitude of evoked DA release was observed following both cocaine and speedball injections. The maximal effect of intravenously administrated drugs on evoked DA release in the NAc was found to occur one minute after administration, which is consistent with previous findings in our lab (Mateo et al., 2004; Oleson et al., 2009b) and correlates with the time it takes for cocaine to reach peak brain levels (Fowler et al., 1998). Kinetic analyses of the electrically evoked DA signals recorded during cocaine and speedball time courses indicated that both effects are associated with an increase in the apparent Km for DA uptake. Importantly, it was previously indicated that the cocaine-induced increase in the electrically evoked DA release measured by FSCV in vivo is preferentially due to the decrease in DA reuptake (increase in the apparent Km ) (Mateo et al., 2004; Oleson et al., 2009a; Oleson et al., 2009b). Since the Km changes were indistinguishable between cocaine and speedball groups, the possibility that heroin makes cocaine more efficient in inhibiting DA uptake when these two drugs are combined can be ruled out. Because Km was unaffected by heroin, the elevation in Km due to speedball is likely attributable to the cocaine component alone. Therefore, changes in the affinity of the DAT cannot be involved in the potentiating effect of speedball on extracellular DA concentrations.
The magnitude of the increase in evoked DA concentrations was significantly greater following cocaine in comparison with speedball administration. At first glance, these voltammetric results may appear contradictory to previously published microdialysis data (Hemby et al., 1999; Smith et al., 2006; Zernig et al., 1997), but the findings are in fact compatible. Microdialysis provides an assessment of the steady state extracellular levels of DA in the region of interest, but with relatively poor temporal resolution (minutes) (Jones et al., 1999). In contrast, FSCV does not allow assessment of basal DA concentrations, but detects evoked DA concentrations with subsecond resolution, allowing DA dynamics to be measured in real time (Budygin and Jones, 2008). Therefore, prompt adaptive changes in DA release and uptake can be detected with this approach. Taking this into account, the attenuated amplification in electrically evoked DA effluxes observed following speedball administration compared to cocaine alone is likely due to feedback induced by greater accumulation of DA in NAc extracellular space after the speedball administration.
Several mechanisms can be considered in this regard. Post-synaptic D2DA receptors in the NAc provide indirect feedback to DA neurons via long pathways (Hommer and Bunney, 1980), while pre-synaptic D2DA receptors on dopaminergic terminals and somatodendrites function as autoreceptors (Bunney et al., 1991; Starke et al., 1989; Wolf and Roth, 1990). Pre-synaptic D2 autoreceptors maintain dopaminergic homeostasis via feedback mechanisms, which include regulation of DA release (Cardozo and Bean, 1995; Herdon et al., 1987; Uchimura et al., 1986; Wu et al., 2002). For example, microdialysis assessment of DA in wild type and D2 receptor knockout mice (D2R−/−) following acute injections of cocaine or morphine showed that D2R−/− mice exhibited a significantly greater increase in [DA]e in the striatum than wild type mice (Rouge-Pont et al., 2002). In vitro voltammetric assessment found an increase in electrically evoked DA release when amphetamine was applied in the presence of the D2 antagonist sulpiride compared to amphetamine alone. Moreover, a greater peak DA amplitude from amphetamine was observed in striatal slices from D2R−/− mice in comparison with wild type mice (Schmitz et al., 2002). Even though amphetamine is known to elicit DA overflow in the NAc (Fischer and Cho, 1979; Jones et al., 1998; Raiteri et al., 1979; Sulzer et al., 1995; Sulzer et al., 1993), stimulation-dependent DA release is decreased by amphetamine (Jones et al., 1998), mostly due to release-regulating D2 autoreceptor activation which inhibits electrically evoked DA release (Schmitz et al., 2002). Similarly, markedly increased extracellular DA induced by cocaine (~400 %) and speedball (~1000%) likely activates release-regulating autoreceptors, decreasing electrically evoked DA release. However, post-synaptic D2 receptors which provide release inhibition via long, indirect feedback loops can be also involved in this effect. Since the magnitude of NAc [DA]e is greater following speedball compared to cocaine administration, D2 feedback initiated by DA overflow from cocaine would be of lesser magnitude compared to speedball. Therefore, electrically evoked DA release detected by voltammetry is reduced following speedball administration compared to cocaine alone.
In agreement with this speculation, the dose of heroin used in this study did not significantly affect electrically evoked DA release. As mentioned previously, heroin administration increases dopaminergic cell firing rates via activation of mu opiate receptors on VTA GABAergic neurons causing hyperpolarization and removal of tonic inhibition on dopaminergic cells (Johnson and North, 1992; Leone et al., 1991; Self et al., 1995). In vivo extracellular electrophysiological recordings have shown that acute heroin decreases VTA GABAergic cell firing rates (Steffensen et al., 2006) and microdialysis studies have shown modest increases in NAc [DA]e following heroin administration (Leri et al., 2003b; Wise et al., 1995a; Zernig et al., 1997). Perhaps a higher dose of heroin would better facilitate detection of heroin-induced increases in extracellular DA levels, which would likely be observable as a decrease in electrically evoked DA release. In agreement with such a suggestion, we observed decreased electrically evoked DA release following high doses of ethanol (Budygin et al., 2001; Jones et al., 2006), which reliably increased basal DA levels measured by microdialysis (Di Chiara and Imperato, 1988; Weiss et al., 1993; Weiss et al., 1996; Yoshimoto et al., 1992).
DA D2 receptor activation not only inhibits release but can also upregulate uptake through DAT (Cass and Gerhardt, 1994; Hoffman and Donovan, 1994; Wu et al., 2002). The attenuated increase in electrically evoked DA efflux observed after speedball administration could be a consequence of other feedback mechanisms resulting from D2 autoreceptor induced upregulation of DAT. However, no evidence was obtained in our experiments to support this hypothesis. Indeed, the maximal uptake rate was not notably increased following cocaine and its combination with heroin.
Although these data revealed significant changes in electrically evoked DA overflow in response to the enhanced NAc [DA]e elicited by speedball, mechanistically they cannot explain the potentiation of this drug combination, since the ultimate outcome of D2 DA autoreceptor-mediated feedback is a decrease in extracellular DA concentration. At the same time, we have clearly demonstrated that alterations in DAT affinity cannot contribute to the potentiation of [DA]e observed with speedball. Therefore, our voltammetric results, when combined with published microdialysis and electrophysiological data, indicate that the combination of cocaine-induced competitive inhibition of the DAT (apparent Km increase, but not Vmax changes) and the modest increase in the DA release elicited by heroin via the enhancement of firing rate, explains the synergistic increase in extracellular DA concentrations induced by speedball in the NAc.
Acknowledgments
The research for this study was funded by DA012498 (SEH) and DA021634 (EAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Budygin EA, Jones SR. Electrochemical characterization of dopamine transporters. In: Trudell ML, Izenwasser S, editors. Dopamine Transporters: Chemistry, Biology, and Pharmacology. John Wiley and Sons, Inc; Hoboken: 2008. pp. 97–121. [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J Neurophysiol. 1995;74:1137–1148. doi: 10.1152/jn.1995.74.3.1137. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Craddock SG, Rounds-Bryant JL, Flynn PM, Hubbard RL. Characteristics and pretreatment behaviors of clients entering drug abuse treatment: 1969 to 1993. Am J Drug Alcohol Abuse. 1997;23:43–59. doi: 10.3109/00952999709001686. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse. 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Greberman SB, Wada K. Social and legal factors related to drug abuse in the United States and Japan. Public Health Rep. 1994;109:731–737. [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Herdon H, Strupish J, Nahorski SR. Endogenous dopamine release from rat striatal slices and its regulation by D-2 autoreceptors: effects of uptake inhibitors and synthesis inhibition. Eur J Pharmacol. 1987;138:69–76. doi: 10.1016/0014-2999(87)90338-4. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Donovan H. D1 and D2 dopamine receptor antagonists reverse prepulse inhibition deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 1994;115:447–453. doi: 10.1007/BF02245567. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Bunney BS. Effect of sensory stimuli on the activity of dopaminergic neurons: involvement of non-dopaminergic nigral neurons and striato-nigral pathways. Life Sci. 1980;27:377–386. doi: 10.1016/0024-3205(80)90185-x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Caron MG. Application of microdialysis and voltammetry to assess dopamine functions in genetically altered mice: correlation with locomotor activity. Psychopharmacology (Berl) 1999;147:30–32. doi: 10.1007/s002130051137. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-year follow-up of cocaine use among treated opioid addicts. Have our treatments helped? Arch Gen Psychiatry. 1987;44:281–284. doi: 10.1001/archpsyc.1987.01800150101012. [DOI] [PubMed] [Google Scholar]
- Leone P, Pocock D, Wise RA. Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacol Biochem Behav. 1991;39:469–472. doi: 10.1016/0091-3057(91)90210-s. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003a;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003b;28:2102–2116. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, Morgan D, Roberts DC, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–2842. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmacol. 1997;5:113–118. doi: 10.1037//1064-1297.5.2.113. [DOI] [PubMed] [Google Scholar]
- Near JA, Bigelow JC, Wightman RM. Comparison of uptake of dopamine in rat striatal chopped tissue and synaptosomes. J Pharmacol Exp Ther. 1988;245:921–927. [PubMed] [Google Scholar]
- Ng JP, Hubert GW, Justice JB., Jr Increased stimulated release and uptake of dopamine in nucleus accumbens after repeated cocaine administration as measured by in vivo voltammetry. J Neurochem. 1991;56:1485–1492. doi: 10.1111/j.1471-4159.1991.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Salek J, Bonin KD, Jones SR, Budygin EA. Real-time voltammetric detection of cocaine-induced dopamine changes in the striatum of freely moving mice. Neurosci Lett. 2009a;467:144–146. doi: 10.1016/j.neulet.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 2009b;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology (Berl) 1993;112:343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979;208:195–202. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz CG, Vlahov D, Anthony JC, Graham NM. Comparison of self-reported injection frequencies for past 30 days and 6 months among intravenous drug users. J Clin Epidemiol. 1994;47:191–195. doi: 10.1016/0895-4356(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Self DW, McClenahan AW, Beitner-Johnson D, Terwilliger RZ, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to heroin self-administration. Synapse. 1995;21:312–318. doi: 10.1002/syn.890210405. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Dissociation of the actions of uptake blockers upon dopamine overflow and uptake in the rat nucleus accumbens: in vivo voltammetric data. Neuropharmacology. 1989;28:1383–1388. doi: 10.1016/0028-3908(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202:139–151. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Uchimura N, Higashi H, Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986;375:368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988a;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wightman RM, May LJ, Michael AC. Detection of dopamine dynamics in the brain. Anal Chem. 1988b;60:769A–779A. doi: 10.1021/ac00164a001. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995a;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995b;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001a;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001b;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zernig G, O'Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337:1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman JB, Wightman RM. Simultaneous electrochemical measurements of oxygen and dopamine in vivo. Anal Chem. 1991;63:24–28. doi: 10.1021/ac00001a005. [DOI] [PubMed] [Google Scholar]


