Abstract
Increases in vesicular glutamate transporter (VGLUT) levels are observed after a variety of insults including hypoxic injury, stress, methamphetamine treatment, and in genetic seizure models. Such overexpression can cause an increase in the amount of glutamate released from each vesicle, but it is unknown whether this is sufficient to induce excitotoxic neurodegeneration. Here we show that overexpression of the Drosophila vesicular glutamate transporter (DVGLUT) leads to excess glutamate release, with some vesicles releasing several times the normal amount of glutamate. Increased DVGLUT expression also leads to an age-dependent loss of motor function and shortened lifespan, accompanied by a progressive neurodegeneration in the postsynaptic targets of the DVGLUT-overexpressing neurons. The early onset lethality, behavioral deficits, and neuronal pathology require overexpression of a functional DVGLUT transgene. Thus overexpression of DVGLUT is sufficient to generate excitotoxic neuropathological phenotypes and therefore reducing VGLUT levels after nervous system injury or stress may mitigate further damage.
Keywords: VGLUT, glutamate, excitotoxicity, Drosophila, neurodegeneration, disease model
Introduction
The vesicular glutamate transporter (VGLUT) is responsible for packaging glutamate into synaptic vesicles. Vertebrates contain three VGLUT family members, with either VGLUT1 or VGLUT2 expressed in most glutamatergic neurons at maturity. In a number of disease models associated with excitotoxicity there is increased expression of vesicular glutamate transporters (VGLUTs). In a rat absence seizure model, VGLUT2 is significantly increased in the cortex (Touret et al., 2007). Likewise, hypoxic injury causes an increase in VGLUT1 protein in rat hippocampus (Kim et al., 2005). Stressed animals also show more VGLUT1 protein on vesicles (Raudensky and Yamamoto, 2007).
We and others have previously shown that increasing the levels of VGLUT protein results in increased glutamate release (Daniels et al., 2004, Moechars et al., 2006, Wilson et al., 2005, Wojcik et al., 2004). In Drosophila, overexpression of the single fly VGLUT ortholog DVGLUT leads to larger vesicles that each release more glutamate after fusion (Daniels et al., 2004). Since elevated extracellular glutamate is a critical factor that contributes to excitotoxic neurodegeneration after stroke or other brain injury, we wondered whether overexpression of VGLUT is sufficient to cause neurodegeneration. Most of the negative effects of high extracellular glutamate are mediated through glutamate receptors. In neurons expressing glutamate receptors, receptor activation can increase intracellular calcium levels and activate apoptosis.
Here we show that overexpression of Drosophila VGLUT (DVGLUT) in glutamatergic neurons results in huge spontaneous glutamate release events. The prevalence of these huge events correlates with DVGLUT expression level and with the onset of lethality. Weaker overexpression results in adult flies that exhibit progressive behavioral deficits including loss of coordinated motor behavior and a progressive neuropathy of target neurons. DVGLUT transport function is required for these effects since overexpressing mutant transgenes does not cause these phenotypes. These results show that DVGLUT overexpression is sufficient to generate behavioral defects, premature lethality, and excitotoxic neurodegeneration in Drosophila and suggest possible therapeutic targets for excitotoxicity in humans.
Materials and Methods
Fly stocks
Flies were maintained on food made from cornmeal, molasses, and agar in a temperature- and humidity-controlled incubator at 25 °C. Genes were expressed in glutamatergic neurons using the driver dvglutCNSIII-Gal4 (Daniels et al., 2008). UAS-GFP:DVGLUT flies have been described previously (Daniels et al., 2008). UAS-mCD8::GFP was used as a control (Bloomington stock number 5139). The UAS-GFP:DVGLUT A470V point mutant is from Grygoruk et al., 2010. For the electrophysiology experiments in figure 1, w1118, BG380-Gal4, and UAS-DVGLUT2 flies were used (Daniels et al., 2004) and w1118, UAS-GFP:DVGLUT, and UAS-GFP:DVGLUT A470V flies were used in figure 4.
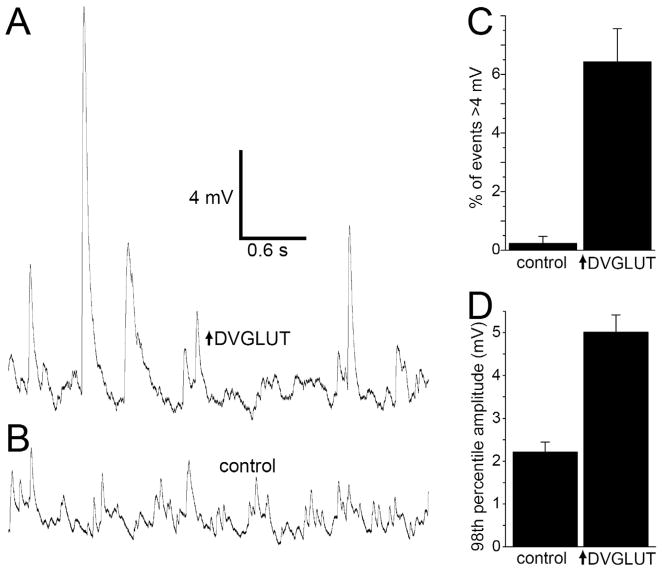
Figure 1.
Large spontaneous events occur in muscles of animals overexpressing DVGLUT in motoneurons. A. Intracellular voltage recording in an animal overexpressing DVGLUT showing numerous large events (>4 mV), in the absence of extracellular Ca2+ and in the presence of 1 μM TTX and after cutting the motoneuron axon. B. Sample trace from a control muscle. C. Large events are much more frequent in larvae overexpressing DVGLUT (p<0.0001, Student’s t-test, n=12 cells with 70 events for each genotype). D. The largest 2% of events is larger in larvae overexpressing DVGLUT (p<0.0001, Student’s t-test, n=12 cells with 70 events for each genotype).
Figure 4.
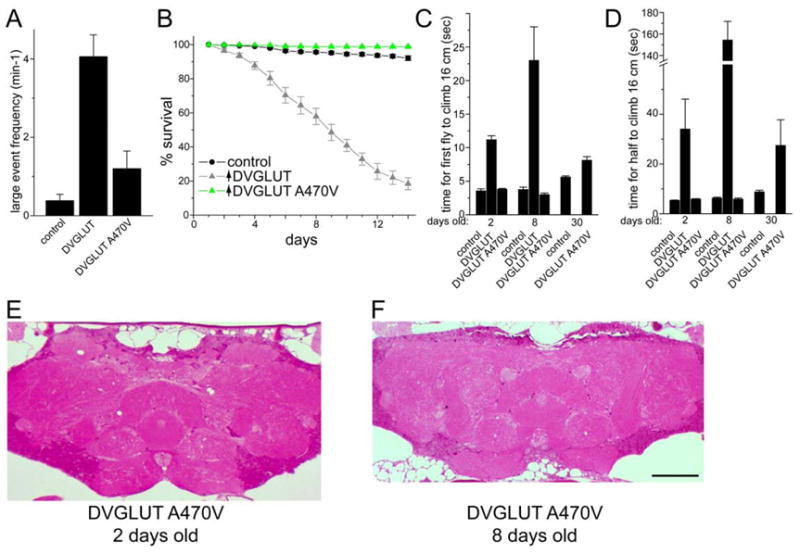
Phenotypes of DVGLUT overexpression in glutamatergic neurons requires DVGLUT function. Overexpression of the mutant DVGLUT protein containing a single amino acid change at position 470 from alanine to valine (A470V) is significantly less deleterious than overexpression of wild type DVGLUT protein. A. Large spontaneous event frequency is not significantly increased in flies overexpressing DVGLUT A470V (p>0.06 between control and A470V; n=20 cells in WT, n=37 cells for DVGLUT overexpression, and n=15 cells for A470V overexpression). B. Expression of DVGLUT A470V does not reduce survival rate. C and D. Expression of DVGLUT A470V does not significantly decrease climbing ability at 2 or 8 days after eclosion, although a slight difference can be seen after 30 days. The time for the first fly to climb 16 cm is shown in C, and the time for 5 of 10 flies to climb 16 cm is presented in D, used as a indication of the best and average times, respectively. E and F. Plastic sections of adult brains from 2 days old (E) and 8 day old (F) flies overexpressing DVGLUT A470V stained with basic fucshin and toluidine blue. Notice the low occurrence of vacuolar degeneration compared with figure 3B and 3D. Scale bar represents 50 μm.
Lifespan analysis
Male flies were collected every 24 hours and transferred to a new food vial. Up to 25 flies were housed together in a vial. Flies were transferred to new food vials at least 5 times a week and more often if the food started to get moist. The number of flies that died was recorded each day, subtracting out any flies accidentally lost. The survival plot in figure 2 represents the average of three experiments using a total of 926 control flies (FRTG13, UAS-mCD8::GFP/+; dvglutCNSIII-Gal4/+) and 1001 DVGLUT overexpressing flies (UAS-GFP:DVGLUT/+; dvglutCNSIII-Gal4/+).
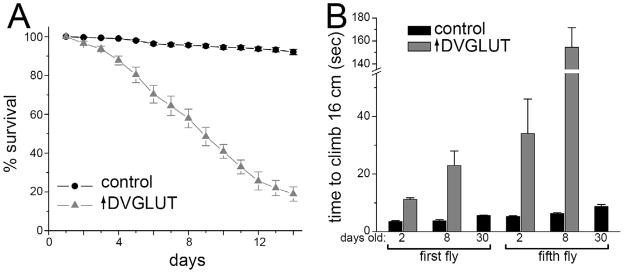
Figure 2.
Overexpression of DVGLUT leads to decreased lifespan and progressive motor deficits. A. Survival over a two-week period was significantly decreased in male flies overexpressing DVGLUT compared with flies overexpressing GFP (control). B. Flies overexpressing DVGLUT in glutamatergic neurons exhibit progressive motor impairment. The time it took the fastest fly to climb 16 cm (labeled first fly) and the time it took half of the flies (labeled fifth fly) to climb 16 cm were measured to obtain both the group best and a measure of the population average, respectively. Flies were measured on 2, 8, and 30 days after eclosing.
Climbing assay
Motor performance was assayed by the same method described by Martinez et al. (2007). Briefly, 10 male flies of the indicated age were collected under brief CO2 anesthesia and allowed to recover for several hours at room temperature. All tests were performed between 2:30 and 3:30 PM to minimize circadian variability. The flies were placed into a graduated cylinder, tapped to the bottom, and the time for the flies to climb past a line 16 cm above the bottom. A white light source was positioned above the top of the cylinder. Each group of flies was subjected to two sets of four consecutive trials and the average for each set was recorded. Each genotype was tested on at least 2 different days.
Immunohistochemistry
Immunostaining was performed as previously described (Daniels et al., 2008). In brief, flies were anesthetized on ice and then heads and probosci cut off with a scalpel. Heads were then placed in cold, fresh 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2 containing 0.2% Triton-X 100 (TX) for 3 hours on ice. Afterwards, the brains were dissected away from the head cuticle and transferred to 0.1 M phosphate buffered saline (PBS) overnight at 4 °C. The next day, primary anti-DLG antibody (University of Iowa, DSHB) diluted 1:100 in PBS + 0.75% TX was added for 10 hours at room temperature. Secondary goat anti-mouse-Cy3 antibody (Jackson ImmunoResearch, West Grove, PA) was used at a concentration of 1:500 diluted in PBS + 0.25% TX and incubated overnight at 4 °C. Brains were then washed several times with PBS + 0.1% TX and then equilibrated with 70% glycerol in water before mounting in Vectashield medium (Vector Laboratories, Burlingame, CA). For measuring DVGLUT levels at the larval neuromuscular junction, larvae were dissected in HL-3 saline and fixed for 5 minutes in Bouin’s fixative. After eviscerating, the body walls were blocked in PBS + 0.1% TX for 1 hour and then incubated with a 1:10,000 dilution of rabbit anti-DVGLUT and a 1:1000 dilution of goat anti-HRP-Cy3 (Jackson Immunoresearch) overnight. After two rinses with PBS + 0.1% TX, goat anti-rabbit Alexa488 (Jackson Immunoresearch) was added at 1:1000 for 2 hours. After two more rinses with PBS +0.1% TX, the preparations were equilibrated in 70% glycerol and mounted in Vectashield (Vector Laboratories, Burlingame, CA). The neuromuscular junction on muscle 4 was imaged using epifluorescence with a mounted camera and the images were quantified using the Measure RGB function in Image J (NIH), subtracting background from a nearby muscle region.
Plastic sections
Heads were dissected as described above and fixed with fresh 4% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2 for 3 hours on ice. Heads were then washed and stored in PBS at 4 °C for up to a week before embedding in plastic. Heads were dehydrated through an ethanol series, then in propylene oxide and then overnight in a 1:1 mix of propylene oxide in Epon 812 (hard formulation; Ted Pella Inc., Redding, CA) under -5 inches Hg vacuum. The next day, the samples were changed into fresh Epon resin, left on a rotator for several hours, and then placed in fresh Epon in coffin molds. The resin was cured at 60 °C for 48 hours and sectioned with a diamond knife (Micro Star Technologies, Huntsville, TX) on a Leica EM UC6 ultramicrotome (Leica Microsystems, Wetzlar, Germany) at 1 μm thickness. Sections were melted onto slides and stained with a combination of toluidine blue O and basic fuchsin (epoxy tissue stain; Electron Microscopy Sciences, Hatfield, PA). Slides were imaged on a Zeiss Axioplan2 microscope (Carl Zeiss, Thornwood, NY), using a 20x Neofluar objective and a color AxioCam camera.
Electrophysiology
Recordings were done essentially as previously described (Daniels et al., 2004). Briefly, wandering third instar larvae were dissected in HL-3 containing (in mM), NaCl, 70; KCl, 5; MgCl2, 20; NaHCO3, 10; trehalose, 5; sucrose, 115; and HEPES, 5 with pH adjusted to 7.20. Tetrodotoxin (TTX) was added to a final concentration of 1 μM. The larvae were eviscerated and the segmental nerves were cut and brain removed before washing with saline and recording. Sharp glass electrodes filled with 3 M KCl were used to impale muscle 6 in segments A3 and A4. For each cell, the first 70 miniature events were measured. To obtain the frequency of minis larger than 4 mV (shown in figure 4), we counted only the number of large events in a 60 second record for each cell.
Statistics and graphs
Student’s t-test was performed when comparing two genotypes and one-way ANOVA was performed when comparing multiple genotypes. Statistical tests were performed and graphs were created in Origin 7.0 (OriginLab Corp., Northampton, MA) or Excel (Microsoft, Redmond, WA).
Results
DVGLUT overexpression causes “huge minis”
Previously, we demonstrated that increasing the levels of the Drosophila vesicular glutamate transporter (DVGLUT) increases presynaptic glutamate release. When DVGLUT is overexpressed at the larval neuromuscular junction, there is a shift in the population of single vesicle response (mini) amplitudes to larger values, indicating increased vesicular glutamate content (Daniels et al., 2004). Similar results have been reported for vertebrate VGLUT overexpression (Moechars et al., 2006; Wilson et al., 2005; Wojcik et al., 2004). We also observed some strikingly large events that could exceed 10 mV in amplitude, an order of magnitude larger than in wild type (figure 1A). These huge events persist after severing the axon, blocking action potentials with TTX, and removing Ca2+ from the extracellular solution, demonstrating that they are not due to calcium-dependent evoked release. To quantify these events, we counted the frequency of spontaneous events with amplitudes of 4 mV or greater under these conditions. This category includes only approximately 0.02% of minis in control cells, but >6% of events in animals overexpressing DVGLUT (figure 1C, p<0.0001, n=12 cells with 70 events each). Not only are these large events much more frequent, they are also much larger in mutants, with an average 98th percentile amplitude of 5.0 +/− 0.4 mV in mutants versus 2.2 +/− 0.2 mV in controls (p<0.00001, n=12 cells for each, figure 1D).
The frequency of large release events correlates with DVGLUT expression level. We varied the DVGLUT expression level by driving a UAS-DVGLUT transgene in motoneurons with either the weaker Gal4 driver dvglutCNSIII-Gal4 or the stronger driver BG380-Gal4. The weaker driver lead to a 35 +/− 6% increase in synaptic DVGLUT levels compared to wild type while the stronger driver lead to a 68 +/− 12% increase in synaptic DVGLUT (p<0.001 vs. control for both drivers, and p<0.03 between the two drivers, n=8 for each genotype). The frequency of minis larger than 4 mV correlated with the expression level of DVLUT, with a frequency of 1.7 +/− 0.4 per minute in controls, 3.9 +/− 0.9 per minute with dvglutCNSIII-Gal4 (p<0.05), and 16 +/− 3 per minute with BG380-Gal4 (p<0.001 vs. control, p<0.01 vs. dvglutCNSIII-Gal4). Hence, the frequency of these large spontaneous events increases with increasing DVGLUT dosage.
Age-dependent decline in motor function and premature lethality with DVGLUT overexpression
Overexpression of DVGLUT in neurons results in lethality that also correlates with DVGLUT expression level. Driving UAS-DVLGUT with the strong BG380-Gal4 line described above results in death during larval stages, while driving with the weaker Gal4 line dvglutCNSIII-Gal4 resulting in lethality within the first few weeks of adulthood (see below). Why would overexpression of DVGLUT lead to lethality? Although DVGLUT overexpression causes production of large spontaneous release events (figure 1), evoked release remains constant, presumably due to a homeostatic response (Daniels et al., 2004). Since other mutants increase the mean mini amplitude but have not been reported to show early lethality (Petersen et al., 1997; Zhang et al., 1998), we propose that the giant spontaneous events are responsible for the lethality in mutants where DVGLUT is overexpressed. Such giant spontaneous events could be more damaging to central synapses, which usually receive weak inputs, than to muscles, which are bombarded with glutamate from thousands of vesicles during normal locomotion.
To more closely replicate the VGLUT upregulation seen in the vertebrate models as well as to minimize the effects of DVGLUT expression in other neuron types, we limited DVGLUT overexpression to glutamatergic neurons by using the dvglutCNSIII-Gal4 line (Daniels et al., 2008). This driver line results in weaker expression and leads to lethality during the first few weeks of adult life (figure 2A). By 9 days, approximately 50% of the DVGLUT-overexpressing flies have died, compared with 5% percent of controls (flies overexpressing GFP alone). Flies overexpressing DVGLUT are uncoordinated and cannot fly, which is consistent with Drosophila models of neurodegeneration that show age-dependent declines in motor behavior. To assess motor behavior, we used a simple climbing test (Martinez et al., 2007) performed with flies at different ages. Flies normally show robust negative geotaxis and are also attracted to light. For these experiments, flies were tapped down to the bottom of a graduated cylinder and then timed as they climbed up the sides towards a light source. We recorded both the time for the first fly and for five of the ten flies to cross a line 16 cm from the bottom of the tube. We use the first fly and the fifth fly as an indication of the best and average performance of a genotype, respectively. Two-day-old control flies took about 4 seconds to climb 16 cm, while flies overexpressing DVGLUT were significantly slower, with the fastest fly taking approximately 11 seconds (figure 2B). The difference between genotypes was magnified at 8 days with mutants taking about 23 seconds and controls still taking only about 4 seconds. At 30 days, controls climb slightly slower, taking about 6 seconds to reach 16 cm, although this is still faster than overexpressing flies took at 2 days. DVGLUT-overexpressing flies died before they could be tested at 30 days. Hence, increased DVGLUT expression in glutamatergic neurons leads to a progressive motor defect.
Widespread degeneration in adult brains
To determine whether this age-dependent decline in viability and motor performance had any concomitant neuropathology, we examined brain sections from adult flies at various ages for evidence of neurodegeneration. Control brain sections show compact and evenly stained neuropil (synapse and dendrite-rich regions) at all ages, with a few holes appearing by 30 days (figure 3A and C, data not shown). In contrast, flies overexpressing DVGLUT have extensive vacuolization in neuropil regions beginning at 2 days and becoming more severe at 8 days (figure 3B and D). This progressive degeneration occurs throughout most regions of the neuropil.
Figure 3.
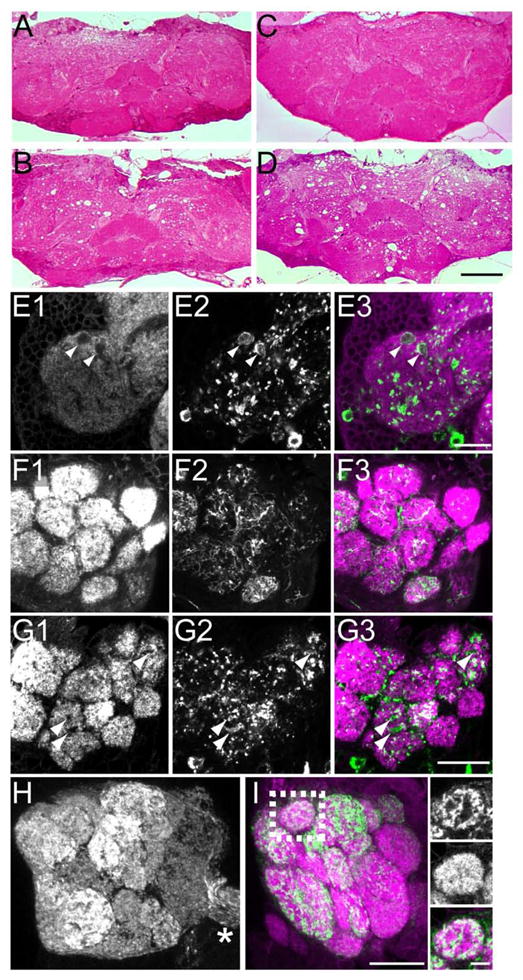
Pronounced neurodegeneration occurs in flies overexpressing DVGLUT in glutamatergic neurons. A–D. 1 μm plastic sections stained with toluidine blue and basic fuchsin. Horizontal sections of controls (A and C) and mutants (B and D) at 2 days (A and B) and 8 days (C and D). Holes in neuropil regions are apparent in 2 day-old mutants (B) and become more severe at 8 days (D). Scale bar represents 50 μm. E1-3. Thin confocal stack from a whole-mount adult brain demonstrating holes in the medial inferior lateral protocerebrum (milpr) by immunostaining for postsynaptic marker Dlg (E1). GFP fluorescence from GFP:DVGLUT (E2) shows that holes in Dlg staining are filled by presynaptic GFP. E3 is the merged image. Scale bar represents 10 μm. F1-3. Single confocal section of the antennal lobe in a control stained with anti-Dlg (F1). GFP fluorescence from mCD8::GFP (F2) shows the expression pattern of dvglutCNSIII-Gal4 in the antennal lobe. Large holes in the antennal lobe glomeruli are not seen opposite GFP-positive terminals. F3 shows the merged image. G1-3. Single confocal section from the antennal lobe of a fly overexpressing GFP:DVGLUT with dvglutCNSIII-Gal4 stained with anti-Dlg (G1). Holes in Dlg staining within glomeruli (arrowhead) colocalize with enlarged GFP-positive presynaptic terminals (G2, green in merged image), indicating loss of postsynaptic targets of DVGLUT-expressing terminals. G3 shows the merged image. Scale bar represents 20 μm (for F and G). H and I. Or83b-Gal4 expression of GFP:DVGLUT in olfactory receptor neurons (ORNs) does not kill ORNs or cause degeneration in the antennal lobe. H. Robust GFP signal from GFP:DVGLUT is seen in the antennal lobe glomeruli and in the axons of the antennal nerve (asterisk), indicating that ORN axons and terminals are intact when expressing GFP:DVGLUT (2D maximum projection of confocal stack). I. Projection of a thin confocal stack stained with anti-Dlg (magenta) and with GFP fluorescence in green. Insets show a single confocal section of the boxed area with uniform Dlg staining, indicating lack of degeneration of postsynaptic ORN targets. Scale bar in I represents 20 μm and in the inset represents 5 μm.
In studies of vertebrate excitotoxic neurodegeneration, it is the postsynaptic target neurons (which express glutamate receptors) that exhibit pathology. If the degeneration we observe is excitotoxic, we would expect 1) that the degeneration requires overexpression of an enzymatically-active DVGLUT, 2) that the degeneration is due to expression of DVGLUT in glutamatergic neurons, and 3) that the target neurons are injured, and not the neurons overexpressing DVGLUT. We test these three predictions below.
Overexpression of DVGLUT could lead to degeneration due to increased DVGLUT function and excess glutamate release, or it could induce a less specific toxicity due to the increased protein expression. If DVGLUT function is required for lethality, then overexpression of non-functional DVGLUT transgenes should suppress this lethality. Since there is little known about residues important for VGLUT function, we performed an in vivo chemical mutagenesis screen to find DVGLUT mutants that are viable following expression from the strong BG380-Gal4 driver (Grygoruk et al., 2010). From this screen we isolated approximately 50 independent mutants that change amino acids in a GFP-tagged DVGLUT transgene. This collection includes transgenic flies in which the mutant DVGLUT protein is still highly expressed and correctly localizes to the synapse. We have compared the consequences of overexpressing such a mutant, GFP:DVGLUT(A470V), in which the alanine at position 470 is mutated to a valine, to a wild type DVGLUT transgene. In contrast to wild type DVGLUT, overexpression of the mutant GFP:DVGLUT(A470V) does not lead to the release of the huge spontaneous synaptic events (figure 4A). In addition, high-level expression of mutant DVGLUT does not cause motor deficits, early lethality, or brain pathology (figure 4), so excess mutant DVGLUT protein does not lead to a non-specific toxicity. This mutant protein is expressed at a similar level as the wild type protein as assessed by quantifying anti-GFP immunofluorescence at the neuromuscular junction. Using the BG380-Gal4 driver, GFP levels were 69 +/− 8 for the wild-type GFP:DVGLUT compared with 47 +/− 8 for GFP:DVGLUT(A470V) (not significant, p>0.06, n=8 for each genotype). Using the dvglutCNSIII-Gal4 line, levels were also not significantly different (37 +/− 5 versus 24 +/− 5; p>0.1, n=8 for each genotype). Although the mutant transgene tends to express somewhat less protein than the wild type transgene for each driver, the levels of GFP:DVGLUT(A470V) driven by the stronger BG380-Gal4 are higher than the levels of GFP:DVGLUT protein driven by the weaker dvglutCNSIII-Gal4 line, and yet most GFP:DVGLUT(A470V)/BG380-Gal4 flies live for more than a month while the majority of the GFP:DVGLUT/dvglutCNSIII-Gal4 die before two weeks of age. Hence, much of the difference between these two transgenes is likely due to functional differences in the proteins they encode, although the difference in expression levels may also contribute to phenotypic differences. These findings suggest that the transporter activity of DVGLUT is required for these phenotypes.
To investigate whether degeneration requires DVGLUT expression in glutamatergic neurons, we focused on the antennal lobes. In these structures cholinergic olfactory receptor neurons (ORNs), GABAergic interneurons, and glutamatergic neuron terminals that likely originate from interneurons innervate the dendrites of cholinergic projection neurons (PNs) (Daniels et al., 2008, Masse et al., 2009). When the GFP-tagged DVGLUT is driven in the glutamatergic neurons by dvglutCNSIII-Gal4, there is degeneration in the antennal lobe as shown by loss of the postsynaptic marker DLG and by the appearance of holes in tissue sections (figure 3G). Does this degeneration require expression of DVGLUT in glutamatergic neurons or could we achieve the same phenotype by expressing DVGLUT in the neighboring cholinergic olfactory receptor neurons (ORNs)? When we overexpress the GFP-tagged DVGLUT in ORNs with Or83b-Gal4 (Kreher et al., 2005), GFP:DVGLUT is well expressed and is targeted to the synapses in the antennal lobe (figure 3H), but no degeneration is apparent either by immunostaining with DLG or by histological analysis (figure 3I and data not shown). Hence, DVGLUT must be expressed in glutamatergic neurons to induce degeneration.
Furthermore, when GFP:DVGLUT is overexpressed by dvglutCNSIII-Gal4, we observe swellings of GFP-positive terminals apposed to holes where staining for the postsynaptic marker DLG is absent (figure 3E). We also see this same phenotype in the antennal lobe when GFP:DVGLUT is expressed in the glutamatergic interneurons (figure 3G). Thus the presynaptic cells expressing DVGLUT survive while the postsynaptic targets of the glutamatergic neurons degenerate. Taken together, these results demonstrate that increased DVGLUT expression in glutamatergic neurons damages the postsynaptic target neurons, as is seen in vertebrate models of excitotoxic injury. In addition, this degeneration requires expression of a functional DVGLUT protein in glutamatergic neurons. The progressive neurodegeneration likely causes the decline in motor behavior and the premature lethality. Thus overexpression of DVGLUT generates an in vivo model of excitotoxic neurodegeneration with behavioral and neuropathological phenotypes, and demonstrates that increased transporter expression is sufficient to cause excitotoxic degeneration.
Discussion
VGLUT expression increases in a number of disease models, yet it is unclear whether this upregulation contributes to the subsequent pathologies. For example, the increase in VGLUT1 after methamphetamine treatment may change the synaptic weight of corticostriatal circuits and damage striatal dopaminergic terminals (Mark et al., 2007). We and others have previously shown that increased VGLUT expression leads to a shift in the population of mini amplitudes to larger values indicating that more glutamate is released from each vesicle (Daniels et al., 2004; Moechars et al., 2006; Wilson et al., 2005; Wojcik et al., 2004). Since excess extracellular glutamate can be toxic, we tested whether increasing VGLUT levels is sufficient to cause excitotoxicity.
Here we show that overexpression of the Drosophila vesicular glutamate transporter (DVGLUT) in glutamatergic neurons leads to neurodegeneration. These flies show enhanced spontaneous release of very large amounts of glutamate at the neuromuscular junction that correlates with level of DVGLUT overexpression. Flies overexpressing DVGLUT also die prematurely and exhibit pronounced neurodegeneration. This neurodegeneration is likely excitotoxic because 1) expression of functional DVGLUT is required, 2) degeneration occurs only when DVGLUT is overexpressed in glutamatergic neurons, and 3) the degeneration occurs in the postsynaptic targets of the neurons overexpressing DVGLUT whereas the DVGLUT-expressing neurons do not die.
Glutamate is the most widely used excitatory neurotransmitter in the vertebrate CNS. We have previously shown that glutamatergic synapses are much more common than previously appreciated in the Drosophila CNS (Daniels et al., 2008). As in vertebrates, reduced glutamate clearance can cause neurodegeneration in flies (Rival et al., 2002). In vertebrates, excitotoxic neurodegeneration is thought to involve increased calcium entry downstream of calcium-permeable NMDA and some AMPA receptors. The Drosophila genome encodes many glutamate receptor subunits with high homology to vertebrate AMPA, kainate, and NMDA families (Littleton and Ganetzky, 2000). Some of the ionotropic receptor subtypes have been studied at the glutamatergic larval neuromuscular junction (reviewed in DiAntonio, 2006), and a few have also been found in the adult CNS, including an NMDA receptor homolog DNMDAR-1 (Völkner et al., 2000). Thus the essential components are present in the Drosophila CNS to mediate glutamate excitotoxicity.
In our previous examination of DVGLUT overexpression at the larval neuromuscular junction, we did not observe overt signs of degeneration in our electron microscopic analysis. Muscles may be less sensitive to excess glutamate release because they routinely encounter high levels of extracellular glutamate. During an in vivo muscle contraction event, motoneurons fire bursts of about 20 action potentials at approximately 20 Hz and release on the order of 100 vesicles per action potential onto a given muscle. Central neurons, on the other hand, likely receive far less intense glutamatergic stimulation, and so might be less able to tolerate the release of excess glutamate.
Our model of excess glutamate release may be more similar to a chronic injury model such as amyotrophic lateral sclerosis (ALS) rather than the excitotoxicity following stroke or brain injury since there is no discrete lesion event, despite the obvious differences in disease etiology. Nevertheless, our model may be used to study conserved excitotoxic mechanisms and also in searches for other genes that modify the observed phenotypes. In addition, this model may provide insights into the pathophysiological effects of increased VGLUT levels that accompany seizures, hypoxic injury, stress, and methamphetamine or antidepressant treatment (Kim et al., 2005; Mark et al., 2007; Raudensky and Yamamoto, 2007; Tordera et al., 2005; Touret et al., 2007).
Acknowledgments
We would like to thank the DiAntonio lab for support, Howard Wynder for advice and use of his ultramicrotome, and Xiaolu Sun and Sylvia Johnson for excellent technical support. This work was supported by NIH grants NS054467 to R.W.D. and NIH grants NS051453 and DA020812 to A.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49(1):11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24(46):10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in the Drosophila larval and adult CNS. J Comp Neurol. 2008;508(1):131–152. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- DiAntonio A. Glutamate receptors at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:165–179. doi: 10.1016/S0074-7742(06)75008-5. [DOI] [PubMed] [Google Scholar]
- Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmaclology. 2000;39(9):1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Grygoruk A, Fei H, Daniels RW, Miller BR, DiAntonio A, Krantz DE. A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo. J Biol Chem. 2010;285(10):6867–6878. doi: 10.1074/jbc.M109.073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65(4):529–534. doi: 10.1212/01.wnl.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- Karunanithi S, Marin L, Wong K, Atwood HL. Quantal size and variation determined by vesicle size in normal and mutant Drosophila glutamatergic synapses. J Neurosci. 2002;22:10267–10276. doi: 10.1523/JNEUROSCI.22-23-10267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Kwak SE, Kim JE, Won MH, Choi HC, Song HK, Kwon OS, Kim YI, Choi SY, Kang TC. Bilateral enhancement of excitation via up-regulation of the vesicular glutamate transporter subtype 1, not subtype 2, immunoreactivity in the unilateral hypoxic epilepsy model. Brain Res. 2005;1055(1–2):122–130. doi: 10.1016/j.brainres.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46(3):445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26(1):35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine –induced glutamate release. J Neurosci. 2007;27(25):6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2007;67(6):778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19(16):R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Tosenmund C, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26(46):12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135(1):129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iché M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol. 2004;14(6):599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Tordera RM, Pei Q, Sharp T. Evidence for increased expression of the vesicular glutamate transporter VGLUT1 by a course of antidepressant treatment. J Neurochem. 2005;94(4):875–883. doi: 10.1111/j.1471-4159.2005.03192.x. [DOI] [PubMed] [Google Scholar]
- Touret M, Parrot S, Denoroy L, Belin MF, Didier-Bazes M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007;8:69. doi: 10.1186/1471-2202-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkner M, Lenz-Böhme B, Betz H, Schmitt B. Novel CNS glutamate receptor subunit genes of Drosophila melanogaster. J Neurochem. 2000;75(5):1791–1799. doi: 10.1046/j.1471-4159.2000.0751791.x. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25(26):6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21(6):1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]