Abstract
Cryptosporidium parvum induces the formation of an actin-dense plaque which is essential for the successful invasion of epithelial cells. Host molecules that are involved in the regulation of this cytoskeleton reorganization are unknown. Here we identified that calcium-dependent thiol protease calpain is critical for regulating parasite-induced actin polymerization. C. parvum invasion induced activation of calpain. Inhibition of calpain activity by overexpression of the endogenous inhibitor calpastatin diminished the formation of the actin-dense plaque and decreased the initial invasion of parasites. Our data indicates a key role of calpain activity of host cell in C. parvum infection via regulating cytoskeleton reorganization.
Keywords: CRYPTOSPORIDIUM PARVUM, CALPAIN, INVASION
1. Introduction
Cryptosporidiosis is a clinically significant human enteric infection associated with acute diarrhea in the general population. Cryptosporidium infection can be life-threatening in individuals with immunodeficiencies such as AIDS patients, because of severity and chronicity of the diarrhea, and lack of effective therapy [1]. Infection begins with ingestion of oocysts, which sporulate in the intestine releasing four infectious sporozoites. Sporozoites then invade the apical surface of the intestinal epithelium to initiate infection. The sporozoites do not penetrate deeply into the host cells but rather stays in an intracellular but extracytosolic niche separated from the enterocyte cytosol by an electron-dense band whose formation is characterized by the deposition of host actin during the initial invasion [2].
Some studies have revealed the importance of calcium signaling in Cryptosporidium parvum infection of host cells [3, 4]. Calcium depletion with EDTA inhibits the formation of actin-dense plaques and blocks the early invasion of the parasites [4]. Calpain are intracellular Ca2+-activated cysteine proteases. Members of the calpain family are believed to function in various biological processes, among them, cytoskeletal remodeling [5]. There are two ubiquitous calpain isoforms that are defined by their in vitro requirement of calcium for activation: μ-Calpain (calpain 1) requires calcium concentrations in the micromolar range for activation, and m-calpain (calpain 2) requires calcium concentrations in the millimolar range. Calpain 1 and calpain 2 comprise the large 80-kDa catalytic subunits of separate heterodimers; each isoform associating with a common 30-kDa small subunit, calpain 4 [6, 7]. Both forms (μ- and m-calpain) are inactive heterodimers undergoing autolytic activation in response to Ca2+ [8, 9]. In this study, we examined the role of a calcium-dependent thiol protease calpain of host cells in the early invasion of C. parvum.
2. Material and Methods
2.1. Cell lines and parasites
Human intestinal adenocarcinoma Caco-2 line was purchased from American Type Culture Collection (ATCC). Calpastatin overexpression Caco-2 cell line CI.11 and empty vector-transfected CI.9 control line were kindly provided by Dr. Ira Herman (Tufts Medical School, MA). Cells were grown in DMEM medium containing 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin G, and 50 μg/ml streptomycin sulfate. For infection, cells were transferred to 6-well plates and monolayers grown to 80% confluence in 5% CO2 and 95% humidity. C. parvum isolate MD [10] were propagated in our laboratory. Approximate 106 excysted oocysts excysted using 0.8% sodium taurocholate added to monolayers and sporozoites were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) in some experiments [11].
2.2. Flow cytometry and confocal microscopy
Flow cytometry was performed using FACSCalibur and CellQuest software (BD Biosciences, Mountain View, CA). Cell monolayers were inoculated with newly excysted CFSE-labeled sporozoites or mock-inoculated with an equal amount of heat-killed parasites. For FACS analysis, cells were detached by treating them with 0.05% trypsin and 0.02% EDTA solution (Invitrogen) at 37°C in a C O2 incubator for 5 to 10 min. Cells were collected, washed twice with PBS and fixed with 4% formaldehyde in PBS for 20 minutes before being analyzed by a flow cytometer. In some experiments, unlabeled sporozoites were used to infect cells. Cells were fixed and permeabilized for 15 minutes with 0.5% Triton X-100 in PBS containing 1% BSA before wash and incubation with Cryptosporidium-specific monoclonal antibody JF4 (generated in our laboratory) for 15 min at room temperature. Cells were washed and then incubated with PE conjugated goat anti-mouse secondary antibody (BD Biosciences) before FACS analysis. For confocal imaging analysis, cells on coverslips were exposed to CFSE-labeled parasites for 1 hour. The cells were then washed, permeabilized, and fixed followed by Alexa-568-conjugated pholoidin (Invitrogen) staining. The actin polymerization was visualized under a Leica TCS SP2 laser-scanning microscope (Leica Microsystems Inc., Bannockburn, Illinois) and data were analyzed using Leica LcsLife confocal software.
2.3. Calpain activity
Calpain activity was measured using a fluorogenic calpain substrate succinyl-leucyl-leucyl-valyl-tyrosyl-7-amino-4-methylcoumarin (suc-LLVY-AMC) as described previously [12]. For determining calpain autocleavage, cells were lysed and western blotting was performed using specific primary antibody against N-terminus of domain I of Calpain 1 (EMD biosciences) and detected using horseradish peroxidase-conjugated secondary antibodies (Invitrogen).
3. Results
3.1. Calpain is activated during the initial invasion of C. parvum
First, we investigated the calpain activation during the initial invasion of the parasite. C. parvum invasion induced a time-dependent autolysis of domain I of the 80 kDa catalytic subunit of calpain 1 (μ-form), whereas the 30 kDa of regulatory subunit remained intact (Fig. 1A). The mock infection by heat inactivated C. parvum failed to induce the cleavage of calpain, suggesting that the active invasion was necessary for the induction of autolysis. Moreover, the calpain activity of Caco 2 cells was increased to over three fold when exposed to live C. parvum for 1 hour as compared to medium treatment (Fig. 1B). The calpain activity of the cells essentially remained unchanged when exposed to the same amount of heat killed C. parvum (Fig. 1B). Therefore these data indicated that the active invasion of C. parvum induced the activation of calpain in the host cells.
Fig.1.
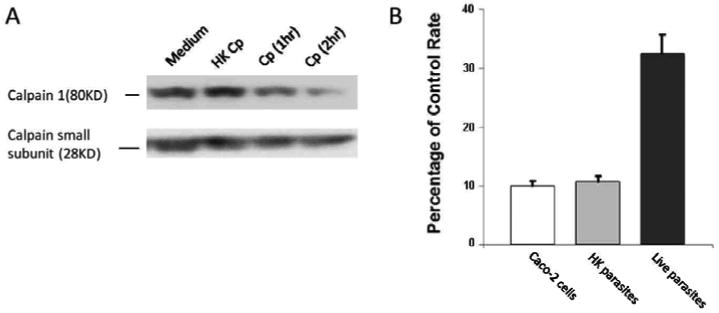
Calpain activity during the initial invasion of C. parvum.
A. Western blot analysis showing time dependent autolysis of the 80 kDa of catalytic subunit of calpain 1 after initial C. parvum (Cp) infection. B. Calpain activity of Caco-2 cells exposed to heat killed or live C. parvum for 1 hour. The data are representative of three independent experiments.
3.2. Calpain activity is associated with C. parvum-induced formation of actin dense plaque
Next we investigated whether calpain activity plays some roles in C. parvum-induced formation of actin dense plaque in host cells. We employed a calpastatin-overexpressed Caco 2 cell line CI.11, which has a decreased expression of calpain 1 and consequently a diminished calpain activity as compared to empty plasmid-transfected control CI.9 cells [13]. As shown in Fig. 2, C. parvum invasion of CI.9 cells induced actin polymerization at the site of infection whereas heat-killed parasites did not (Fig. 2A and 2B). C. parvum invasion of CI.11 cells also induced formation of actin dense plaque as determined by fluorochrome-conjugated phalloidin staining (Fig. 2C, pointed by arrows). However, the intensity of red fluorescence (actin staining) that overlaid with green fluorescence (sporozoite staining) in CI.11 cells was significantly lower than that in CI.9 cells (Fig. 2B and 2C, histograms), indicating that calpastatin overexpression cells had a diminished ability to form actin dense plaque during the initial invasion of C. parvum.
Fig.2.
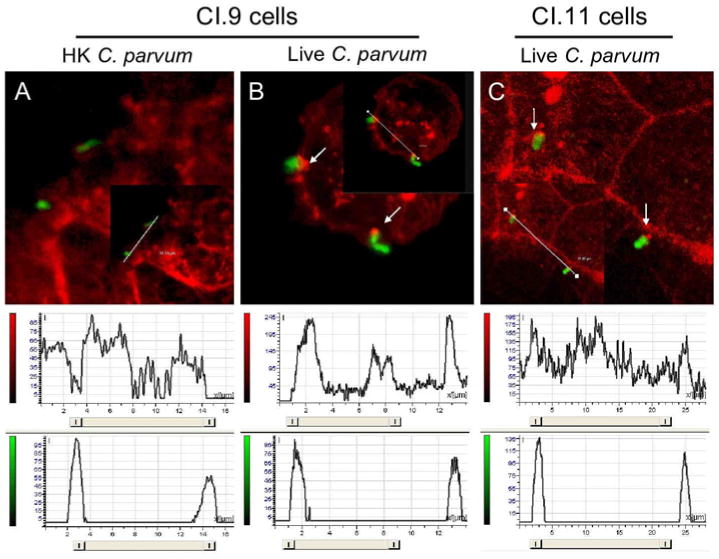
Analysis of actin-dense plaque formation at the site of C.parvum infection by confocal microscopy. Red fluorescence (actin staining). Green fluorescence (sporozoite staining). Below, histograms from fluorescence intensity profiles along the white lines as analyzed by Leica LcsLife confocal software. A. CI.9 cells exposed to heat killed C. parvum sporozoites. B. CI.9 cells exposed to live C. parvum sporozoites. C. CI.11 cells exposed to live C. parvum sporozites. The data are representative of three independent experiments.
3.3 Blocking calpain activity inhibits the host invasion of C. parvum
Because the induction of actin dense plaque is critically important for the host cell invasion by C. parvum, we investigated the infectivity of C. parvum in calpastatin overexpression CI.11 and its control CI.9 cells. Figure 3A shows the infection rate of CFSE-labeled C. parvum sporozoites to the culture cells after 1 hour of incubation. It is was detected a significant higher percentage of CFSE-positive cells in live C. parvum infected CI.9 cells compared to that in CI.11 cells, as determined by FACS analysis, whereas heat killed parasites had similar non-specific binding between these cells (Fig. 3A). Similar results were observed when cells were exposed to the parasites for a longer time (Fig. 3B). Exposure to cells with C. parvum for 8 or 24 hours resulted in a higher infection rate in both cell lines, as determined by specific anti-Crytosporidium antibody staining and FACS analysis (Fig. 3B).
Fig.3.
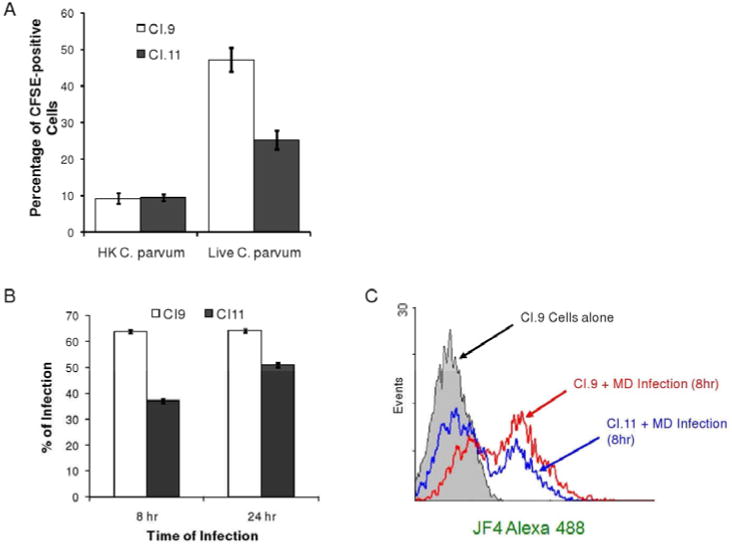
Infection rate of CFSE-labeled C. parvum sporozoites in CI.9 and CI.11 cells. A. Infection rate of heat killed and live C. parvum sporozoites in CI.9 and CI.11 cells after 1 hour of incubation. B. Infection rate of live C. parvum sporozoites in CI.9 and CI.11 cells after 8 and 24 hours of incubation. C. FACS analysis of CI.9 and CI.11 cells infected with live C. parvum sporozoites after 8 hours. The data are representative of three independent experiments.
4. Discussion
In this study we examined the role of calpain in host cell invasion by Cryptosporidium parvum sporozoites. Calpain has been implicated in cytoskeletal remodeling, including disruption of cell-matrix interactions at the rear of the cell during crawling [14] and lamellipodial and protrusion formation during spreading [12]. These examples illustrate the role of calpain in remodeling dynamic actin filament structures at the periphery of the cell. Since C. parvum infection induces host cell cytoskeleton reorganization [2] we investigated the calpain activation during the initial invasion of C. parvum. It is widely known that both forms of calpain (μ- and m-calpain) are inactive heterodimers undergoing autolytic activation in response to Ca2+ [8, 9]. In our study we found that the active invasion of C. parvum was necessary for the induction of autolysis since the mock infection by heat inactivated parasites failed to induce the cleavage of calpain. Indeed, this active invasion induced a time-dependent autolysis of domain I of the 80 kDa catalytic subunit of calpain 1leading to its activation in the host cell.
As it has been pointed out before, for a successful Cryptosporidium invasion the host cell cytoskeleton needs to be reorganized. A unique structure is formed at the host-parasite interface during invasion, containing an electron-dense band. Several studies indicate that this dense plaque is a sharply circumscribed aggregation of F-actin that is intimately associated with the host cell plasma membrane, particularly early in the invasion process. An approach was developed to study the role of calpain in cytoskeletal remodeling was to create stable transfectants of established cell lines that overexpress calpastatin, calpain's physiological inhibitor [12]. Calpastatin inhibits the ubiquitous calpain isoforms calpain 1 and 2. Calpastatin is specific for calpain, regulates no other protease and is the inhibitor of choice for implicating calpain in biological processes. Thus, the data indicated that the calpain activation is important for the parasite-induced actin polymerization and the formation of actin-dense plaque. These results demonstrated that C. parvum had a diminished ability to invade cells with a low calpain activity.
Taken together, the results shown in this study suggest that calpain activation plays an important role in the early invasion of C. parvum. Actin-dense plaque formation in the host cells as a response of the initial parasite invasion is depending upon calpain activation in the host cell. Calpain activation is Ca2+-dependent but the intracellular Ca2+ level, which is generally 1 μM at most, even in stimulated cells, and never reaches the high concentration range at which the calpains become active [5]. These authors suggest that maybe other molecules such as protein inhibitors as well as phospholipids [15] are required to modulate the Ca2+-sensitivity of calpains. Perhaps, calpain activation in the host cell during initial C. parvum invasion is modulated by apical organelle discharge releasing a complex mixture of molecules which are contained in these secretory vesicles. This apical organelle discharge has been demonstrated to be involved in the invasion of apicomplexan parasites such as Toxoplasma gondii and Plasmodium spp., [16] but the mechanisms of apical organelle discharge by C. parvum and its role in host cell invasion are unclear. The inhibition of apical organelle discharge by C. parvum sporozoites blocks parasite invasion but not attachment to host cells [17]. The role of apical organelle discharge in the parasite invasion and its possible involvement in the host cell calpain activation might be supported by the findings in the present study. We found that live, but not heat-inactivated parasites induced the cleavage of calpain, suggesting that the active invasion was necessary for the activation of calpain in Caco 2 cells. Further research will be required to clarify the mechanisms involved in host cell calpain activation by the active invasion of C. parvum.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI071300 and K01DK076549.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tzipori S. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:187–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 2.Elliot DA, Clark DP. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infect Immun. 2000;68:2315–2322. doi: 10.1128/iai.68.4.2315-2322.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu G, Keithly JS. Molecular analysis of a P-Type ATPase from Cryptosporidium parvum. Mol Biochem Parasitol. 1997;90:307–316. doi: 10.1016/s0166-6851(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 4.Chen XM, O'Hara PO, Huang BQ, Nelson JN, Lin JJ, Zhu G, Ward HD, Larusso NF. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton and intracellular calcium dependent and required for host cell invasion. Infect Immun. 2004;72:6806–6816. doi: 10.1128/IAI.72.12.6806-6816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khorchid A, Ikura M. How calpain is activated by calcium. Nat Struct Bio. 2002;9:239–241. doi: 10.1038/nsb0402-239. [DOI] [PubMed] [Google Scholar]
- 6.Carafoli E, Molinari M. Calpain: a protease in search of a function? Biochem Biophys Res Commun. 1998;247:193–203. doi: 10.1006/bbrc.1998.8378. [DOI] [PubMed] [Google Scholar]
- 7.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Tsuji S, Kubota S, Kimura Y, Imahori K. Autolysis of calcium-activated neutral protease of chicken skeletal muscle. J Biochem. 1981;90:275–278. doi: 10.1093/oxfordjournals.jbchem.a133656. [DOI] [PubMed] [Google Scholar]
- 9.Melloni E, Pontremoli S. The Calpains. Trends Neurosci. 1989;12:438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- 10.Okhuysen PC, Rich SM, Chappell CL, Grimes KA, Widmer G, Feng X, Tzipori S. Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-gamma knockout mice. J Infect Dis. 2002;185:1320–1325. doi: 10.1086/340132. [DOI] [PubMed] [Google Scholar]
- 11.Feng H, Nie W, Bonilla R, Widmer G, Sheoran A, Tzipori S. Quantitative tracking of Cryptosporidium infection in cell culture using CFSE. J Parasitol. 2006;74:3342–3346. doi: 10.1645/GE-853R.1. [DOI] [PubMed] [Google Scholar]
- 12.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, Mier JW, Maki M, Herman IM. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potter DA, Srivangam A, Fiacco KA, Brocks D, Hawes J, Herndon C, Maki M, Acheson D, Herman IM. Calpain regulates enterocyte brush border actin assembly and pathogenic Escherichia coli-mediated effacement. J Biol Chem. 2003;278(32):30403–30412. doi: 10.1074/jbc.M304616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttenlocher A, Palecek SP, Lu Q, Zhan W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Biol Chem. 1997;272:32719–32722. [Google Scholar]
- 15.Saido TC, Shibata M, Takenawa T, Murogushi H, Suzuki K. Positive regulation of mu-calpain action by polyphosphoinositides. J Biol Chem. 1992;207:24585–24590. [PubMed] [Google Scholar]
- 16.Sam-Yellowe TY. Rhaptory organellea of the Apicomplexa: their role in host cell invasion and intracellular survival. Parasitol. Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 17.Potter DA, Srirangam A, Fiacco KA, Brocks D, Hawes J, Herndon C, Maki M, Acheson D, Herman IM. Calpain regulates enterocyte brush border actin assembly and pathogenic Escherichia coli-mediated effacement. J Biol Chem. 2003;278:30403–30412. doi: 10.1074/jbc.M304616200. [DOI] [PMC free article] [PubMed] [Google Scholar]


