Abstract
The current definition of Major Depressive Disorder (MDD) emerged from efforts to create reliable diagnostic criteria for clinical and research use. However, despite decades of research, the neurobiology of MDD is largely unknown, and treatments are no more effective today than they were 50–70 years ago. We propose that the current conception of depression is misguiding basic and clinical research. Redefinition is necessary and may include a focus on a more narrowly defined set of core symptoms. However, we conclude that depression is better defined as the tendency to enter into, and inability to disengage from, a negative mood state rather than the mood state per sé. The implications for future clinical and basic research are discussed.
Introduction
If a fright or despondency lasts for a long time,
it is a melancholic affection.
Hippocrates, Aphorisms, Section 6, no. 23
Depression has been recognized as a distinct and serious condition for over 2000 years. Although definitions have varied, the core construct of depression has remained remarkably intact: a condition of despondency and anergia akin to normal sadness, but critically different in its severity, duration and/or lack of a triggering event. In modern psychiatry, Major Depressive Disorder (MDD) is broadly defined as a syndrome including episodes of persistent negative mood (feeling down, sad, blue, etc.) or anhedonia (a loss of interest in pleasurable activities) plus a combination of additional emotional, psychological and somatic symptoms (Box 1) [1]. This constellation of symptoms must persist for at least two weeks and not be secondary to an obvious substance, medical condition, or other psychiatric disorder; nor can the symptoms be better explained by normal grief. This definition emerged from efforts to develop reliable diagnostic criteria for use in clinical and research settings, culminating in the Diagnostic and Statistical Manual of the American Psychiatric Association (currently in its 4th edition [DSM-IV-TR]) [1].
BOX 1. DSM-IV-TR diagnostic criteria for a major depressive episode.
Must have 5 or more of the following symptoms for a duration of 2 or more weeks. Furthermore, one of these 5 symptoms must consist of depressed mood or anhedonia.
| Mood/Thought | Somatic/Vegetative |
|---|---|
| Depressed mood | Appetite/weight changes |
| Anhedonia | Sleep disturbances |
| Worthlessness or guilt | Changes in psychomotor activity |
| Poor concentration, indecisiveness | Fatigue |
| Thoughts of death, suicidal ideation |
In this Opinion piece, we describe the inadequacy of available antidepressant treatments and conclude a shift is needed in the definition of depression and the research agenda going forward. We first provide a brief overview of the epidemiology of depression, including the high prevalence of treatment-resistant depression (TRD). We then present a brief history of antidepressant treatment development and emphasize that, after several decades of research, antidepressant efficacy has not substantially improved. We next critically consider the most commonly posited explanation for this, namely the clinical heterogeneity of the disorder suggesting different biological subtypes of depression. We conclude that the current construct of depression, as defined for the purposes of clinical and basic research, has lost its utility. We note that focusing investigation towards specific symptoms considered core to the illness may be more fruitful in elucidating the neurobiology of the disease and developing improved treatments. Moreover, we propose that the illness “depression” may be better defined as the inability to disengage from a negative mood state and the tendency to inappropriately re-enter this state: i.e., the tendency to get “stuck in a rut”. These positions are supported by emerging data on deep brain stimulation (DBS) in the treatment and study of depression.
The Extent and Cost of Depression
The U.S. lifetime prevalence of MDD is 17%, with twice as many women affected as men [2]. Median age of symptom onset is 30 years, though the disorder may arise at almost any age [2]. MDD is moderately heritable (ranging from 30%–40%)[3], supporting a role for both genetic and environmental risk factors. MDD is the leading cause of years lost due to disability worldwide and the third overall contributor to the worldwide burden of disease (but projected to be the biggest contributor by 2030)[4].
A number of treatments are available for depression including medications, psychotherapy and various somatic treatments such as electroconvulsive therapy [ECT], vagus nerve stimulation [VNS] and transcranial magnetic stimulation [TMS]. Unfortunately, up to two-thirds of patients remain symptomatic following first-line treatment [5] and a third fail to achieve remission (defined as full resolution of depressive symptoms) after four established treatments [6]; approximately 10%–20% of depressed patients may show virtually no improvement despite multiple, often aggressive treatments. Thus, a conservative estimate places the U.S. prevalence of TRD at 1%–3%. Importantly, this estimate does not include the prevalence of dysthymia or treatment-resistant bipolar depression.
Even among those patients who achieve remission with antidepressant treatment, recurrence is the rule rather than the exception: the probability of another depressive episode is >60% after the first, >70% after the second, and >90% after the third [1]. Relapse rates may be somewhat lower among patients maintained on antidepressant medications [7, 8] and those receiving psychotherapy [9, 10]. However, in a recent, large, multi-center trial assessing the efficacy of sequential treatments for MDD, the 6 month relapse rate was especially high (between 34% and 83%)[6]. Notably higher relapse rates were seen in patients with greater treatment resistance (even if remission was achieved).
A Century of Antidepressant Treatment Research: Limited Progress
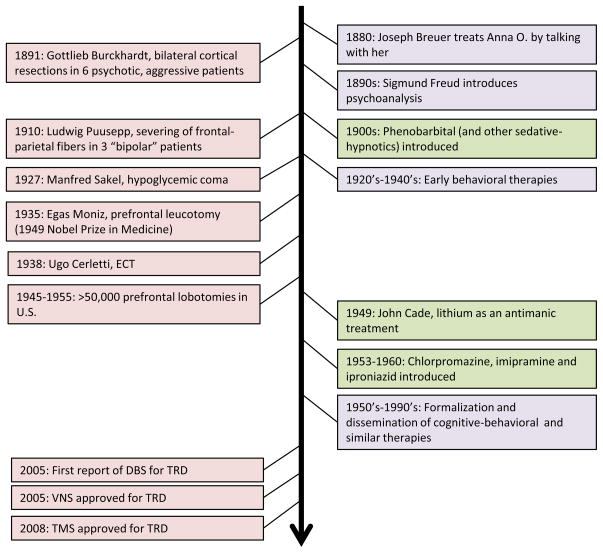
In the absence of effective pharmacological treatments, attempts to treat malignant psychiatric illness have often pursued an aggressive course (Figure 1). This began with an early attempt to treat psychosis with cortical resection [11] and peaked with the mid-20th century popularity of prefrontal leucotomy – the surgical ablation of prefrontal white matter tracts – for multiple neuropsychiatric indications [12]. These treatments were based on a rudimentary understanding of the neural circuitry of emotional/behavioral regulation derived from animal and human brain injury data [13] and the hypothesis that severing white matter connections between cortical and subcortical structures would diminish aberrant behavior. Modern ablative strategies include subcaudate tractotomy, anterior capsulotomy, anterior cingulotomy, and limbic leucotomy – approaches that employ more focal disruption of white matter tracts using stereotactic neurosurgical techniques. Observational data suggest efficacy in 25%–50% of patients across a variety of treatment-refractory psychiatric disorders, including depression and obsessive-compulsive disorder (OCD)[14].
Figure 1.
Milestones in the development of psychiatric treatments. DBS=deep brain stimulation; ECT=electroconvulsive therapy; TMS=transcranial magnetic stimulation; VNS=vagus nerve stimulation. Treatment categories: psychotherapy (purple), medication (green), other somatic interventions (pink).
Side effects can include cognitive detriments, personality changes, and seizures [14]. A number of non-surgical but still relatively aggressive treatments were also employed in the early 20th century for severe psychiatric illness, including malarial pyrotherapy [15], camphor-induced seizures [16], and hypoglycemic coma [17]. In general, these treatments were developed to treat “schizophrenia” though formal, reliable diagnostic criteria were not employed. Also, while these treatments were often associated with convulsions, this was not necessarily the presumed mechanism of action at the time (except for camphor-induced seizures). Developed within this context, ECT was introduced in 1938. Over time, ECT has become established as the most effective acute treatment for a depressive episode, with remission rates over 50% [18–20]. However, cognitive side effects can be significant and occasionally persistent [21], and relapse remains a significant problem [18].
For many years, these remained the only treatments available for patients with severe psychiatric illness. However, in the mid-20th century, an explosion of psychopharmacologic research occurred. Contemporary with the discovery of the antimanic properties of lithium [22, 23] (now a standard first-line treatment for bipolar disorder) and the antipsychotic/antimanic properties of chlorpromazine [24, 25] (a treatment for schizophrenia), the first antidepressant medications were identified in the mid-1950’s. These included the tricyclic compound imipramine [26] and the monoamine oxidase inhibitor (MAOI) iproniazid [27, 28], leading to the “monoamine hypothesis” of depression [29–31]. Research in depression then largely shifted to delineating the neurochemical bases of the disorder and helped establish a role for serotonin, norepinephrine and dopamine dysfunction in the pathophysiology and treatment of depression. Medications modulating monoaminergic neurotransmission have consistently shown response rates (defined as a ≥50% decrease in depression severity from baseline) of approximately 60%.
Other neuromodulatory systems have also been explored as potential drug targets. Abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis have been clearly identified in depressed patients, consistent with findings that trauma and psychosocial stress predisposes an individual to depression, suggesting the stress hormone axis as a possible target for antidepressant medications [32, 33]. Abnormalities within the hypothalamic-pituitary-thyroid axis have also been linked with depression [34], and thyroid hormone augmentation is an accepted strategy for TRD, though with limited efficacy [35]. Dysfunction of glutamate and γ-aminobutyric acid (GABA) systems have been found in depressed patients and certain animal models of depression, and these systems have been suggested as targets for further treatment development [36]. The NMDA glutamate receptor antagonist ketamine, for instance, has been shown to have acute antidepressant effects in severely depressed patients, though relapse occurs relatively quickly [37–39]; and riluzole (an agent that appears to inhibit glutamate release, increase glutamate reuptake and block glutamate receptors) has been shown to have antidepressant efficacy [40]. More recently, the antimuscarinic agent scopolamine has been shown to have acute antidepressant effects [41]. The opioid system has also been proposed as a target of antidepressant treatment [42]. Inflammatory processes have been implicated in depression, and anti-inflammatory therapies may have antidepressant efficacy [43]. Nociceptive neurokinins may also play a role in the neurobiology of depression [44]. Finally, an increasing number of studies are focusing on dysregulation of second messenger systems, gene transcription, various neurotrophic factors, cell turnover and epigenetic mechanisms in mood disorders, including depression [45–47]. It is encouraging to see this expansion of psychopharmacological research beyond a fairly limited focus on the monoaminergic systems. Unfortunately, this work has yet to yield treatments that substantially change the overall response rates and course of illness for depressed patients [48].
In conjunction with the development of somatic treatments, formal psychotherapeutic approaches to depression have evolved (Figure 1). Psychoanalysis was developed in the late 19th century [49], and the 20th century saw a dramatic increase in types of psychotherapy, many influenced by advances in behavioral psychology. The most studied psychotherapies for depression include cognitive behavioral therapy (CBT)[50] and interpersonal psychotherapy [51], with reported efficacy virtually identical to that of antidepressant medications used as monotherapy (e.g., response rates of approximately 60%).
More recently, treatments have been developed to specifically target medication-resistant depression. VNS was approved by the U.S. Food and Drug Administration (FDA) in 2005 for the treatment of depression not responding to at least four antidepressant treatments. VNS does not have acute antidepressant efficacy, but has a 30% response rate (15% remission rate) with 12 months of open-label treatment [52, 53]. Although relapse rates over 1–2 years may be as high as 56% [54, 55], VNS patients appear to relapse less often than patients receiving only “treatment-as-usual” [54, 56, 57]. Repetitive TMS was FDA approved in 2008 for depression not responding to one antidepressant medication. TMS has consistently demonstrated statistically significant antidepressant effects [58–60]. However, response rates are generally less than 30%, efficacy appears limited in patients failing more than one adequate antidepressant treatment [61], and long-term relapse rates following the acute course are unknown but likely to be similar to those seen with ECT [62].
More invasive antidepressant treatments have also been revisited in the past few years. Subcallosal cingulate (SCC) DBS was associated with a 60% response rate in TRD patients following 6 months of chronic, high frequency stimulation in an uncontrolled, open-label study [63]; these effects were largely maintained over an additional 6 months [63]. DBS of other targets may also have antidepressant efficacy. These include the ventral anterior internal capsule/ventral striatum (40% response rate following 6 months of chronic stimulation, 53% response rate at last follow-up)[64], nucleus accumbens (50% response rate following 6 months of chronic stimulation)[65], inferior thalamic peduncle [66], and habenula [67]. Aside from DBS, epidural stimulation of bilateral medial and dorsolateral prefrontal cortices was associated with remission in 3 of 5 TRD patients following 7 months of treatment [68]. It should be emphasized that, despite encouraging early results with these various surgical interventions, these data are extremely preliminary and based on small, open-label, uncontrolled studies.
In sum, currently available treatments are modestly effective, but treatment resistance and recurrence remain significant problems. Importantly, current medications are no more effective than those introduced over 50 years ago. Recently approved somatic treatments (VNS, rTMS) have shown limited short- or long-term efficacy. The most effective treatment for a major depressive episode (MDE) is still ECT – developed over 70 years ago – though relapse remains a significant problem. Recent data suggest focal neuromodulation of various brain regions (via DBS or direct cortical stimulation) may have benefit in extremely treatment-resistant patients; however, these data are very preliminary.
One Syndrome, Many Faces: Clinical Heterogeneity in Depression
A common explanation for the limited efficacy of antidepressant treatments is that “depression” likely refers to multiple diseases, each with a distinct neurobiology. In support of this, the clinical heterogeneity of depression is frequently cited: age of onset and family history can vary widely, and presenting symptoms are highly pleomorphic. For example, although it is most common for patients to present with decreased sleep and appetite, the reverse can occur and is often associated with “atypical depression” in which normal mood reactivity and hedonic responses are generally maintained. Some patients may have a more classic “melancholic” presentation associated with severe anhedonia, absent mood reactivity, profound psychomotor retardation or agitation, and pronounced early morning awakening (among other symptoms). Comorbid anxiety and somatic complaints are common in depressed patients, and often resolve with successful treatment, though these symptoms are not part of the formal diagnostic criteria; conversely, many patients with a primary anxiety disorder diagnosis (Generalized Anxiety Disorder, OCD and/or Post-Traumatic Stress Disorder) frequently present with clinically significant depressive symptoms that often meet criteria for MDD. Patients with bipolar disorders can present with depressive episodes that are phenomenologically indistinguishable from those presenting in MDD, yet the primary diagnoses are viewed as distinct.
It is commonly presumed that the clinical heterogeneity of depression reflects biological heterogeneity, such that specific interventions may be needed for specific (clinical) subtypes of depressed patients. This view has led to attempts to define clinically significant depressive subtypes based on phenomenological differences. Early apparent success was seen with the recognition that patients with atypical depression may preferentially respond to MAOIs vs. tricyclic antidepressants (TCAs) [69]; however, a substantial number of patients with atypical depression show a very good response to a TCA, thus limiting the utility of this subtype for clinical and research purposes [70]. Similarly, it is generally accepted that patients with bipolar depression should be initially treated with a “mood stabilizer”, such as lithium or an anticonvulsant [71, 72]. However, compared with typical mood stabilizers, monotherapy with a typical antidepressant medication may be as or more effective in achieving and maintaining euthymia in bipolar disorder, at least in patients with bipolar II disorder (consisting of depressive and milder hypomanic episodes, but not more severe manic episodes) [73, 74]. Furthermore, it is not clear that the neurobiology of unipolar vs. bipolar depression is significantly different [75]. Therefore, the definition of depressive subtypes based on clinical criteria has not led to improved overall response rates, nor has this helped identify biologically distinct subtypes to guide further treatment development.
Variability also exists in antidepressant treatment response across depressed patients, and even within clinically defined subtypes. Some patients fail to respond to one medication, but will show an excellent response to a second (sometimes very similar) medication [76]. Others may not respond well to medications, but achieve clinical remission with psychotherapy [77], and vice versa; many patients may need both to achieve remission (ie. a full resolution of all symptoms) [78]. Finally, it is widely acknowledged, although less well studied, that some patients may do very well with a particular medication early in their illness, but either relapse during maintenance treatment or show limited response to the same medication in a later episode. To this point, it is noted that the vast majority of patients enrolled in studies of TRD have recurrent depression – implying they achieved remission during one or more prior depressive episodes [53, 64, 79].
It must be recognized, however, that few studies (clinical or basic) have emphasized clinical subtypes. In human studies, patients that meet DSM criteria for MDD are generally included for treatment and neurobiological studies regardless of phenomenological heterogeneity; post-hoc analyses of subtypes often include too few patients to draw meaningful conclusions. In animal studies, models are often judged by how closely these approximate the full syndrome of depression (while necessarily focusing on symptoms that can be reproduced in an animal). Additionally, new animal models may only become accepted to the extent they reproduce behavioral effects with treatment seen in previously tested animal models. Yet, no animal model mirrors the full syndrome of a DSM-defined depressive episode or the phenomenological heterogeneity of MDD. Critically, current animal models do not have face validity for important aspects of MDD, including a relapsing/recurring course, tachyphylaxis, and the potential transformation (within a patient) from a treatment-responsive to a treatment-resistant illness.
Rethinking Depression and Its Treatment
Working within an accepted model and definition of depression, the development of DBS of the SCC for TRD was based on the hypothesis that Brodmann Area 25 (BA25) served as a key node in a distributed network of brain regions involved in mood regulation [80, 81]. Activity in BA25 was noted to increase with sad mood, and a decrease in BA25 activity was a consistent finding associated with successful antidepressant response to a number of somatic treatments [80]. According to this hypothesis, the many symptoms of depression were due to abnormalities in several discrete, but interconnected neural systems, and BA25 was a critical region that had direct connections to most of these circuits.
Proof-of-principle testing helped confirm the hypothesis that directly modulating BA25 activity and white matter tracts connecting this brain region to other nodes in a presumed depression network would have antidepressant effects in TRD patients [63, 79]. However, only 60% of patients responded. With DBS for patients with severe, treatment-resistant movement disorders [eg. Parkinson’s disease (PD)], nearly all patients show clinically significant improvement (of motor symptoms) with chronic stimulation [82–84].
The critical question then becomes: if BA25 is a critical node in a depression network, whose activity and connectivity with other brain regions is specifically dysfunctional in TRD, why were response and remission rates not higher? One simple answer is, as above: depression is a complex disorder with multiple neurobiological etiologies – thus, only patients with “BA25-dependent” TRD may respond to this treatment. We reject this possibility for several reasons: (1) Several decades of neurobiological research have failed to identify clinically meaningful biological subtypes of “depression” as currently defined. (2) The vast majority of TRD patients have recurrent illness [i.e., they previously remitted with adequate (standard) treatment]. Thus, at an earlier stage of illness, TRD patients show response to the same biological interventions as non-TRD patients. TRD then cannot be simply defined as a “non-monoaminergic” depression. Further, this highlights the recurrence and potential malignant transformation of the disorder over time – and the limits of current treatments to address this. (3) Nearly all TRD patients enrolled in the SCC DBS study showed at least some (>30%) improvement, and these effects persisted over time [63], suggesting that modulation of a BA25-associated network leads to sustained positive effects on mood regulation in nearly all patients. Thus, we conclude: that BA25 and connected brain regions are involved in the regulation of negative mood, and that modulation of this network via focal stimulation is likely to impact mood regulation in a potentially beneficial way in most patients. However, while this beneficial mood effect is necessary for the full syndrome of depressive symptoms to resolve, it may not in itself be sufficient to achieve complete remission in all patients.
With this supposition in mind, we propose another answer: the syndrome of depression, as currently defined, is too broad to serve as a useful construct for treatment development, especially for more focal treatments. By analogy, PD is recognized as a complex neurological syndrome (with motor, sleep, cognitive, mood and autonomic abnormalities), and it is widely recognized that certain treatments may improve a subset of symptoms but not affect, or even worsen, others. For example, subthalamic DBS can improve the core motor symptoms of PD [82], but may not significantly affect postural instability, mood or cognition [85, 86] – and negative effects on mood and cognition have been reported [87, 88]. A similar approach to depression would identify specific symptoms or subsets of symptoms (e.g. depressed mood/anhedonia vs. sleep vs. anxiety) as targets for treatment; the efficacy of a particular treatment could then be gauged by its effect on these symptoms, rather than using improvement in the entire syndrome as the primary efficacy measure. A version of this approach has been recently suggested (using a dimensional construct for the depressive syndrome) as a way to develop novel antidepressant treatment strategies [89]. Such approaches should not diminish the goal of achieving full symptomatic and functional recovery. But, they may be more useful in developing specific treatment targets for clinical and basic research studies.
However, it is our opinion that a re-definition of depression should go further. We recognize that the observed symptoms of “depression” clearly track together in presentation and improvement with treatment, including domains of mood (e.g., sadness, irritability, anxiety), cognition (eg. guilt, low self-esteem, poor concentration/attention, decreased/increased psychomotor speed, hopelessness, suicidal ideation), and somatic state (eg. sleep/appetite/libido disturbances, somatic anxiety, motoric retardation/agitation). But, we conclude that this depressive state, as currently defined, is not abnormal per se, but reflects an etiologically non-specific response to stress/distress (e.g., physical or emotional stress, infection, inflammation, etc.) that includes a stereotypical set of emotional, cognitive-behavioral and somatic responses. This is supported by the currently accepted clinical criteria, where the diagnosis of a MDE can only be made if the presentation is not clearly due to a state caused by withdrawal from an addictive substance, effects of a medical illness, effects of a medication or normal bereavement highlighting the non-specific nature of the phenomenology.
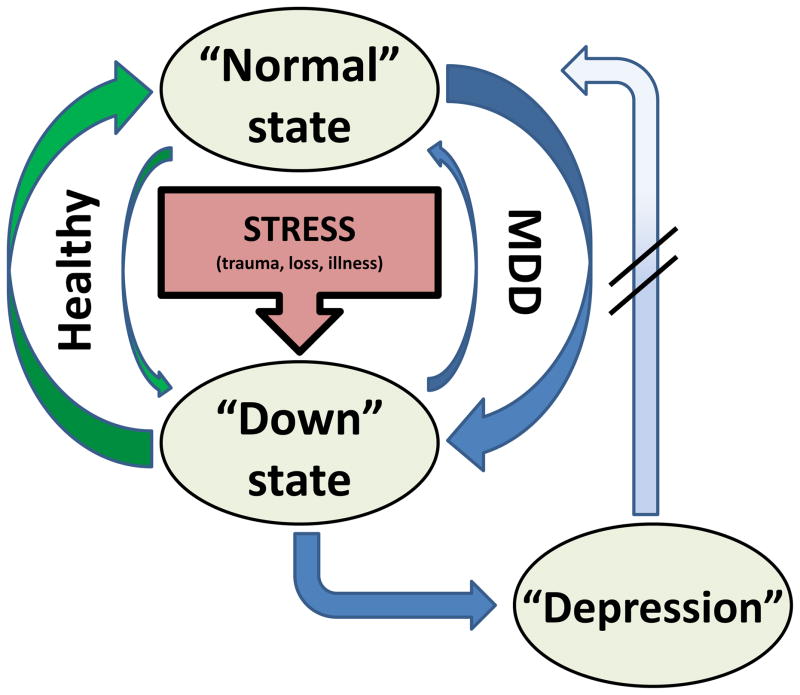
We instead propose that the primary abnormality of depression is not the depressive state itself, but rather the inability to appropriately regulate that state (Figure 2): depressed patients become “depressed” without a trigger, become more severely “depressed” following a trigger (e.g, death of a loved one) than is considered normal, and/or remain “depressed” for longer than would be expected. By analogy, with cardiac arrhythmias (e.g., Wolff-Parkinson-White syndrome, sick sinus syndrome, etc.), the actual arrhythmia (e.g., tachycardia) is the symptomatic presentation that may need acute treatment, but treatment of the underlying disease requires addressing the tendency of the system to enter an aberrant rhythm it cannot exit. Similarly, we conceptualize the depressive state as an aberrant neural rhythm, but the depressive disorder as the brain’s tendency to go into and stay in that rhythm inappropriately.
Figure 2.
Proposed model of depression: “stuck in a rut”. In response to a stressor, there is a mood reaction, i.e., a shift from the normal, euthymic state to a “down” state that includes the symptoms associated with the syndrome of depression, grief, sickness behavior, etc. The return to the normal state is achieved through mood regulation. In healthy, non-depressed individuals, the tendency to enter the down state is relatively low, and when it occurs, the return to normal is relatively quick and complete (green arrows). In individuals with Major Depressive Disorder (MDD), the pressure to enter the down state is relatively high (and may even occur in the absence of a clear stressor), but the ability to return to normal is impaired (blue arrows). During a depressive episode, the down state becomes more established, and the individual may have even greater difficulty returning to the normal state without external intervention (psychotherapy, medication, etc.). This model emphasizes that the down state itself is not abnormal. Instead, it is the tendency to enter and get stuck in this state that defines depression. Thus, the neurobiology of depression should be that of mood reaction and regulation rather than the mood state per se.
Such a view has important implications for treatment development. With cardiac arrhythmias, a number of diverse interventions with different mechanisms of action (e.g., most medications, cardioversion, time [i.e., spontaneous recovery]) may return the system to the physiological state, but others (eg. ablation, pacing, certain medications) may be needed to help maintain the “normal” state over time. Likewise, diverse “antidepressant” treatments (e.g., various medications, psychotherapy, ECT, or TMS) can shift a patient out of the depressive state but may not necessarily prevent re-entry into that state. This is especially true of treatments with purported acute “antidepressant” effects (such as sleep deprivation [90], ketamine [38], and scopolamine [41]), where a positive mood/behavioral change is seen within minutes to hours, but effects are short-lived (with return of depressive symptoms within hours or days). Other treatments, with mechanisms of action distinct from those of more acute treatments, may be needed to prevent the abnormal recurrence of the depressive state. For example, certain psychotherapies may provide positive steps in this direction [9, 10, 91–93]. It has been suggested that such treatments may work by strengthening the cognitive cortical-subcortical systems responsible for regulating mood [94–96]).
In light of our proposed re-definition of depression, we suggest a number of directions that may prove useful to take with future research on MDD (Box 2). One important focus of such research should be on identifying interventions that not only alter the underlying state, but actually prevent the brain from entering or remaining in that state at inappropriate times. Although highly preliminary, emerging data for DBS suggest that such a treatment intervention may be possible. With SCC DBS, there are immediate changes in mood with initial acute stimulation that may or may not persist, with resolution of the full syndrome of the depressive episode requiring weeks to months [63, 79]. But critically, once patients are well, they appear to stay well over time: although the published follow-up data are currently limited to one year of treatment, it is notable that >70% of patients responding to SCC DBS at 6 months were still responders at 12 months [63]. Though not described in detail, similar results may be seen with ventral capsule/ventral striatal DBS for TRD [64]. Further, if stimulation ceases (e.g., the device is turned off or the battery depletes), patients will almost invariably return to the depressive state [63, 64, 97].
Box 2. Future directions.
Further investigate the neurobiological bases for normal and abnormal mood regulation (rather than the depressed state per se)
Further investigate the neurobiology of treatment resistance and depressive relapse
Explore strategies to redefine the key endpoints for clinical trials, recognizing that specific treatments may only target a subset of depressive symptoms
Develop animal models with face validity for key elements of MDD, such as relapse, tachyphylaxis and treatment resistance.
It is hoped that less invasive strategies to help prevent depressed patients from becoming “stuck in a rut” can be developed, which do not require brain surgery. However, it is our opinion that such strategies are likely to be very different than the treatments currently under development that focus primarily on shifting patients out of the depressive state. An important focus for future clinical and basic research in light of our proposed re-definition of depression would be the regulation of mood states, rather than the states themselves. Several imaging studies have implicated abnormal emotional processing (not just abnormal mood state) in the pathophysiology of depression [94, 96, 98]. Furthermore, as discussed above, recent data suggest that cognitive-behaviorally based and chronobiological therapies (which can be effective in TRD patients [77, 99] and may decrease recurrence risk [9, 10, 92, 93]), may function via specific neurobiological routes related to mood regulation [96, 100, 101]. With respect to the development of future animal models, an emphasis on symptom breakthrough/recurrence is needed, since there are currently no accepted animal models of TRD or highly recurrent depression. Such models are needed to better identify and develop novel treatment targets.
Summary
Accurately and precisely defining depression is a non-trivial problem of critical importance. Current models have outlasted their utility as a basis for treatment development, a conclusion primarily based on the observation that antidepressant treatments today are no more effective than those available 50–70 years ago despite intensive neurobiological investigation. Although a full discussion is beyond the scope of this article, similar issues have been described in the study and treatment of other psychiatric disorders, including schizophrenia and OCD [102–105], highlighting the challenges of a fully phenomenologically-based nosology.
The limitations of the current definition of depression for clinical and basic science research purposes are essentially two-fold: (1) focusing on the full syndrome of depression as the target of treatment; and (2) emphasizing the acute resolution of symptoms rather than the maintenance of normal mood regulation over time. As an alternative, we propose the following main arguments. Firstly, treatment development should be guided by the recognition that different depressive symptoms may need to be targeted with different treatments. Secondly, the goal of antidepressant treatment should ultimately be the maintenance of normal mood regulation over the long-term. Preliminary data from DBS trials suggest that the latter point may at least be a possibility with such a treatment regime. However, this is obviously a highly invasive treatment that, if validated, will only be appropriate for a select few patients with the most severe forms of TRD. Still, by building on the knowledge gained from such treatments, it is hoped that the neuroscience community will re-focus efforts on developing less-invasive interventions for MDD that achieve the same degree of short- and long-term effectiveness.
Glossary
- Atypical depression
A clinical subtype of depression defined by normal to increased mood reactivity together with increased sleep, increased appetite, rejection sensitivity, and a heavy, leaden feeling in the limbs
- Bipolar Disorder
A mood disorder characterized by periods of euthymia (normal mood state), major depressive episodes (see below), manic or hypomanic episodes, and/or mixed episodes (occurrence of a major depressive and manic episode concurrently)
- Chlorpromazine
An antipsychotic medication with a primary mechanism of action of blocking dopaminergic receptors (though it also has affects on other neurotransmitter systems); chlorpromazine and other antipsychotics are primarily used in the treatment of psychotic disorders (e.g., schizophrenia), but have also shown efficacy as augmentation medications for treatment-resistant depression
- Cognitive Behavioral Therapy (CBT)
A form of psychotherapy that attempts to correct cognitive distortions and dysfunctional behaviors that are posited to cause or contribute to emotional illness
- Deep Brain Stimulation (DBS)
A neurosurgical treatment where one or more electrodes are inserted into a specific brain structure and focal electrical current is delivered to the surrounding brain tissue; stimulation parameters are typically controlled via a computer/battery pack implanted subcutaneously (often in the chest wall)
- Diagnostic and Statistical Manual of Mental Disorders (4th Edition, Text Revision)(DSM-IV-TR)
A publication of the American Psychiatric Association that provides standardized criteria for diagnosing psychiatric disorders
- Dysthymia
A mood disorder characterized by 2–4 depressive symptoms occurring nearly every day for more than two years; although associated with fewer depressive symptoms than a MDE, dysthymia is equally distressing and disabling due to its chronic nature
- Electroconvulsive Therapy (ECT)
A treatment that involves the transcranial delivery of electricity to induce a generalized seizure under general anesthesia; a treatment course will typically include 2–3 treatments per week over 3–4 weeks
- Lithium
A psychotropic medication used in the treatment of bipolar disorder where it has shown antimanic and antidepressant properties, as well as the ability to prevent mood episodes over time; lithium is also an accepted augmentation medication for MDD; the primary mechanism of action for lithium is unknown
- Major Depressive Disorder (MDD)
A mood disorder characterized only by major depressive episodes; also referred to as unipolar depression (to distinguish it from depression in the context of bipolar disorder)
- Major Depressive Episode (MDE)
A mood episode defined by the presence of depressed mood or anhedonia, plus a combination of additional emotional, cognitive, behavioral, and/or somatic symptoms (see Box 1)
- Manic Episode
A mood episode defined by elevated or irritable mood plus a combination of additional symptoms (grandiosity, racing thoughts, decreased need for sleep, increased rate and volume of speech, increased goal-directed activity, and/or increased participation in pleasurable but risky activities)
- Monoamine oxidase inhibitor (MAOI)
A class of antidepressant medications with a primary mechanism of action of inhibiting the enzyme monoamine oxidase leading to an increase in available monoaminergic neurotransmitters (serotonin, norepinephrine, dopamine)
- Melancholic depression
A subtype of depression associated with profound anhedonia, severe weight loss, significant psychomotor agitation or retardation, severe insomnia with early morning awakening, excessive guilt and diurnal variation (mood is often somewhat improved later in the day)
- Monoaminergic hypothesis
A classic neurobiological hypothesis that proposes depression to arise from dysfunction of the monoaminergic neurotransmitters (ie. serotonin, norepinephrine, dopamine)
- Subcallosal cingulate (SCC)
A ventral portion of the anterior cingulate gyrus that is one target for DBS as a treatment for TRD
- Serotonin Norepinephrine Reuptake Inhibitor (SNRI)
A class of antidepressant medications with a primary mechanism of action of blocking the reuptake of serotonin and norepinephrine via presynaptic transporters thereby increasing the availability of both neurotransmitters
- Selective Serotonin Reuptake Inhibitor (SSRI)
A class of antidepressant medications with a primary mechanism of action of blocking the reuptake of serotonin via the presynaptic transporter thereby increasing the availability of serotonin
- Tricyclic Antidepressants (TCA)
A class of antidepressant medications with a primary mechanism of action of blocking the reuptake of norepinephrine (and to a lesser degree serotonin) via presynaptic transporters thereby increasing the availability of these neurotransmitters
- Transcranial Magnetic Stimulation (TMS)
A non-invasive technique that uses a rapidly changing magnetic field to depolarize cortical neurons; TMS can either be delivered as a single pulse or in a repetitive train (rTMS)
- Treatment-Resistant Depression (TRD)
A depressive episode (in the context of either MDD or bipolar disorder) that has not responded to adequate antidepressant treatment
- Vagus Nerve Stimulation (VNS)
A technique that involves implanting an electrode on a vagus nerve and stimulating via a subcutaneous computer/battery back
Footnotes
Disclosures
Dr. Mayberg has a consulting agreement with St. Jude Medical Inc, which has licensed her intellectual property to develop SCC DBS for the treatment of severe depression (US 2005/0033379A1, co-inventor Andres Lozano, MD, PhD). Dr. Mayberg has current grant funding from the Dana Foundation, NARSAD, National Institute of Mental Health (NIMH), Stanley Medical Research Institute, and Woodruff Foundation.
Dr. Holtzheimer has received grant funding from the Dana Foundation, Greenwall Foundation, NARSAD, National Institute of Mental Health (NIMH) (K23 MH077869), National Institutes of Health Loan Repayment Program, Northstar, Inc., Stanley Medical Research Institute, and Woodruff Foundation; he has received consulting fees from AvaCat Consulting, St. Jude Medical Neuromodulation and Oppenheimer & Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders - Text Revision (DSM-IV-TR) 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 4.WHO. The global burden of disease: 2004 update. Geneva: 2008. [Google Scholar]
- 5.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 7.Gelenberg AJ, Trivedi MH, Rush AJ, et al. Randomized, placebo-controlled trial of nefazodone maintenance treatment in preventing recurrence in chronic depression. Biol Psychiatry. 2003;54(8):806–17. doi: 10.1016/s0006-3223(02)01971-6. [DOI] [PubMed] [Google Scholar]
- 8.Keller MB, Trivedi MH, Thase ME, et al. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) Study: Outcomes from the 2-year and combined maintenance phases. J Clin Psychiatry. 2007;68(8):1246–56. doi: 10.4088/jcp.v68n0812. [DOI] [PubMed] [Google Scholar]
- 9.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417–22. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 10.Fava GA, Ruini C, Rafanelli C, et al. Six-Year Outcome of Cognitive Behavior Therapy for Prevention of Recurrent Depression. Am J Psychiatry. 2004;161(10):1872–1876. doi: 10.1176/ajp.161.10.1872. [DOI] [PubMed] [Google Scholar]
- 11.Burckhardt G. On cortical resection as a contribution to the operative treatment of psychosis. Psychiatrie psychischgerichtliche Medizin. 1891;47:463–548. [Google Scholar]
- 12.Jasper HH. A historical perspective. The rise and fall of prefrontal lobotomy. Adv Neurol. 1995;66:97–114. [PubMed] [Google Scholar]
- 13.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- 14.Sachdev P, Sachdev J. Sixty years of psychosurgery: its present status and its future. Aust N Z J Psychiatry. 1997;31(4):457–64. doi: 10.3109/00048679709065065. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NN. Artificial Fever as a Therapeutic Procedure. Cal West Med. 1936;44(5):357–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Meduna LJ. Die Konvulsionstherapie der Schizophrenie. Psychr Neurol Wochenschr. 1935;37:317–319. [Google Scholar]
- 17.Sakel M. The Origin and Nature of the Hypoglycemic Therapy of the Psychoses. Bull N Y Acad Med. 1937;13(3):97–109. [PMC free article] [PubMed] [Google Scholar]
- 18.Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Arch Gen Psychiatry. 2006;63(12):1337–44. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66(7):729–37. doi: 10.1001/archgenpsychiatry.2009.75. [DOI] [PubMed] [Google Scholar]
- 20.The UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. The Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 21.Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244–54. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- 22.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Medical Journal of Australia. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 23.Schou M, Juel-Nielsen N, Stromgren E, et al. The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry. 1954;17(4):250–60. doi: 10.1136/jnnp.17.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bower WH. Chlorpromazine in psychiatric illness. N Engl J Med. 1954;251(17):689–92. doi: 10.1056/NEJM195410212511703. [DOI] [PubMed] [Google Scholar]
- 25.Winkelman NW., Jr Chlorpromazine in the treatment of neuropsychiatric disorders. J Am Med Assoc. 1954;155(1):18–21. doi: 10.1001/jama.1954.03690190024007. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn R. The treatment of depressive states with G 22355 (imipramine hydrochloride) Am J Psychiatry. 1958;115(5):459–64. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- 27.Kiloh LG, Child JP, Latner G. A Controlled Trial of Iproniazid in the Treatment of Endogenous Depression. Journal of Mental Science. 1960;106(444):1139–1144. doi: 10.1192/bjp.106.444.1139. [DOI] [PubMed] [Google Scholar]
- 28.Bailey SD, Bucci L, Gosline E, et al. Comparison of iproniazid with other amine oxidase inhibitors, including W-1544, JB-516, RO 4-1018, and RO 5-0700. Ann N Y Acad Sci. 1959;80:652–68. doi: 10.1111/j.1749-6632.1959.tb49243.x. [DOI] [PubMed] [Google Scholar]
- 29.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 30.Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13(6):483–94. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 31.Prange AJ., Jr The Pharmacology and Biochemistry of Depression. Dis Nerv Syst. 1964;25:217–21. [PubMed] [Google Scholar]
- 32.Arborelius L, Owens MJ, Plotsky PM, et al. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 33.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 34.Musselman DL, Nemeroff CB. Depression and endocrine disorders: focus on the thyroid and adrenal system. Br J Psychiatry Suppl. 1996;(30):123–8. [PubMed] [Google Scholar]
- 35.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 36.Krystal JH, Sanacora G, Blumberg H, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7 (Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 37.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 38.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 39.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 40.Sanacora G, Kendell SF, Levin Y, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61(6):822–5. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432–8. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegadoren KM, O’Donnell T, Lanius R, et al. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides. 2009;43(5):341–53. doi: 10.1016/j.npep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bondy B, Baghai TC, Minov C, et al. Substance P serum levels are increased in major depression: preliminary results. Biol Psychiatry. 2003;53(6):538–42. doi: 10.1016/s0006-3223(02)01544-5. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunsberger JG, Austin DR, Chen G, et al. Cellular mechanisms underlying affective resiliency: the role of glucocorticoid receptor- and mitochondrially-mediated plasticity. Brain Res. 2009;1293:76–84. doi: 10.1016/j.brainres.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holtzheimer PE, 3rd, Nemeroff CB. Emerging treatments for depression. Expert Opin Pharmacother. 2006;7(17):2323–39. doi: 10.1517/14656566.7.17.2323. [DOI] [PubMed] [Google Scholar]
- 49.Freud S, Breuer J. Studien über Hysterie. Leipzig and Vienna: Deuticke; 1895. [Google Scholar]
- 50.Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62(9):953–9. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 51.de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255(2):75–82. doi: 10.1007/s00406-004-0542-x. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355–63. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–54. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Nahas Z, Marangell LB, Husain MM, et al. Two-Year Outcome of Vagus Nerve Stimulation (VNS) for Treatment of Major Depressive Episodes. J Clin Psychiatry. 2005;66(9):1097–1104. doi: 10.4088/jcp.v66n0902. [DOI] [PubMed] [Google Scholar]
- 55.Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38(5):651–61. doi: 10.1017/S0033291707001924. [DOI] [PubMed] [Google Scholar]
- 56.Dunner DL, Rush AJ, Russell JM, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688–95. doi: 10.4088/jcp.v67n0501. [DOI] [PubMed] [Google Scholar]
- 57.Sackeim HA, Brannan SK, John Rush A, et al. Durability of antidepressant response to vagus nerve stimulation (VNSTM) Int J Neuropsychopharmacol. 2007:1–10. doi: 10.1017/S1461145706007425. [DOI] [PubMed] [Google Scholar]
- 58.Holtzheimer PE, 3rd, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2001;35(4):149–69. [PubMed] [Google Scholar]
- 59.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 60.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 61.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 62.Dannon PN, Dolberg OT, Schreiber S, et al. Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals--preliminary report. Biol Psychiatry. 2002;51(8):687–90. doi: 10.1016/s0006-3223(01)01274-4. [DOI] [PubMed] [Google Scholar]
- 63.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Jimenez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–93. doi: 10.1227/01.neu.0000170434.44335.19. discussion 585–93. [DOI] [PubMed] [Google Scholar]
- 67.Sartorius A, Kiening KL, Kirsch P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 68.Nahas Z, Anderson BS, Borckardt J, et al. Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biol Psychiatry. 2010;67(2):101–9. doi: 10.1016/j.biopsych.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quitkin FM, Stewart JW, McGrath PJ, et al. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry. 1993;(Suppl)(21):30–4. [PubMed] [Google Scholar]
- 70.Thase ME. Atypical depression: useful concept, but it’s time to revise the DSM-IV criteria. Neuropsychopharmacology. 2009;34(13):2633–41. doi: 10.1038/npp.2009.100. [DOI] [PubMed] [Google Scholar]
- 71.APA. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;(supp):159. [PubMed] [Google Scholar]
- 72.Baldessarini RJ, Vieta E, Calabrese JR, et al. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143–57. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- 73.Agosti V, Stewart JW. Efficacy and safety of antidepressant monotherapy in the treatment of bipolar-II depression. Int Clin Psychopharmacol. 2007;22(5):309–11. doi: 10.1097/YIC.0b013e3280c28410. [DOI] [PubMed] [Google Scholar]
- 74.Amsterdam JD, Shults J. Efficacy and safety of long-term fluoxetine versus lithium monotherapy of bipolar II disorder: a randomized, double-blind, placebo-substitution study. Am J Psychiatry. 2010;167(7):792–800. doi: 10.1176/appi.ajp.2009.09020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 77.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739–52. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 78.Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462–70. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 79.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119(4):717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’ disease. Mov Disord. 2010;25(5):578–86. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 83.Lyons KE, Pahwa R. Deep Brain Stimulation and Tremor. Neurotherapeutics. 2008;5(2):331–338. doi: 10.1016/j.nurt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5(2):320–330. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133(Pt 1):215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 86.Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7(7):605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- 87.Zahodne LB, Okun MS, Foote KD, et al. Cognitive declines one year after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change. Clin Neuropsychol. 2009;23(3):385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Voon V, Krack P, Lang AE, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. 2008;131(Pt 10):2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katz MM, Bowden CL, Frazer A. Rethinking depression and the actions of antidepressants: uncovering the links between the neural and behavioral elements. J Affect Disord. 120(1–3):16–23. doi: 10.1016/j.jad.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 91.Frank E, Kupfer DJ, Wagner EF, et al. Efficacy of interpersonal psychotherapy as a maintenance treatment of recurrent depression. Contributing factors. Arch Gen Psychiatry. 1991;48(12):1053–9. doi: 10.1001/archpsyc.1991.01810360017002. [DOI] [PubMed] [Google Scholar]
- 92.Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48(6):593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- 93.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 94.Siegle GJ, Steinhauer SR, Thase ME, et al. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 95.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 96.Segal ZV, Kennedy S, Gemar M, et al. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63(7):749–55. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- 97.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–77. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 98.Elliott R, Rubinsztein JS, Sahakian BJ, et al. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 99.Fava GA, Savron G, Grandi S, et al. Cognitive-behavioral management of drug-resistant major depressive disorder. J Clin Psychiatry. 1997;58(6):278–82. doi: 10.4088/jcp.v58n0608b. quiz 283–4. [DOI] [PubMed] [Google Scholar]
- 100.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 101.Farb NA, Anderson AK, Mayberg H, et al. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carpenter WT, Bustillo JR, Thaker GK, et al. The psychoses: cluster 3 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med. 2009;39(12):2025–42. doi: 10.1017/S0033291709990286. [DOI] [PubMed] [Google Scholar]
- 103.Conn PJ, Tamminga C, Schoepp DD, et al. Schizophrenia: moving beyond monoamine antagonists. Mol Interv. 2008;8(2):99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- 104.Starcevic V, Brakoulias V. Symptom subtypes of obsessive-compulsive disorder: are they relevant for treatment? Aust N Z J Psychiatry. 2008;42(8):651–61. doi: 10.1080/00048670802203442. [DOI] [PubMed] [Google Scholar]
- 105.Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(2):228–38. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]