Abstract
Carbonic anhydrase IX (CAIX) is a zinc metalloenzyme that catalyzes the reversible hydration of CO2. CAIX is overexpressed in many types of cancer, including breast cancer, but is most frequently absent in corresponding normal tissues. CAIX expression is strongly induced by hypoxia and is significantly associated with tumor grade and poor survival. Herein, we show that hypoxia induces a significant increase in CAIX protein in MDA-MB-231 breast cancer cells. Using a unique mass spectrophotometric assay, we demonstrate that CAIX activity in plasma membranes isolated from MDA-MB-231 is correlated with CAIX content. We also show that CAIX exists predominantly as a dimeric, high-mannose N-linked glycoprotein. While there is some evidence that the dimeric form resides specifically in lipid rafts, our data do not support this hypothesis. EGF, alone, did not affect the distribution of CAIX into lipid rafts. However, acute EGF treatment in the context of hypoxia increased the amount of CAIX in lipid rafts by about 5-fold. EGF did not stimulate tyrosine phosphorylation of CAIX, although EGFR and down-stream signaling pathways were activated by EGF. Interestingly, hypoxia activated Akt independent of EGF action. Together, these data demonstrate that the active form of CAIX in the MDA-MB-231 breast cancer cell line is dimeric but that neither lipid raft localization nor phosphorylation are likely required for its dimerization or activity.
Keywords: carbonic anydrase IX, MDA-MB-231 breast cancer cells, hypoxia, EGFR, lipid rafts, dimerization
1. Introduction
Carbonic anhydrase (CA) is a family of zinc metalloenzymes that catalyze the reversible hydration of CO2: CO2+ H2O ↔ HCO3−+ H+. To date, 16 isoforms have been identified in mammals, of which three are not catalytically active (CA-RP VIII, X and XI)[1]. The 13 catalytically active isoforms are further divided into 4 groups based on tissue distribution and subcellular localization. CAI, II, III, VII, and XIII are expressed in the cytosol. CAVA and CAVB are expressed in mitochondria, but show unique tissue distribution. CAVI is the only secreted CA and found in salivary gland of a number of mammalian species. There are five membrane-associated CA isoforms: CAIV, CAIX, CAXII, CAXIV and CAXV. CAXV is not found in humans.
Two CA isoforms (CAIX and CAXII) are associated with and overexpressed in many solid tumors and cancer cell lines [2,3]. CAIX was first sequenced and characterized as MN/CA9 in HeLa cells by Pastorek et al. [4]. CAIX is a transmembrane N-linked glycoprotein that contains 422 amino acids and four distinct domains: an N-terminal proteoglycan-like domain, a conserved extracellular catalytic domain, a transmembrane region, and an intracellular C-terminal, cytoplasmic domain. The activity of the catalytic domain of CAIX is nearly identical to that of the well studied and very active cytoplasmic isoform of this enzyme, CAII [5]. CAIX expression is mostly restricted to pre malignant of malignant cells, including breast cancer, and rarely in normal tissues or benign lesions cells [6–10] Gastric tumors are unique in that different parts of the normal mucosa express CAIX [11,12]. CAIX expression, which is regulated by the HIF-1 transcription factor complex, is strongly induced by hypoxia and is significantly associated with tumor grade, reduced survival and poor prognosis in breast cancer [13]. We and others have shown that CAIX activity is associated with extracellular acidosis [14–17]. Increased acidity is not permissive to normal cell growth and leads in vivo to apoptosis [18]. Yet cancer cells appear to establish a new “set-point” which allows them to tolerate an interstitial pH of around 6.8 [19]. Work by Gaten by and Gillies suggest that this change in set point is critical to tumor biology because acid will flow along concentration gradients from the tumor to adjacent normal tissue causing normal cell death, disruption of the extracellular matrix, promotion of angiogenesis, and loss of immune response to tumor antigens, and resistance to therapeutic drugs [20,21]. Because of their increased tolerance to extracellular pH, tumor cells are readily able to colonize this adjacent damaged normal tissue providing a mechanism for continued invasion and growth.
Triple negative breast cancer (TNBC) is a descriptor for a subtype of breast cancer that does not express the estrogen receptor, progesterone receptor, or HER2receptor [22]. However, many of these cancers overexpress the EGF receptor (HER1), including the MDA-MB-231 cells [23]. Recent evidence has shown that the cytoplasmic domain of CAIX possesses a tyrosine target for the EGF receptor (EGFR) kinase and participates in the PI3 kinase signaling pathway in renal clear cell carcinoma (RCC) [24]. The authors proposed that CAIX translocates from the bulk phase plasma membrane to lipid rafts to form dimer sin response to EGF stimulation and directly mediate down-stream signaling. Lipid rafts are unique, cholesterol-rich microdomains within the plasma membrane that sequester signaling proteins to amplify intracellular signaling events.
In the current study, we took advantage of the constitutive expression of EGFR and hypoxia-inducible expression of CAIX in the MDA-MB-231 breast cancer cell line to examine the role of hypoxia and EGF in CAIX regulation. Our studies revealed that hypoxia-induced CAIX exists primarily in a dimeric form in the membrane and is responsible for most of the CA activity in isolated plasma membranes. Little CAIX resides in lipid rafts in either control or hypoxia-exposed cells indicating its localization to lipid rafts is not required for activity. In addition, CAIX is not phosphorylated in response to EGF, although EGF induced a 5-fold increase in CAIX translocation to lipid rafts. Hypoxia increased Akt phosphorylation independent of EGF action suggesting that hypoxia engages unique mechanisms to initiate similar signaling paths. Taken together, our data suggest that CAIX in the MDA-MB-231 cells is active as a dimer and does not require lipid rafts or phosphorylation for either its activity or dimerization.
2. Material and methods
2.1. Cell culture
The MDA-MB-231 cell line was a gift from Dr. Kevin Brown (University of Florida) and plated at a density of 8 × 104 cells/per 10cm dish in 8mL of DMEM (Gibco) containing 10% FBS (Atlanta Biological). Cells were grown in a humidified atmosphere containing 5% CO2 for 3 days before treatment with 100 μM desferoxamine mesylate (DFO) or exposure to hypoxia (1% O2, 5% CO2, and balance N2) in a humidified Modulator Incubator Chamber (MIC-101, Billups-Rothenberg, Inc) for 16 h. Parallel normoxic cells were incubated in a humidified atmosphere at 37°C in air with 5% CO2.
2.2 Plasma membrane isolation
To isolate plasma membranes, we have used a modification of the method published by Sennoune et al. [25]. Cells were washed three times with Buffer A (Tris, 10mM; EDTA, 1mM; NaCl, 150 mM; PMSF, 1 mM; pH 7.4) and then scraped into the same buffer. After centrifugation at 1000 × g for 7 min, the supernatant was removed and the pellet was resuspended in 3 mL of Buffer B (Tris, 10mM; EDTA, 1mM; NaCl, 5 mM; pH 7.4) and incubated on ice for 10 min. The cell suspension was then homogenized in a Potter Elvehjem homogenizer with 15 up and down strokes. Cell debris was collected by centrifugation at 500 × g for 5 min. The supernatant was removed and kept on ice. Two mL of Buffer B was added to the pellet which was then re-homogenized. After centrifugation at 250 × g for 5 min, this second supernatant was combined with the first supernatant. Five mL of Buffer C (Tris, 160mM; EDTA, 20mM; NaI, 2 M; MgCl2, 5 mM; pH 7.4) was added to the above solution and stirred on ice for 10 min. Twenty mL of Buffer D (Tris, 10mM; EDTA, 1mM; pH 7.4) was added to the above solution. After mixing, the solution underwent ultracentrifugation at 105,000 × g for 45 min at 4 °C. The pellet was washed 3 times with Buffer D. After resuspending the pellet with homogenizing buffer (Tris, 50mM;EGTA, 1mM; sucrose, 250mM; pH 7.4), the solution was loaded on a step gradient of 20 and 40% sucrose (20% or 40% sucrose, Tris, 10mM; EDTA, 1mM; pH 7.4) and centrifuged at 200k × g for 1 hour at 4 °C. The interface between the steps (plasma membrane s) was collected and resuspended in Buffer D. After centrifugation at 100k × g for 30 min at 4 °C, the pellet was resuspended in homogenizing buffer and kept in −80 °C. Protein recovery was determined by a modification of the Lowry method [26].
2.3. Membrane inlet mass spectrometry (MIMS) to determine CAIX activity
An Extrell EXM-200 mass spectrophotometer was used to measure directly the content of CO2 through a membrane which is permeable to CO2 but not to bicarbonate. This allows an estimation of the rate of exchange of 18O from CO2and bicarbonate to water at chemical equilibrium as previously described [27]. This process is catalyzed by and is a measure of carbonic anhydrase activity. In our plasma membrane samples, this activity is exclusively CAIX.
2.4. CAIX Oligomerization
MDA-MB-231 cells were exposed to hypoxia for 16 hours. Total membranes were isolated as previously described [28] with minor modifications. Briefly, cells were washed 3 times with 5ml Krebs Ringer Phosphate buffer (NaCl, 128mM; KCl, 4.7mM; MgSO4, 1.25 mM; CaCl2, 1.25mM; sodium phosphate, 5mM; pH, 7.4) and incubated for 10 min. Cells were then homogenized in a Potter Elvehjem homogenzer in a Tris–based buffer (TES1p) containing 20 mM Tris-HCl, 25 mM sucrose, 1 mM EDTA and protease inhibitor (Sigma). A total membrane fraction was collected at 212,000 × g. The pellet was washed once with TES1 and resuspend in TES1p. Fifty μg of total membrane protein from hypoxic cells was denatured in 0.5 % SDS in a final volume of 30 μL for 10 min at 100 °C. The samples were brought to 60 μL with 50 mM sodium phosphate buffer, pH 7.5, 1% NP-40 and incubated at 37 °C for 2 hours. Proteins were separated on 10 % polyacrylamide gels in the presence or absence of 1% β-mecaptoethanol. Proteins were then transferred to nitrocellulose and probed for CAIX using the M75 monoclonal antibody developed by Pastorekova et al. [29]. For some experiments, we also used CAIX antibodies purchased from Novus Biologicals (NB100-417). Densitometry of individual bands in these experiments, and all other immunoblot analyses, was performed using UnScanIt (Silk Scientific).
2.5 CAIX Glycosylation
To determine CAIX glycosylation, 50 μg of total membrane protein (or cell lysates) from hypoxic MDA MB-231 cells was denatured in 40 mM DTT/0.5 % SDS in a final volume of 30 μL for 10 min at 100°C. For N-glycosidase F digestion, the samples were brought to 60 μL in 50 mM sodium phosphate, pH 7.5, 1% NP-40, containing 2 μL N-glycosidase F (1000 U) (Biolabs) and incubated at 37°C for 2 hours. For endoglycosidase H digestion, denatured protein samples were brought to 60 μl in 50 mM sodium citrate, pH 5.5, containing 2 μL endoglycosidase (1000 U) (Biolabs) and incubated at 37°C for 2 hours. Twenty μL of 4X sample dilution buffer was added to each of the samples which were then loaded onto a 10% SDS-PAGE gel for protein separation followed by western blot analysis for CAIX expression using the M75 monoclonal antibody.
2.6. Localization of CAIX in lipid rafts
Total membranes from MDA-MB-231 cells were isolated as described above. Five mg of total membrane protein was extracted in 1.0 mL ice-cold MBS buffer (25 mM MES and 150 mM NaCl, pH 6.5) containing 1% Triton X-100 (TX-100) and supplemented with protease inhibitor. The samples were mixed end-over-end for 20min at 4 °C and then homogenized with 10 strokes in a Dounce homogenizer. The homogenizing flask was rinsed with 0.5mL MBS/TX-100 and added to the extracted sample. The samples were then mixed with 1.5ml of 80% sucrose. The samples, now at 40% sucrose, were placed at the bottom of centrifuge tubes and overlaid with 6mL of 30% sucrose in MBS and 3.5mL of 5% sucrose in MBS. After centrifugation at 240,000 × g in a Beckman SW41 rotor for 18h, 1.0 mL fractions were collected into tubes containing protease inhibitor at 4°C by upward displacement using 60% sucrose. The fractions were mixed and 500μL of ice-cold, 30% tricholoroacetic acid was added to each fraction collected. The samples were mixed and incubated on ice for30 min. The protein precipitate was collected at 16,000 × g for 10min after which it was washed 2 times in 1ml of ice-cold acetone. The precipitate was then dissolved in 65 μL of 0.1% SDS. Total membrane protein (100 μg) and precipitated protein from fractions 2–13 from the sucrose gradients were resolved on a 10%SDS-PAGE gel under reducing conditions. The proteins were transferred to nitrocellulose membrane and probed for CAIX, GLUT1 [characterized previously [30]], caveolin (Transduction Laboratories # C13630/L5), and transferrin receptor (Alpha Diagnostics, #TFR12-M).
2.7. Phosphorylation of EGFR and Akt
MDA-MB-231 cells, grown to 50% confluence in 10 cm culture dishes, were exposed to serum-free medium overnight under normoxic or hypoxic condition s. Recombinant EGF (rEGF Santa Cruz) was dissolved in 10 mM acetic acid containing0.1% BS A at a stock concentration of 10 μg/mL, EGF (16 nM, final concentration) was added to the serum-starved, control and hypoxic cells for specific times as indicated in the figure legends. Immediately after treatment, cells were placed on ice, washed with ice-cold PBS (10 mM sodium phosphate salts, 120 mM NaCl, pH 7.4), and lysed in RIPA buffer [1% NP-40, 10mM phosphates buffer, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, 0.5 mM phenylmethyl sulfonyl fluoride (PMSF) and protease inhibitor (Roche Diagnostics), pH, 7.4]. Cell lysates were clarified by centrifugation at 16,000 × g for 15 minutes at 4°C. Protein concentration of the clarified supernatants was determined using a modification of the Lowry assay. Proteins were separated on an 8% PAGE gel and transferred to a nitrocellulose membrane for western blot analysis. The following antibodies were used in the analysis: EGFR (Cell Signaling Technology #2232); pEGFR (Y1173: Santa Cruz Biotechnology #sc-101668); Akt1 (Sigma #p1601); pAkt (S473)(Cell Signaling Technology #D9E); Map kinase/Erk2 (Calbiochem #442700); pErk1/2 (Biolabs #9106).
2.8. Phosphorylation of CAIX
Cells were treated as described above from which total membranes were prepared. Total membrane proteins were lysed in RIPA buffer. Then, lysates were processed for immunoprecipitation. Briefly, lysed samples (1mg protein) were treated with 50 μL of a 50% suspension of protein A/G plus-agarose (Santa Cruz) for 1h at 4° C to eliminate non-specific protein binding. The beads were removed by centrifugation at 3000, × g for 1 min. To the precleared supernatants, 50 μL of a 50% solution of protein A/G plus-agarose beads and 3 μg of a (goat) polyclonal antibody against CAIX (R&D Systems #AF2188) were added and subjected to gentle end-over-end mixing overnight at 4° C. The immune complexes were collected by centrifugation at 3000 × g for 5 min. Immunoprecipitates were subjected to gel electrophoresis and blotted onto nitrocellulose membranes. Phosphorylated CAIX was detected with antibody against phosphotyrosine (Santa Cruz Biotechnology, #sc-7020). Total CAIX was detected with the M75 monoclonal antibody.
3. Results
3.1 Hypoxia increases CAIX expression and activity in MDA-MB-231 cells
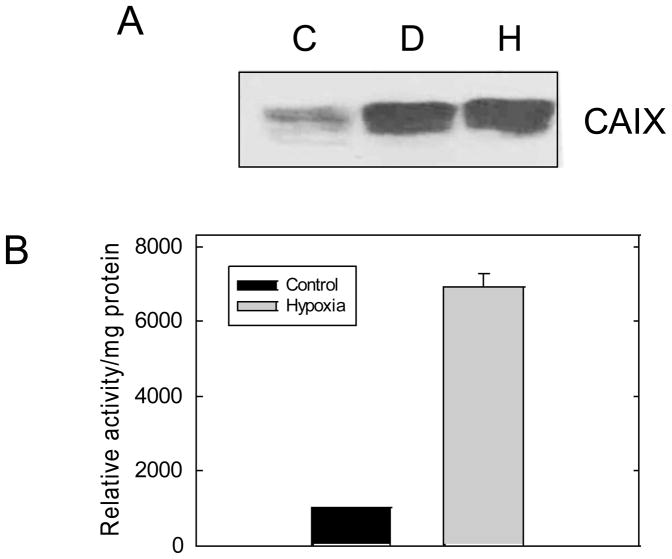
Hypoxic-dependent expression of CAIX expression was investigated by Western immunoblot. By densitometric analysis, CAIX expression was increased by 6-fold in total membranes by desferoxamine mesylate (D), an iron chelator which mimics the effect of hypoxia, or hypoxia (H) compared to controls(C) (Figure 1A). We have previously shown that CAIX is the only membrane-associated CA in MDA-MB-231 cells [14] which allows us to unequivocally assay CAIX activity in plasma membranes. Using 18O isotopic exchange between CO2 and water detected by mass spectrometry, we demonstrate that there is a 7-fold increase in CA activity in plasma membranes isolated from hypoxic cells relative to controls (Figure 1B). This demonstrates that there is a strong correlation between CAIX expression and activity.
Figure 1. Hypoxia increases CAIX expression and activity in MDA-MB-231 cells.
Panel A: Total membranes were collected from control cells or cells exposed to DFO or hypoxia for 16 hours. Western blot analysis of CAIX expression was performed using the NB-100 antibody. This blot is typical of at least four independent experiments. C: Control; D: DFO (desferoxamine mesylate), H: Hypoxia. Panel B: Plasma membranes were isolated from control or hypoxic cells and CAIX activity was measured by 18O exchange. These data represent duplicate experiments.
3.2 Membrane-bound CAIX is a dimer
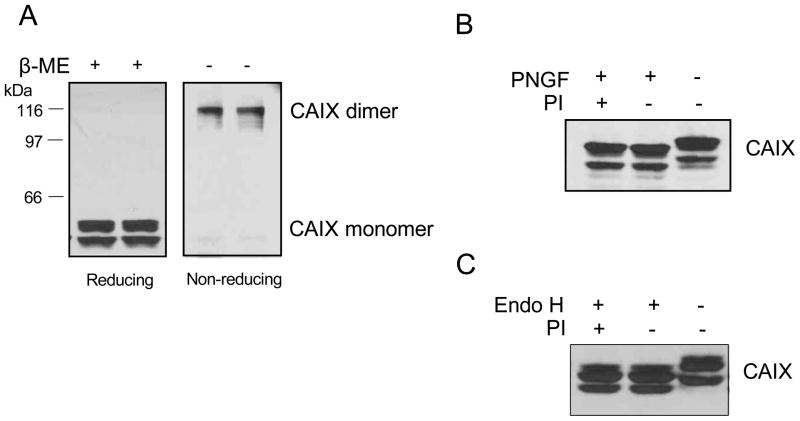
It has been suggested that CAIX in renal carcinoma cells is translocated to lipid rafts where it forms a dimeric structure, a process that is controlled by EGF action [24]. To investigate the oligomerization status of CAIX in hypoxic MDA-MB-231 cells, total membranes were analyzed by SDS-PAGE under reducing and non-reducing conditions (Figure 2A). Under reducing conditions, CAIX migrated as a 54/58kDa doublet which has been observed by many investigators [31]. Under non-reducing conditions, CAIX migrated as a single, higher molecular weight band which represents 90% of the CAIX pool. Taking into consideration the activity data (Figure 1B), we conclude that the dimeric form of CAIX represents most of the activity in MDA-MB-231 cells. We also investigated the glycosylation state of CAIX through endoglycosidase digestion. N-glycosidase F (PNGF) releases the entire N-linked glycan while endoglycosidase H (endo H) releases the glycan only if the structure is high mannose or a hybrid form, but not a complex structure. The 54/58 doublets were both sensitive to PNGF showing more rapid migration of these species in SDS-PAGE gels (Figure 2B). This indicates that the 54kDa protein is not a deglycosylated form of the 58kDa protein. In other words, the 54kDa species may be a truncated isoform of CAIX or the 58kDa form may be post-translationally modified by mechanisms in addition to glycosylation. Further, both forms were completely sensitive to endo H indicating that the glycans were of high mannose structure.
Figure 2. Oligomerization and glycosylation of CAIX in MDA-MB-231 cells.
Panel A: Total membranes were isolated from MDA-MB-231 cells exposed to hypoxia for 16 h. Fifty μg of total membrane protein were separated on an SDS-PAGE gel in the presence or absence of 1% β-mercaptoethanol (β-ME). CAIX expression was detected by western blotting using the M75 monoclonal antibody. Panel B: Total membranes were isolated from MDA-MB-231 cells exposed to hypoxia for 16 h. Fifty μg of protein was digested with 2 μL N-glycosidase F (PNGF) in the presence of absence of protease inhibitor (PI) for 2 hours at 37°C. Panel C: Cell lysates were isolated from MDA-MB-231 cells exposed to hypoxia for 16 h. Fifty g of protein was treated with 2 μL endoglycosidase H (endo H) in the presence or absence of protease inhibitor (PI) for 2 hours at 37°C. CAIX expression was detected by western blotting using the M75 monoclonal antibody. These blots represent duplicate experiments.
3.3 CAIX activity does not require its localization to lipid rafts
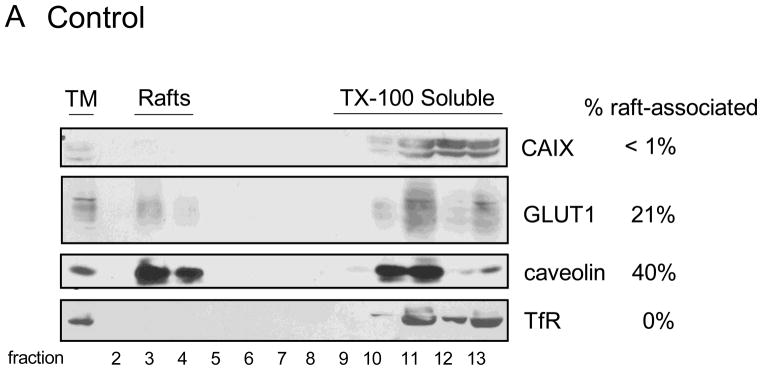
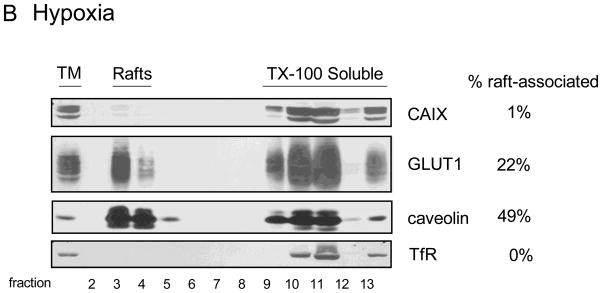
Lipid rafts are microdomains within the plasma membrane that serve as signaling platforms. These are enriched in specific lipids and are comprised of a select set of proteins that reside in, or transiently associate with, these domains. We have shown that GLUT1, in 3T3-L1 adipocytes, is associated with lipid rafts [28] and show that the same is true for GLUT1 in MDA-MB-231 cells. In Figure 3, fractions 3 and 4 from sucrose density gradients represent plasma membrane vesicles derived from lipid rafts. These vesicles are resistant to disruption by Triton X-100 (TX-100) because of the high cholesterol and sphingolipid content of the lipid rafts. The fractions that are labeled as “TX-100 Soluble” represent membrane proteins that were not protected from detergent extraction. Thus, these proteins were not in lipid rafts to begin with and were dissolved by detergent treatment. We have used the identification of caveolin as a marker of lipid rafts and the transferrin receptor as a protein excluded from lipid rafts and thus solubilized by TX-100 exposure. Neither caveolin nor transferrin receptor expression or localization was influenced by hypoxia (compare Figure 3B to 3A). The localization of GLUT1 to lipid rafts in both control and hypoxic cells represents about 25% of the total pool despite a significant 6-fold increase in expression in response to hypoxia. We would interpret this to mean that hypoxia does not have a specific influence on GLUT1 localization within the plasma membrane. CAIX was not detected in lipid rafts in control cells (Figure 3A), perhaps a result of the low total CAIX expression. Hypoxia increased the total expression of CAIX by 5-fold in this experiment (Figure 3B) with only a small increase in the amount of CAIX associated with lipid rafts, about 1.1% of the total CAIX pool. With a 7-fold increase in CAIX activity in response to hypoxia (Figure 1B), it is unlikely that the small shift of CAIX to lipid rafts accounts for this change in activity. Thus, lipid raft association does not to appear to play a major role in regulating CAIX activity under hypoxic conditions.
Figure 3. Association of CAIX and GLUT1 with lipid rafts.
MDA-MB-231 cells were exposed to normoxic (Panel A) or hypoxia (Panel B) conditions for 16 h. Total membranes were collected and extracted with 1% TX-100. Detergent-resistant membranes (lipid rafts) were separated from extracted protein by flotation on sucrose gradients (fractions 2 through 13 as numbered at the bottom of each panel). Expression of CAIX, GLUT1, caveolin and transferrin receptor is indicated. The values to the right of the figures represent the percent of the total pool that migrates in lipid rafts. TM = total membrane fraction; Rafts = lipid raft-containing membranes; TX-100 Soluble = membrane proteins extracted by TX-100. These data are representative of at least triplicate experiments.
3.4 EGF induces CAIX translocation to lipid rafts but not its phosphorylation
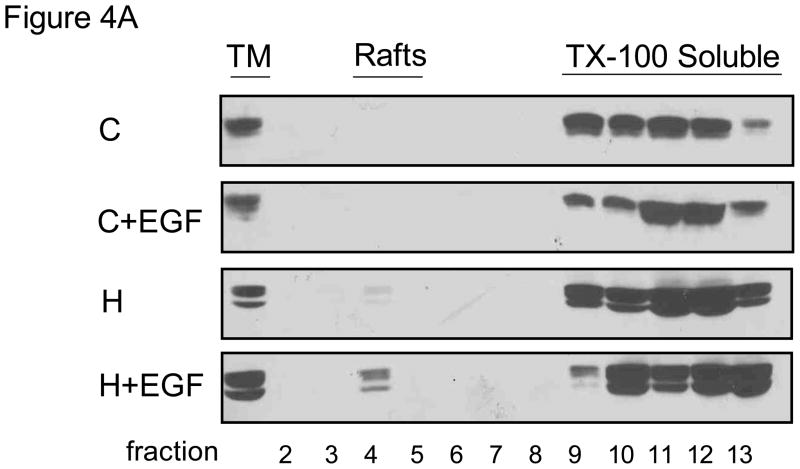
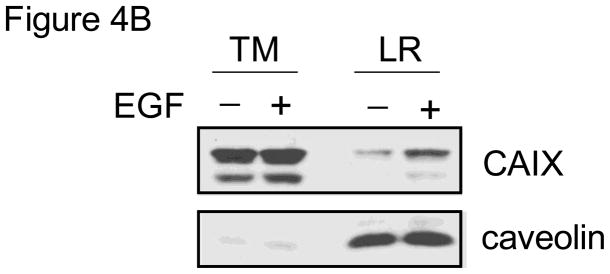
We next determined the effect of EGF on CAIX distribution within the plasma membrane. In Figure 4A, we show only CAIX expression. Consistent with data in Figure 3A, little CAIX was detected in lipid rafts from control cells. EGF, under normoxic conditions, did not cause any translocation of CAIX to lipid rafts. Hypoxia increased the amount of CAIX, again by only a small amount, while the combination of hypoxia and EGF stimulation increased the amount of CAIX by 5-fold relative to hypoxia alone. In this particular experiment, it appeared that there was a little more CAIX in the TM fraction isolated from hypoxic cells treated with EGF. This could of course influence the amount of CAIX in the lipid raft fraction. We repeated this experiment and confirm in Figure 4B that CAIX expression did not change in the presence of short-term exposure to EGF. Together these data suggest that EGF induces CAIX translocation to lipid raft only in the context of hypoxia, although the pool associated with lipid rafts is relatively small (about 5%) compared to the total.
Figure 4. EGF-dependent localization of CAIX.
Panel A: MDA-MB-231 cells were serum-starved overnight under normoxic or hypoxic conditions and then stimulated with EGF (16nM) for 30 min. Total membranes were collected and then lysed with TX-100. Detergent resistant proteins (lipid rafts) were separated from extracted proteins. CAIX expression in each sample was detected by western blotting using the M75 monoclonal antibody. TM= total membrane fraction; Rafts = lipid raft-containing membranes; TX-100 Soluble = membrane proteins extracted by TX-100. Panel B: MDA-MB-231 cells were exposed to hypoxia for 16 hours and then treated with EGF (16 nM) for 30 min. Total membranes were prepared from which lipid rafts were isolated. CAIX and caveolin expression were detected by western blotting. TM = total membrane proteins; LR = lipid raft containing membranes.
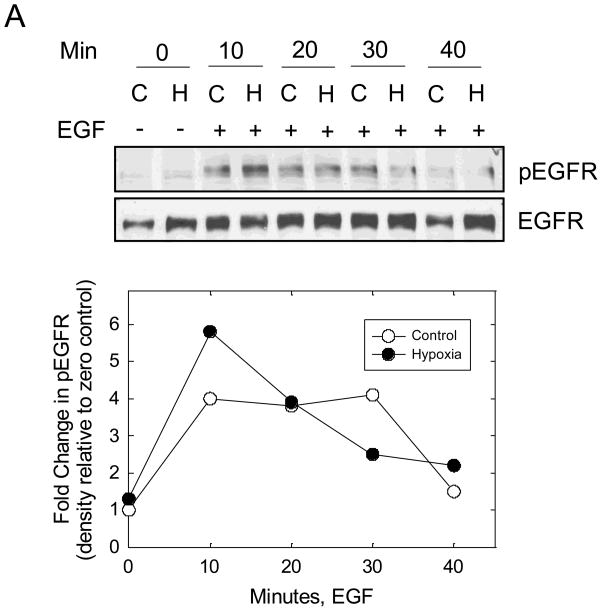
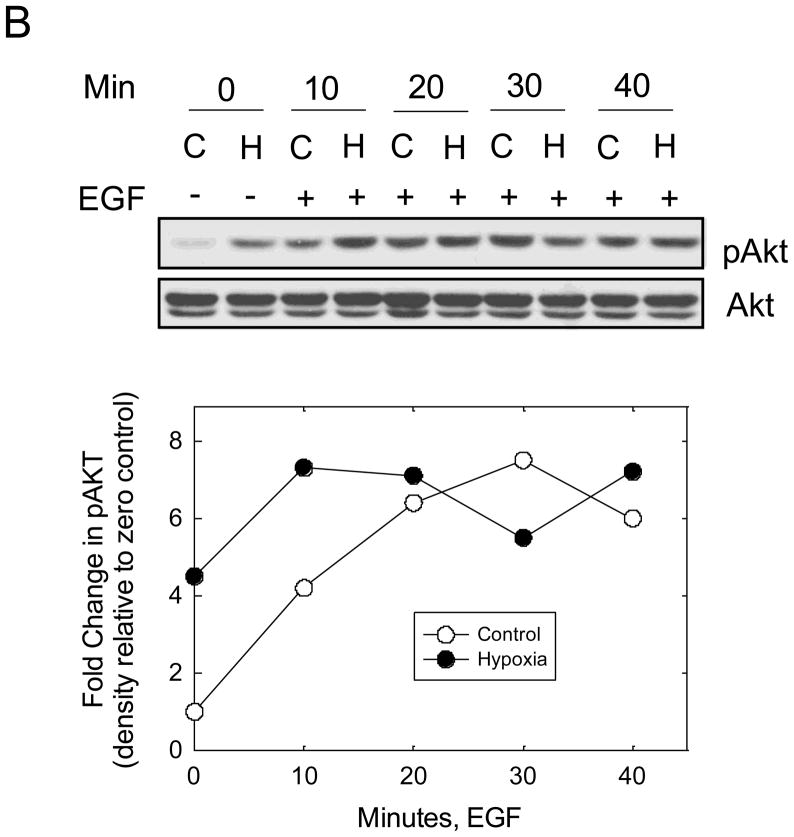
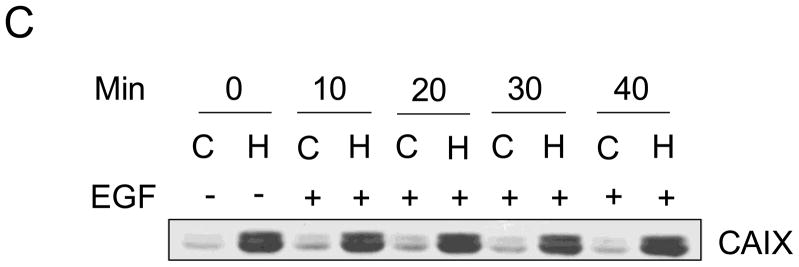
Recent data suggest that EGF stimulates CAIX tyrosine phosphorylation in the cytoplasmic domain in renal carcinoma cells which leads to down-stream activation of the PI3-kinase pathway [24]. To investigate this possibility in MDA-MB-231 cells, we first examined EGF-dependent autophosphorylation of the EGF receptor (EGFR). Cells were serum-starved under hypoxicor normoxic conditions for 16 hours and then stimulated with EGF (16 nM) for specific times ranging from 10 min to 40 min. Under these conditions, we observed a time-dependent phosphorylation of EGFR on Y1173 (Figure 5A). The kinetics of phosphorylation were similar for both the normoxic and hypoxic conditions peaking between 10 and 30 min of EGF exposure. Activation of EGFR led to down-stream phosphorylation of both Akt (Figure 5B) and ERK1/2 (data not shown). Interestingly, Akt phosphorylation was relatively strong in hypoxic cells even in the absence of EGF. Under the conditions of the experiment, EGF did not influence CAIX expression (Figure 5C).
Figure 5. EGF and hypoxia dependent activation of Akt.
MDA-MB-231 cells were exposed to normoxic (C) or hypoxic (H) conditions for 16 h in the absence of serum and then stimulated with 16nM EGF for specific times. Proteins (100 μg) from cell lysates were separated on SDS-PAGE gels and analyzed by western blotting. Panel A represents total and phosphorylated pools of EGFR; Panel B represents total and phosphorylated pools of Akt; Panel C represents the total pool of CAIX. This experiment is representative of two independent experiments.
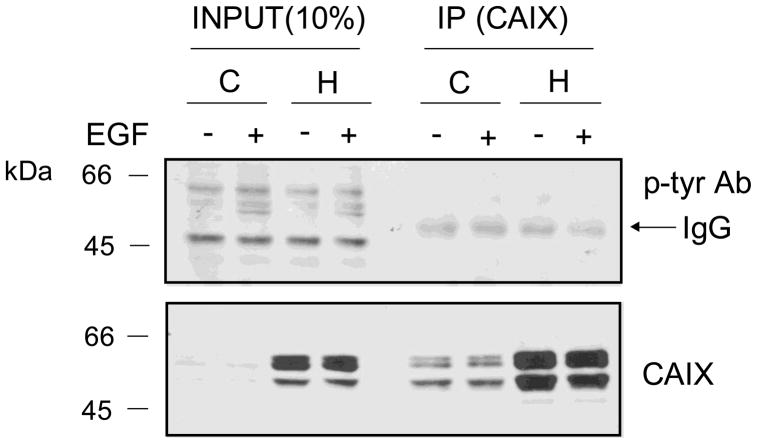
To examine EGF-dependent phosphorylation of CAIX, cells were exposed to hypoxia for 16 hours and then 30min with EGF. CAIX was then immunoprecipitated with a CAIX-specific polyclonal antibody. While there appeared to be several phosphorylated proteins in the cell extracts (input) including those that were EGF-dependent, there were no phosphorylation signals in the CAIX-immunoprecipitated samples (Figure 6). Note the non-specific detection of the heavy chain IgG (arrow). The presence of CAIX protein in the immunoprecipitates was verified by Western blot using the M75 monoclonal antibody. These results indicate that CAIX is not phosphorylated on tyrosine under hypoxic conditions or in an EGF-dependent manner inMDA-MB-231 breast cancer cells.
Figure 6. CAIX phosphorylation in response to EGF stimulation.
MDA-MB-231 cells were exposed to hypoxia or not for 16 h in the absence of serum. EGF (16nM) was added for 30 min after which total membranes were isolated. CAIX was immunoprecipitated with an antibody generated in goat (R&D Systems, #AF2188) followed by western blotting with an anti-phosphotyrosine antibody or the M75mouse monoclonal antibody. C = control, H = hypoxia. These data represent triplicate experiments.
4. Discussion
CAIX expression is upregulated in a wide variety of human tumors [1] under the control of the HIF cascade [32–35]. In breast cancer, CAIX is a marker for hypoxic regions of tumors [36], is associated with poor prognosis [37–39], and is linked to acidification of the tumor microenvironment [17] which favors cancer cell survival and resistance to chemotherapeutic agents [40]. CAIX expression has also been linked to the basal B, triple-negative phenotype [41]. We have shown that the MDA-MB-231 cell line is a model for this phenotype [14] and confirm here that CAIX expression is induced by an hypoxic mimic and by hypoxia, itself. We have also shown previously that CAIX is the only expressed membrane-bound CA isoform in this cell line [14] which allows us to unambiguously assay for CAIX activity. We have measured endogenous CAIX activity, directly, using a novel mass spectrometric technique. Plasma membranes, isolated from MDA-MB-231 cells by a method specifically developed for breast cancer cells [25], exhibited a 7-fold increase in CAIX activity in response to hypoxia which correlated well with the increased expression of CAIX in total membranes.
The selective expression of CAIX that we have observed in MDA-MB-231 cells is clearly different from the expression pattern described by Hsieh et al., also in MDA-MB-231 cells [42]. In their study, the expression of CAXII appeared to be significantly higher than CAIX. Further, the authors showed that knocking down expression of CAXII decreased the invasion and migration capability of the cells. So the question arises as to how the same cell line shows different expression for specific proteins. It is appreciated that cancer arises from a stepwise accumulation of genetic changes that afford an incipient cancer cell the properties of unlimited, self-sufficient growth and resistance to normal homeostatic regulatory mechanisms [43]. This genetic instability is considered to play a key role in the generation of genetic and phenotypic heterogeneity in cancer cells. Recently, Masramon et al. have demonstrated genetic drift in clonal lines originating from isolated (colon) cancer cells [44]. This indicates that genetic instability is not lost in cultured cells and can continue to contribute to genetic and phenotypic differences. It is logical to assume that this genetic drift is responsible for the protein expression differences in the MDA-MB-231 cells used in our studies and those used by Hsieh et al. That CAXII has similar properties to CAIX is not unexpected based on studies from the Poussegeur lab [16]. In these studies, Chiche et al. demonstrated that overexpression of CAIX and CAXII were equally able to regulate intracellular pH in several different cell lines. Thus our data do not detract from the finding by Hsieh et al. that CAXII is involved in regulating migration and invasion.
It was originally proposed that CAIX could form trimers [29]. More recent characterization reveals that CAIX can also exist as a dimer [45]. These later biochemical studies were done with soluble recombinant forms of CAIX (containing exofacial domains) which were expressed in insect cell expression systems which allows protein glycosylation process. Hilvo et al. showed that approximately 50% of the CAIX catalytic domain constructs form dimers while in constructs containing the proteoglycan-like combined with the catalytic domain, the dimers comprised about 60% of the pool [45]. While the catalytic domain construct could adapt both trimeric and dimeric structures, the proteoglycan domain construct preferred the dimer structure. This suggests that the presence of the proteoglycan domain favors dimerization. These investigators also identified two sets of specific sulfhydryl groups that potentially participate in forming intermolecular disulfide bridges. The recently published crystal structure of the soluble form of CAIX has confirmed that the two catalytic domains associate to form a dimer, stabilized by the formation of a single intermolecular disulfide bond [46,47]. While the TM domain does not appear to be required for dimerization, we hypothesized that the extent of dimerization may be affected by its presence. Indeed, in the MDA-MB-231 cells, dimers comprise 90% of the CAIX pool which infers that dimers provide the majority of the CAIX activity. The equal intensity of the 54/58 kDa doublet, which we observed on reducing gels, might suggest that the doublet pair is linked by a disulfide bond in the dimer as the dimeric species that we observed migrated as a single band at about 119kDa.
It was previously shown in HeLa cells that each of the proteins that comprise the 54/58 kDa doublet of CAIX are N-linked glycosylated [4]. We show here that the same is true for CAIX in the MDA-MB-231 cells. The oligosaccharides on both of these doublets are of high mannose character, based on endo H sensitivity, which is atypical for plasma membrane proteins from normal cells. However, this is not uncommon in cancer cells [48] and specifically in breast cancer cells as was shown recently [49]. In the later study, membrane proteins showed a significant increase in GlcNAc2 Man5 structures although the complex glycan structures with multiantennary arrangements were not significantly different. Like CAIX, GLUT1 has a single N-linked glycosylation site but migrates as a broad band suggesting heterogeneous, complex glycosylation which is resistant to endo H digestion [50]. Both GLUT1 and CAIX are up-regulated by hypoxia but appear to be differentially processed in the same cell. This preference for high mannose glycans may be an intrinsic feature of the CAIX structure as recombinant CAIX (constructs containing either the catalytic domain, alone, or in combination with the proteoglycan domain) expressed in a baculovirus-insect cells or in murine cells also exhibit high mannose glycan structures [45].
Dorai et al. demonstrated that EGF stimulates CAIX phosphorylation on tyrosine 449 in the cytoplasmic domain in a renal carcinoma cell line, SKRC-01 [24]. This phosphorylation led to the ability of CAIX to interact with PI3-kinase. In a similar line, SKRC-17, which does not express CAIX, the authors demonstrated that EGF action leading to phosphorylation of Akt was more robust when CAIX was ecoptically expressed. This infers that EGF stimulates CAIX phosphorylation which independently activates the EGF signaling path. However, we were unable to demonstrate EGF-mediated CAIX phosphorylation in the MDA-MB-231 cells. Clearly, the EGFR is expressed in MDA-MB-231 cells, is phosphorylated in the presence of EGF, and initiates down-stream signaling events. There are several reasons why we may not have observed phosphorylation of CAIX. The EGFR is known to reside in lipid rafts in both normal cells and cancer cells [51,52] and to mediate the activation of down-stream signaling pathways in cancer cells when recruited to lipid rafts [53]. In addition, cholesterol levels change EGFR function, trafficking, and activation [54,55]. Dorai et al. demonstrated, indirectly, that phosphorylated CAIX was present in lipid rafts which implies that EGF stimulation causes the recruitment of CAIX to lipid rafts. We were unable to detect any CAIX in lipid rafts in control cells with or without EGF but the levels of CAIX are quite low in normoxic cells. On the other hand, if EGFR is localized to lipid rafts, then the interaction between EGFR and CAIX might not occur. We did observe an EGF-dependent increase in the content of CAIX in lipid rafts under hypoxic conditions which represented about 5% of the total CAIX pool. If this particular pool was phosphorylated, admittedly, it might go undetected. There is also the issue of CAIX function in renal cell carcinoma versus breast cancer cells. CAIX in RCC is not upregulated by the condition of hypoxia, as it is in triple negative breast cancers and MDA-MB-231 cells. Rather, mutations in the prolyl hydroxylase which normally mediates the degradation of HIF1 drives CAIX expression. Thus the environment surrounding RCC cells and breast cancer cells is quite different. Further, CAIX expression in RCC is a positive predictor of survival [6] while CAIX expression in breast cancer is an indicator of poor prognosis [13]. How these differences play out with respect to EGF action is not known at this point.
PTEN mutation or loss, which leads to sustained activation of PI3-kinase, is associated with many forms of cancer, including breast cancer [56]. However, MDA-MB-231 cells do not lack PTEN [57] which means that the PI3-kinase pathway is not constitutively activated. This allowed us to examine both the effect of hypoxia and EGF on this signaling path. EGF, as expected, stimulated Akt phosphorylation by activating EGFR. This is consistent with earlier studies [57]. Our data also showed that hypoxia, independent of EGFR activation, enhanced Akt phosphorylation. Others have shown that this activation is mediated through PI3-kinase [58,59] and leads to protection from apoptosis [58]. Further, hypoxia-induced activation of Akt can be blocked by inhibitors of transcription or protein synthesis, although the levels of Akt are not affected by these treatments [58]. While preparing the present manuscript, Mardilovich and Shaw showed that IRS2 is transcriptionally upregulated by hypoxia in MDA-MB-231 cells [60]. Thus, the cells are poised for activation by the IGF1 signaling system. Yet in our experiments, serum was absent for the duration of hypoxic treatment. Thus, IGF-mediated phosphorylation of IRS2 is not responsible for the hypoxic activation of Akt in our setting.
Akt activation is also involved in localizing GLUT1 to the cell surface and maintaining hexokinase activity [61] which favors aerobic glycolysis in tumor cells [62]. While GLUT1 is transcriptionally upregulated by HIF1α, hypoxia-activated Akt may also help to recruit GLUT1 to the cell surface and maintain the distribution between intracellular membrane stores and the plasma membrane. While there are data suggesting that lipid rafts influence GLUT1 activity [28,63], the increase in localization of GLUT1 to lipid rafts in MDA-MB-231 cells is equivalent to the increased expression of GLUT1 in response to hypoxia. As well, the increase in glucose uptake in response to hypoxia (data not shown) is equivalent to GLUT1 expression. It would seem logical to conclude that GLUT1 activity is a reflection of the total pool.
In conclusion, we have shown that CAIX activity and expression are well correlated in hypoxia-treated MDA-MB-231 cells. EGF recruits CAIX to lipid rafts, in the context of hypoxia, but it is unlikely that this association regulates its oligomerization status or its activity. Further, we did not detect any EGF-dependent CAIX phosphorylation, despite an intact EGF signaling system. Rather, hypoxia activates Akt independently from EGF action to induce what Dorai et al. called the vicious cycle [24]. Thus, hypoxia induces the stabilization of HIF1α which mediates the expression of CAIX through transcriptional activation of its gene, and increases Akt activation which enhances cap-dependent translation of HIF1α to further induce expression of target genes.
CAIX expression in hypoxic MDA-MB-231 cells is correlated with carbonic anhydrase activity
CAIX exists in a high mannose, dimeric glycoprotein
EGF, alone, does not stimulate CAIX translocation to lipid rafts but in the hypoxic environment increases the association of CAIX with lipid rafts by 5-fold
EGF does not stimulate the phosphorylation of CAIX, but otherwise activates the traditional downstream kinases
Hypoxia increases Akt phosphorylation
Acknowledgments
The authors would like to thank Dr. Egbert Oosterwijk for the M75 antibody and Ms Xiao Wei Gu for her excellent assistance in cell culture.
Funding Source: The work in this manuscript was supported in part by grants to SCF from the Department of Defense (BC073020) and NIH (DK45035) and DNS from NIH (GM25154).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Supuran CT. Carbonic Anhydrase: Novel Therapeutic Applications for Inhibitors and Activators. Nature Reviews. 2008;7:168. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 2.Potter CPS, Harris AL. Diagnostic, Prognostic and Therapeutic Implications of Carbonic Anhydrases in Cancer. Br J Cancer. 2003;89:2. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivanov S, Liao S-Y, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of Hypoxia-inducible Cell-surface Transmembrane Carbonic Anhydrases in Human Cancer. Am J Pathol. 2001;158:905. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavsky R, Zat’ovicová M, Liao S, Portetelle D, Stanbridge EJ, Závada J, Burny A, Kettmann R. Cloning and Characterization of MN, a Human Tumor-associated Protein with a Domain Homologous to Carbonic Anhydrase and a Putative Helix-Loop-Helix DNA Binding Segment. Oncogene. 1994;9:2877. [PubMed] [Google Scholar]
- 5.Wingo T, Tu C, Laipis PJ, Silverman DN. The Catalytic Properties of Human Carbonic Anhydrase IX. Biochem Biophys Res Comm. 2001;288:666. doi: 10.1006/bbrc.2001.5824. [DOI] [PubMed] [Google Scholar]
- 6.Bui MHT, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao S-Y, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS. Carbonic Anhydrase IX is an Independent Predictor of Survival in Advanced Renal Clear Cell Carcinoma: Implication fro Prognosis and Therapy. Clin Cancer Res. 2003;9:802. [PubMed] [Google Scholar]
- 7.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic Significance of a Novel Hypoxia-Regulated Marker, Carbonic Anhydrase IX, in Invasive Breast Cancer. J Clin Oncol. 2005;19:3660. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SA, Palmer DH, Ganesan R, Hiller L, Gregory J, Murray PG, Pastorek J, Young L, James ND. Carbonic Anhydrase IX, a Marker of Hypoxia: Correlation with Clinical Outcome in Transitional Cell Carcinoma of the Bladder. Oncol Rep. 2004;11:1005. [PubMed] [Google Scholar]
- 9.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wykoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CML. Carbonic Anhydrase (CAIX) Expression, a Potential New Intrinsic Marker of Hypoxia: Correlations with Tumor Oxygen Measurements and Prognosis in Locally Advanced Carcinoma of the Cervix. Cancer Res. 2001;61:6394. [PubMed] [Google Scholar]
- 10.Swinson DEB, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O’Bryne KJ. Carbonic Anhydrase IX Expression, a Novel Surrogate Marker of Tumor Hypoxia, Is Associated with a Poor Prognosis in Non-Small-Cell Lung Cancer. J Clin Oncol. 2003;21:473. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 11.Pastorekova S, Parkkila S, Parkkila A, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic Anhydrase IX, MN/CAIX: Analysis of Stomach Complementary DNA Sequence and Expression in Human and Rat Alimentary Tracts. Gastroenterology. 1997;112:398. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 12.Saarnio J, Parkkila S, Parkkila A-K, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of Carbonic Anhydrase Isozyme IX (MN/CA IX) in Human Gut Reveals Polarized Expression in Epithelial Cells with the Highest Proliferative Capacity. J Histochem Cytochem. 1998;46:497. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated Carbonic Anhydrase IX Expression Is Associated with Poor Survival in Patients with Invasive Breast Cancer. Br J Cancer. 2007;96:104. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang H, Oosterwijk E, Tu C, Shiverick KT, Silverman DN, Frost SC. Expression and Activity of Carbonic Anhydrase IX Is Associated with Metabolic Dysfunction in MDA-MB-231 Breast Cancer Cells. Cancer Investigation. 2009;27:613. doi: 10.1080/07357900802653464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois L, Lieuwes NG, Maresca A, Thiry A, Supuran CT, Scozzafava A, Wouters BG, Lambin P. Imaging of CA IX with Fluorescent Labelled Sulfonamides Distinguishes Hypoxic and (re)-Oxygenated Cells in a Xenograft Tumour Model. Radiother Oncol. 2009;92:423. doi: 10.1016/j.radonc.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2009;69:358. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 17.Svastova E, Hulikova A, Rafajova M, Zatovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastorekova S. Hypoxia Activates the Capacity of Tumor-associated Carbonic Anhydrase IX to Acidify Extracellular pH. FEBS Lett. 2004;577:439. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb RA, Giesing HA, Zhu JY, Engler RL, Babior BM. Cell Acidification in Apoptosis: Granulocyte Cology-stimulating Factor Delays Programed Cell Death in Neutrophils by Up-regulating the Vaculolar H+-ATPase. Proc Natl Acad Sci USA. 1995;92:5965. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and Consequences of Tumour Acidity and Implications for Treatment. Molec Med Today. 2000;6:15. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 20.Gatenby RA, Gillies RJ. Why Do Cancers Have High Aerobic Glycolysis? Nat Rev Cancer. 2004;4:891. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 21.Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ. Cellular Adaptations to Hypoxia and Acidosis During Somatic Evolution of Breast Cancer. Br J Cancer. 2007;97:646. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorlie T, Perou CM, Tibshirani R, Aas R, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borrensen-Dale A. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc Natl Acad Sci USA. 2001;98:10869. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe J, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo W, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A Collection of Breast Cancer Cell Lines for the Study of Functionally Distinct Cancer Subtypes. Cancer Cell. 2006;10:515. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP. The Role of Carbonic Anhydrase IX Overexpression in Kidney Cancer. Eur J Can. 2005;41:2935. doi: 10.1016/j.ejca.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in Human Breast Cancer Cells with Distinct Metastatic Potential: Distribution and Functional Activity. Am J Physiol. 2004;286:C1443–C1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 26.Markwell MAK, Haas SM, Lieber LL, Tolbert NE. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal Biochem. 1978;87:206. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 27.Silverman DN. Carbonic Anhydrase: Oxygen-18 Exchange Catalyzed by an Enzyme with Rate-contributing Proton-transfer Steps. Meth Enzymol. 1982;87:732. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Xiao Y-P, Laipis PJ, Fletcher BS, Frost SC. Glucose Deprivation Enhances Targeting of GLUT1 to Lipid Rafts in 3T3-L1 Adipocytes. Am J Physiol. 2004;286:E568–E576. doi: 10.1152/ajpendo.00372.2003. [DOI] [PubMed] [Google Scholar]
- 29.Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J. A Novel Quasi-viral Agent, MaTu, is a Two-Component System. Virology. 1992;187:620. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman HH, Jr, McMahon RJ, Williams MG, Frost SC. Effect of Glucose Deprivation on GLUT1 Expression in 3T3-L1 Adipocytes. J Biol Chem. 1993;268:1320. [PubMed] [Google Scholar]
- 31.Opavsky R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 Gene, A Novel Member of the Carbonic Anhydrase Family: Structure and Exon to Protein Domain Relationships. Genomics. 1996;33:480. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell PH, Wiesener MS, Chang G, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The Tumour Suppressor Protein VHL Targets Hypoxia-inducible Factors for Oxygen-dependent Proteolysis. Nature. 1999;399:271. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 33.Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible Expression of Tumor-associated Carbonic Anhydrase. Cancer Res. 2000;60:7075. [PubMed] [Google Scholar]
- 34.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JAM, van Diest PJ, van der Wall E. Up-regulation of Gene Expression by Hypoxia is Mediated Predominantly by Hypoxia-inducible Factor 1 (HIF1) J Pathol. 2005;206:291. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 35.Ratcliffe PJ, Pugh CW, Maxwell PH. Targeting Tumors through the HIF System. Nat Med. 2000;6:1315. doi: 10.1038/82113. [DOI] [PubMed] [Google Scholar]
- 36.Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL. Expression of Hypoxia-Inducible and Tumor-Associated Carbonic Anhydrases in Ductal Carcinoma in Situ of the Breast. Am J Pathol. 2001;158:1011. doi: 10.1016/S0002-9440(10)64048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Span PM, Bussink J, Manders P, Beex LVAM, Sweep CGJ. Carbonic Anhydrase-9 Expression Levels and Prognosis in Human Breast Cancer: Association with Treatment Outcome. Br J Cancer. 2003;89:271. doi: 10.1038/sj.bjc.6601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Generali D, Fox SB, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield SM, Bruzzi P, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Bottini A, Harris AL. Role of Carbonic Anhydrase IX Expression in Prediction of the Efficacy and Outcome of Primary Epirubicin/Tamoxifen Therapy for Breast Cancer. Endocrine-Related Cancer. 2006;13:921. doi: 10.1677/erc.1.01216. [DOI] [PubMed] [Google Scholar]
- 39.Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouyssegur J, Berra E. HIF-1α and CA IX Staining in Invasive Breast Carcinomas: Prognosis and Treatment Outcome. Int J Cancer. 2007;120:1451. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 40.Cairns R, Papandreou I, Denko N. Overcoming Physiologic Barriers to Cancer Treatment by Molecularly Targeting the Tumor Microenvironment. Mol Cancer Res. 2006;4:61. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 41.Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, Candiloro I, Pezzella F, Gatter KC, Millar EKA, O’Toole SA, McNeil CM, Crea P, Segara D, Sutherland RL, Harris AL, Fox SB. The Key Hypoxia Regulated Gene CAIX is Upregulated in Basal-like Breast Tumours and is Associated with Resistance to Chemotherapy. Br J Cancer. 2009;100:405. doi: 10.1038/sj.bjc.6604844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh M-J, Chen K-S, Chiou H-L, Hsieh Y-S. Carbonic Anhydrase XII Promotes Invasion and Migration Ability of MDA-MB-231 Breast Cancer Cells through the p38 MAPK Signaling Pathway. Eur J Cell Biol. 2010;89:598. doi: 10.1016/j.ejcb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of Cancer. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 44.Masramon L, Vendrell E, Tarafa G, Capella G, Miro R, Ribas M, Peinado MA. Genetic Instability and Divergence of Clonal Populations in Colon Cancer Cells in Vitro. J Cell Sci. 2006;119:1477. doi: 10.1242/jcs.02871. [DOI] [PubMed] [Google Scholar]
- 45.Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A, Scozzafava A, Monti SM, Di Flore A, De Simone G, Lindfors M, Janis J, Valjakka J, Pastorekova S, Pastorek J, Kulomaa MS, Mordlund HR, Supuran CT, Parkkila S. Biochemical Characterization of CA IX, One of the Most Active Carbonic Anhydrase Isozymes. J Biol Chem. 2008;283:27799. doi: 10.1074/jbc.M800938200. [DOI] [PubMed] [Google Scholar]
- 46.Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone C, Scozzafava A, Monti SM, De Simone G. Crystal Structure of the Catalytic Domain of the Tumor-associated Human Carbonic Anhydrase IX. Proc Natl Acad Sci USA. 2009;106:16233. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Simone G, Supuran CT. Carbonic Anhycrase IX: Biochemical and Crystallographic Characterization of a Novel Antitumor Target. Biochim Biophys Acta. 2010;1804:494. doi: 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 48.Wong NK, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the Oligosaccharides Associated with the Human Ovarian Tumor Marker CA125. J Biol Chem. 2003;278:28619. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 49.Goetz JA, Mechref Y, Kang P, Jeng M, Hovotny MV. Glycomics Profiling of Invasive and Non-invasive Breast Cancer Cells. Glycoconj J. 2009;26:117. doi: 10.1007/s10719-008-9170-4. [DOI] [PubMed] [Google Scholar]
- 50.McMahon RJ, Frost SC. Nutrient Control of GLUT1 Processing and Turnover in 3T3-L1 Adipocytes. J Biol Chem. 1995;270:12094. doi: 10.1074/jbc.270.20.12094. [DOI] [PubMed] [Google Scholar]
- 51.Smart EJ, Ying Y, Mineo C, Anderson RGW. A Detergent-free Method for Purifying Caveolae Membrane from Tissue Culture Cells. Proc Natl Acad Sci USA. 1995;92:10104. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, Nanjundan M, Pike LJ, Wiedmer T, Sims PJ. Plasma Membrane Phospholiid Scramblase 1 is Enriched in Lipid Rafts and Interacts with the Epidermal Growth Factor Receptor. Biochem. 2002;41:6338. doi: 10.1021/bi025610l. [DOI] [PubMed] [Google Scholar]
- 53.Nanjundan M, Sun J, Zhao J, Zhou Q, Sims PJ, Weidmer T. Plasma Membrane Phospholipid Scramblase I Promotes EGF-dependent Activation of c-Src through the Epidermal Growth Factor Receptor. J Biol Chem. 2008;278:37413. doi: 10.1074/jbc.M306182200. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Resh MD. Cholesterol Depletion from the Plasma Membrane Triggers Ligand-independent Activation of the Epidermal Growth Factor Receptor. J Biol Chem. 2002;277:49631. doi: 10.1074/jbc.M208327200. [DOI] [PubMed] [Google Scholar]
- 55.Ringerike T, Blystad FD, Lvey FO, Madshus IH, Stang E. Cholesterol is Important in control of EGF Receptor Kinase Activity but EGF Receptors are Not Concentrated in Caveolae. J Cell Sci. 2002;115:1331. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 56.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, Ellis L, El-Naggar A, Frazier M, Jasser S, Langford LA, Lee J, Mills GB, Pershouse MA, Polllack RE, Tornos C, Troncoso P, Yung WKA, Fujii G, Berson A, Bookstein R, Bolen JB, Tavtigian SV, Steck PA. MMACI/PTEN Mutations in Primary Tumor Specimens and Tumor Cell Lines. Cancer Res. 1997;57:5221. [PubMed] [Google Scholar]
- 57.Lu Y, Lin Y, LaPushin R, Cuevas B, Fang X, Yu XS, Davies MA, Kahn H, Furui T, Mao M, Zinner R, Hung M, Steck P, Siminovitch K, Mills GB. The Pten/MMAC/TEP Tumor Suppressor Gene Decreases Cell Growth and Induces Apoptosis and Anoikis in Breast Cancer Cells. Oncogene. 1999;18:7034. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L. Hypoxia Induces the Activation of the Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway in PC12 cells: Protective Role in Apoptosis. J Biol Chem. 2001;276:22368. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 59.Beitner-Johnson D, Rust RT, Hsieh TC, Millhorn DE. Hypoxia Activates Akt and Induces Phosphorylation of GSK-3 in PC12 Cells. Cell Signal. 2001;13:23. doi: 10.1016/s0898-6568(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 60.Mardilovich K, Shaw LM. Hypoxia Regulates Insulin Receptor Substrate-2 Expression toPromote Breast Carcinoma Cell Survival and Invasion. Cancer Res. 2009;69:8894. doi: 10.1158/0008-5472.CAN-09-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-Directed Glucose Metabolism Can Prevent Bax Conformation Change and Promote Growth Factor-independent Survival. Molec Cell Biol. 2003;23:7315. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The Biology of Cancer:Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metabolism. 2008;7:11. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Rubin D, Ismail-Beigi F. Distribution of Glut1 in Detergent Resistant (DRMs) and Non-DRM Domains: Effect of Treatment with Azide. Am J Physiol. 2003;285:C377–C383. doi: 10.1152/ajpcell.00060.2003. [DOI] [PubMed] [Google Scholar]