Abstract
Tumor Endothelial Marker 8 (TEM8) is an integrin-like cell surface protein upregulated on tumor blood vessels and a potential vascular target for cancer therapy. Here, we found that the ability of an anti-TEM8 antibody, clone SB5, to recognize the extracellular domain of TEM8 on the cell surface depends on other host-cell factors. By taking advantage of SB5’s ability to distinguish different forms of cell-surface TEM8, we identified alpha-smooth muscle actin and transgelin, an actin binding protein, as intracellular factors able to alter TEM8 cell surface structure. Overexpression of either of these proteins in cells converted TEM8 from an SB5-exposed to an SB5-masked form and protected cells from SB5-saporin immunotoxins. Because the predominant form of TEM8 on the cell surface is not recognized by SB5, we also developed a new monoclonal antibody, called AF334, which is able to recognize both the SB5-exposed and the SB5-masked forms of TEM8. AF334-saporin selectively killed TEM8-positive cells independent of TEM8 cell surface structure. These studies reveal that TEM8 exists in different forms at the cell surface, a structure dependent on interactions with components of the actin cytoskeleton, and should aid in the rational design of the most effective diagnostic and therapeutic anti-TEM8 monoclonal antibodies.
Keywords: Angiogenesis, endothelial, actin cytoskeleton, TEM8, ANTXR1
1. Introduction
Tumor endothelial Marker 8 (TEM8) is an 85kDa integrin-like cell surface receptor that was originally identified as one of several unrelated genes (called TEM1-TEM9) overexpressed in vascular endothelial cells derived from tumor versus normal colorectal tissues [1]. Subsequent studies have shown that TEM8 is overexpressed in the blood vessels of a variety of human solid tumor types in addition to colorectal cancer [1, 2]. TEM8 is highly conserved, and mouse TEM8 protein, which shares 98% amino acid identity with human TEM8, is also overexpressed in mouse tumor vessels [3]. TEM8 was unique among the original TEMs identified in that it was not detected in the angiogenic vessels of adult ovaries, and in TEM8−/− knockout mice developmental angiogenesis appeared unaffected [1, 2, 4]. However, in tumor challenge studies tumor growth was impaired in TEM8 knockout compared to wildtype mice [4]. Together, these studies suggest that host-derived TEM8 promotes pathological but not physiological angiogenesis. Such specificity makes TEM8 an appealing target for the development of novel anti-angiogenic or vascular disrupting agents. Indeed, current anti-angiogenic agents which have been approved for clinical use can not separate physiological and pathological angiogenesis, and several side effects associated with the use of these agents have been reported [5].
Insights into the physiological functions of TEM8 are beginning to emerge. In vitro studies suggest that TEM8 can bind collagens, such as collagen I and collagen VI which, in turn, can promote the migration of endothelial cells [2, 6]. Migration of cells on extracellular matrix is dependent on actin cytoskeleton reorganization, and recent studies suggests that the TEM8 cytosolic domain may link extracellular matrix molecules to the actin cytoskeleton [7, 8]. However, it is unclear which components of the actin cytoskeleton are involved in binding TEM8 under physiological conditions, and how this binding contributes to TEM8 function. TEM8 may also be involved in collagen uptake through an endocytosis-mediated degradation pathway, as TEM8 knockout mice are viable but display an excess buildup of collagen in select organs [4].
TEM8 shares 58% amino acid identity with CMG2, another cell surface receptor that binds extracellular matrix (ECM) proteins, in this case laminin and collagen type IV [9]. Both TEM8 and CMG2 share an integrin-like von Willebrand factor A domain in their extracellular region. TEM8 and CMG2 have both been found to bind anthrax toxin proteins [10, 11], and have therefore been given the alternative names anthrax toxin receptor 1 (ANTXR1) and ANTXR2, respectively. Protective antigen (PA) is the subunit of anthrax toxin responsible for binding TEM8 or CMG2, and the PA-receptor interaction is critical for toxin entry into cells.
Owing to its high expression in tumor versus normal vessels, TEM8 has been considered as a potential target for anti-tumor therapy based on an anti-angiogenic or vascular targeting approach [12]. Several recent preclinical studies support the idea that TEM8 functions to promote tumor growth and that inhibition of TEM8 may represent a useful anti-tumor strategy. First, a soluble TEM8-Fc fusion protein containing the extracellular domain of TEM8 fused to the Fc region of mouse IgG was found to have potent tumoricidal activity against a variety of human tumor xenografts, presumably by competing for endogenous TEM8 ligand(s) [13]. Second, DNA vaccines against TEM8 have slowed tumor growth in vivo [14, 15]. Third, melanoma tumor growth was found to be impaired in TEM8-deficient mice [4]. Finally, anthrax toxin proteins have been shown to possess potent tumoricidal activity in a number of preclinical studies when judiciously administered at sub-toxic doses [16–18], an activity that appears to be mediated primarily through targeting of the tumor vasculature [19, 20]. Although TEM8, CMG2 or both receptors may be responsible for the anti-tumor activity of anthrax toxin proteins, antibody-based therapeutics directed against a single receptor could potentially have similar efficacy with less toxicity.
In exploratory studies aimed at identifying anti-TEM8 antibodies that may be useful for vascular targeting, we evaluated various antibodies developed against the extracellular domain of TEM8 including those commercially available and the so called “SB” series of anti-TEM8 mAbs [2]. Surprisingly, none of the antibodies tested recognized the predominant form of TEM8 expressed on the surface of live cells, although some of the SB antibodies could recognize a cryptic population of TEM8 expressing cells. Based on its similarity to integrins, which are known to harbor both open and closed conformations, we hypothesized that TEM8 may also have more than one conformation at the cell surface, and that its conformation may be regulated by the expression of other host-cell factors. Here, we set out to identify these host-cell factors by using the SB5 antibody in an unbiased genetic screen, and identified components of the actin cytoskeleton as critical dominant-acting factors capable of regulating TEM8 structure at the cell surface. We also describe a new anti-TEM8 antibody, called AF334 that is able to recognize all forms of TEM8 on the surface of live cells and is a potential tool for TEM8-based therapeutic targeting.
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
293 cells, DLD-1, SK-BR-3, HUVEC and T/G HA-VSMC were from ATCC, HT29 was from the DCTD Tumor Repository at NCI (Frederick, MD), HAECs were from Coriell (Camden, NJ), HMECs were from the CDC (Atlanta, GA), and coronary SMC and uterine SMC were from Lonza (Walkersville, MD). CHO-PR230 (CHO) cells, CHO/TEM8 cells and CHO/CMG2 cells, a generous gift from Dr. Stephan H. Leppla, NIAID, were maintained in Ham’s F12 medium supplemented with 10% FBS unless indicated otherwise. Endothelial cells were cultured in EBM-2 (Lonza), smooth muscle cells in SmGM-2, (Lonza), and all other cells in DMEM supplemented with 10% fetal bovine serum. Endothelial and smooth muscle cell purity was confirmed by RT-PCR using various lineage-specific markers as described in Fig 5.
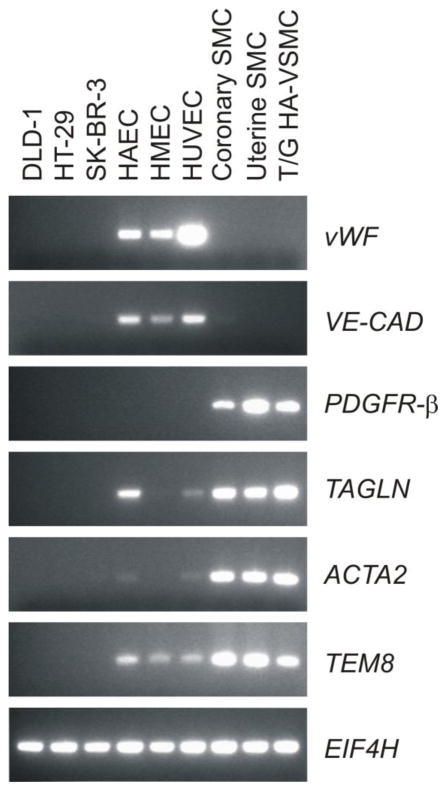
FIGURE 5.
TEM8 is expressed by both smooth muscle cells and endothelial cells. RT-PCR revealed expression of TEM8 in both endothelial cells (HAEC, HMEC and HUVEC) and smooth muscle cells (coronary, uterine and T/G HA-VSMC) but not in colon (DLD-1 and HT29) or breast (SK-BR-3) tumor cells lines. The smooth muscle cell marker PDGFR-β and the endothelial markers von Willebrand factor (vWF) and VE-cadherin (VE-CAD) were used to confirm the purity of the cells. Eukaryotic translation initiation factor 4H (EIF4H) was used as a loading control.
2.2. Incorporation of a FLAG-tag onto TEM8
A two-step PCR-based strategy was used to incorporate a 3xFLAG-tag onto the N-terminus of TEM8 immediately following the signal peptide. In the first step, two separate PCR products were generated on either side of the FLAG insertion site using as template a TEM8/pcDNA3.1 vector containing a CMV-driven full length human TEM8 cDNA (sv1 isoform, Genbank Acc. # AF279145). The primers used for product A were: A-For: 5′-AGGCGTGTACGGTGGGAG-3′ and A-Rev: 5′-CTTGTCATCGTCATCCTTGTAATCGATGTCATGATCTTTATAATC ACCGTCATGGTCTTTGTAGTCCCCGGCGCAGATGAGC-3′ and for product B were: B-For: 5′-GATTACAAGGATGACGATGACAAGCAAGGGGGACGCAGGG-3′ and B-Rev: 5′-CTGGTGAAGTTGATGCAGCG-3′. The primers included a 3x-FLAG tag DNA sequence as indicated by bold lettering, and a small 24bp complementary region of overlap between the two products (underlined). After PCR amplification, the two PCR products were gel purified, mixed, and used as a template in a second PCR reaction that utilized the outside primers: A-For and B-Rev. The resultant PCR product containing the FLAG sequence, product C, was then digested with the restriction enzymes BamH1 and EcoR1 and gel purified to remove the amplicon ends. The parent TEM8/pcDNA3.1 vector was also digested BamH1 and EcoR1, and the vector backbone gel purified. The PCR-generated purified product C was cloned into the vector backbone, and the resulting plasmid, called 3x-FLAG-T8/pcDNA3.1, was verified mutation-free by DNA sequencing.
2.3. Immunomagnetic Bead Selection
SB5 was biotinylated (Thermo Fisher Scientific, Rockford, IL) and mixed with Streptavidin M-280 magnetic Dynabeads (Invitrogen, Carlsbad, CA) to generate SB5-beads. Prior to positive cell selection with SB5-beads, cells were pre-incubated with streptavidin-beads pre-bound to biotin-labeled non-specific IgG to remove any non-specific binders. Following positive selection, cells were expanded and passaged until no bead-bound cells were visible, and the selection was repeated until all cells were SB5-positive by flow cytometry.
2.4. Immunoblotting
Immunoblotting was performed as previously described [2]. Partial epitope mapping of the SB2, SB4, SB8 and SB12 mAbs was performed using GST-TEM8 peptide deletions as described for SB5 [4]. To evaluate cross-reactivity with CMG2, cellular lysates were derived from CHO-PR230 (CHO) cells, CHO/TEM8 cells and CHO/CMG2 cells. To evaluate cross-reactivity with mouse TEM8, mTEM8 cDNA was cloned into the pcDNA3/neo expression vector (Invitrogen), sequence verified, and stably transfected into 293 cells.
2.5. Immunoprecipitation
αSMA was cloned into the expression vector pcDNA3.1/hygro (Invitrogen) and stably transfected into 293/T8-SB5 cells. Total cell lysates were incubated overnight with SB5 anti-TEM8 antibodies, anti-αSMA antibody (Sigma, St. Louis, MO) or nonspecific mouse IgG control antibody at 4°C. Protein G-agarose beads (Roche, Indianapolis, IN) or streptavidin agarose beads (Sigma) were added and incubated at 4°C for 2 hrs. Precipitated proteins were separated by SDS-PAGE and detected by immunoblotting with either SB5 anti-TEM8 antibody, anti-αSMA antibody or anti-FLAG antibody (Sigma, M2 clone) followed by HRP-anti-mouse IgG F(ab′)2 fragment specific (Jackson Immunoresearch).
2.6. Biotinylation experiments
293, 293/TEM8c1, or 293/T8-SB5 cells grown on Poly-D-lysine coated plates were rinsed with cold PBS and labeled with 0.5mM sulfo-NHS-SS-biotin (Thermo Scientific- Pierce) in cold PBS for 40 min. at 4°C according to the manufactures protocol. Immunoprecipitation and immunoblotting of biotinylated proteins was performed as outlined above (section 2.4 and 2.5)
2.7. Immunofluorescence
Cells grown on poly-L-lysine treated chamber slides were chilled on ice and SB5 anti-TEM8 or anti-FLAG mAbs (M2 clone) were added directly to the growth medium for 30 min at 4°C. Cells were washed with cold medium, fixed in cold 4% paraformaldehyde in PBS and labeled with FITC-conjugated goat anti-mouse IgG. To detect TEM8 with SB5 (a mouse IgG1κ) and AF334 (a mouse IgMκ) simultaneously, in this case the primary antibodies were detected with either a DyLight 488-conjugated goat anti-mouse IgG1, Fcγ specific (Jackson, cat # 115-485-205) or a DyLight 594-goat anti-mouse IgM, μ chain specific antibody (Jackson, cat # 115-515-075). For dual-color staining of exogenous TEM8 and αSMA, stably transfected 293/FlagT8-SB5/αSMA cells were incubated with rat-anti-FLAG antibodies (Biolegend), permeabilized with 0.1% Triton-X 100, then incubated with mouse anti-α-SMA mAbs followed by a secondary layer of FITC-linked goat anti-rat and biotin-linked donkey anti-mouse IgG (Jackson) and a third layer of 488-linked donkey anti-FITC and Texas red-streptavidin (Vector). The specificity of staining was verified using the same staining strategy on parent 293 and 293/FlagT8-SB5 cells or by substituting the primary antibodies for isotype matched non-specific IgGs when staining 293/FlagT8-SB5/αSMA cells. Sections were counterstained with DAPI and immunofluorescent images captured using a Nikon Eclipse E600 microscope or a Zeiss LSM510 confocal microscope.
2.8. TEM8 Internalization Experiment
SB5 or control IgG labeled with Cy5.5 (Thermo Fisher Scientific) was added to the culture medium of cells grown on poly-L-lysine treated chamber slides and incubated at 37°C for 3 hr. On ice, cells were rinsed with PBS, fixed with 4% paraformaldehyde, and TEM8 present on the cell surface was detected by post-staining with FITC-conjugated goat anti-mouse IgG antibody.
2.9. Identification of Genes that Regulate SB5-Toxin Sensitivity by Expression Cloning
An MMLV-based cDNA library from uterus (ViraPortXR, Stratagene, Santa Clara, CA) was prepared and used to infect exponential-phase 293/FlagT8-SB5 cells on ten 100 mm plates according to the manufactures recommendations. Virus infected FlagT8-SB5 cells were treated with biotinylated-SB5 and 40 nM TSA and 5 nM saporin-streptavidin [Advanced Targeting Systems (ATS), San Diego, CA]. Surviving colonies were expanded and selected with magnetic Dynabeads (Pan-mouse IgG, Invitrogen) that had been pre-armed with anti-FLAG mAbs. After repeating the SB5-toxin treatment and SB5-bead selection once more, surviving cells were cloned by limiting dilution and genomic DNA from each clone was isolated using the DNeasy blood and tissue kit (Qiagen, Gaithersburg, MD). Each insert was identified by PCR using the primer pair, F: CAGCTTGGATACACGCCG, and R: TGCCAAACCTACAGGTGGG.
2.10. Cell Viability Assay
Cells were plated on poly-L-lysine treated 96-well plates overnight. The next day, biotinylated SB5 and saporin-streptavidin, mouse anti-FLAG mAbs and anti-mouse-IgG saporin conjugates (Mab-ZAP, ATS), or AF334 anti-TEM8 antibodies and anti-mouse IgM-saporin (Anti-M-ZAP, ATS) were pre-incubated together at RT for 20 min and then added to cells. 72hours later, medium was replaced with fresh medium containing 10% AlarmarBlue (Invitrogen) and fluorescence measured at 560nm/590nm (Ex/Em) in a spectrophotometer 4 hours later. Brightfield images were captured 72 hr or 96 hours post-treatment.
2.11. Flow Cytometry
Following trypsinization, at 4°C cells were rinsed in PBS/0.5%BSA, incubated in PBS/0.5%BSA containing primary mAbs, rinsed, incubated with PBS/BSA containing FITC-conjugated secondary (Jackson Immunoresearch, West Grove, PA), rinsed and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
2.12. RT-PCR
RT-PCR was performed using total RNA isolated from cells using RNeasy mini kit (Qiagen) and cDNA was synthesized using Superscript III 1st strand synthesis system (Invitrogen). The primer pairs used were, vWF-F: CGGCAGGTCATCCACGG, vWF-R: CGGACAGCTTGTAGTACCCAG; VE-Cad-F: TGCTAACCCTGCCCAACG, VE-Cad-R: CCTCTCAATGGCGAACACG; PDGFRβ-F: TGTCCCTGTCCGAGTGCTG, PDGFRβ-R: CCAGGATGGCTGAGATCACC; TAGLN-F: CCCATCCTGTCTGTCCGAAC, TAGLN-R: CACGCCATTCTTCAGCCAG; α-SMA-F: GCCGACCGAATGCAGAAG α-SMA-R: GGACATTCACAGTTGTGTGCTAG; TEM8-F: GCCAACGGTAGACGCCTC, TEM8-R: TAGGACCCACAAGGCATCG; Eif4H-F: CGTAGCCAGAAGGAGTTGCC, Eif4H-R: ATGTCCACACGAAGTGACCG.
2.13. Generation of AF334 Anti-TEM8 mAb
CHO cells and CHO cells transfected with full-length human TEM8 (CHO/TEM8) [21] were maintained in alpha MEM supplemented with glutamine, gentamicin, HEPES and hygromycin. CHO/TEM8 cells were enriched for high surface expression of TEM8 by three sequential rounds of flow cytometry sorting (BD FACSAria) by staining cells with PA. PA was prepared as described [22] and conjugated with sulfo-NHS-LC-biotin using a kit (Thermo Fisher Scientific). Cells were labeled in 0.5 mL PBS/1%BSA on ice for 15 min with 10 mcg/mL biotin-PA, rinsed with PBS, incubated with 2 mcg/mL streptavidin-R-PE (BioLegend, San Diego, CA) in PBS/1% BSA, rinsed, and resuspended with PBS. The highest 6% positive cells were selected in each sort. 2.5 million TEM8 surface-enriched CHO/TEM8 cells were administered by intraperitoneal injection in 0.2 mL PBS monthly to BALB/c female two month old mice. Monthly inoculations were repeated three additional times and splenocytes harvested three days after the last boost. Splenocytes were fused 1:1 with OUR-1 mouse myeloma cells in Opti-MEM in the presence of 50% PEG (Invitrogen) following the manufactures protocol. Hybridomas were grown in Opti-MEM supplemented with HAT and 10% FBS. Supernatants were screened by differential flow cytometry binding to CHO/TEM8 versus CHO cells using FITC conjugated goat anti-mouse Ig (Invitrogen). A secondary screen was done using an enzyme-linked immunoassay with membrane preparations made by sonication of CHO or CHO/TEM8 cells as previously described (21). The hybridoma showing the highest supernatant reactivity was subcloned three times by limiting dilution, expanded, cryopreserved, and designated AF334. The AF334 hybridoma produced an IgMκ which reacted specifically with CHO/TEM8 but not CHO or CHO/CMG2 cells by flow cytometry. AF334 also reacted with both 293/mTEM8 and 293/hTEM8 cells, but not 293 cells, demonstrating cross-reactivity with the mouse protein. The AF334 hybridoma maintained stable expression after weaning into serum free PFHM II medium (Invitrogen). Supernatants were concentrated and antibodies were purified by gel filtration.
3. Results
3.1. SB Antibodies are Specific for TEM8 and Recognize the Soluble Extracellular Domain
A panel of mouse mAbs, the “SB” series, was previously developed against the full extracellular domain of human TEM8 using purified recombinant proteins [2]. To determine if these mAbs cross-react with the mouse TEM8 protein (mTEM8), we tested them by western blotting using lysates of 293 cells transfected with a full-length mTem8 expression vector. Four of the five mAbs tested, called SB2, SB4, SB5 and SB12, reacted with both mouse and human TEM8, whereas SB8 was human-specific (Fig. 1A). High cross-species reactivity is not unexpected given the high level of similarity (98%) between the mouse and human proteins. We also tested SB mAbs by western blotting against cells transfected with CMG2, the closest homologue of TEM8, and found that none of the mAbs cross-react (Fig. 1A).
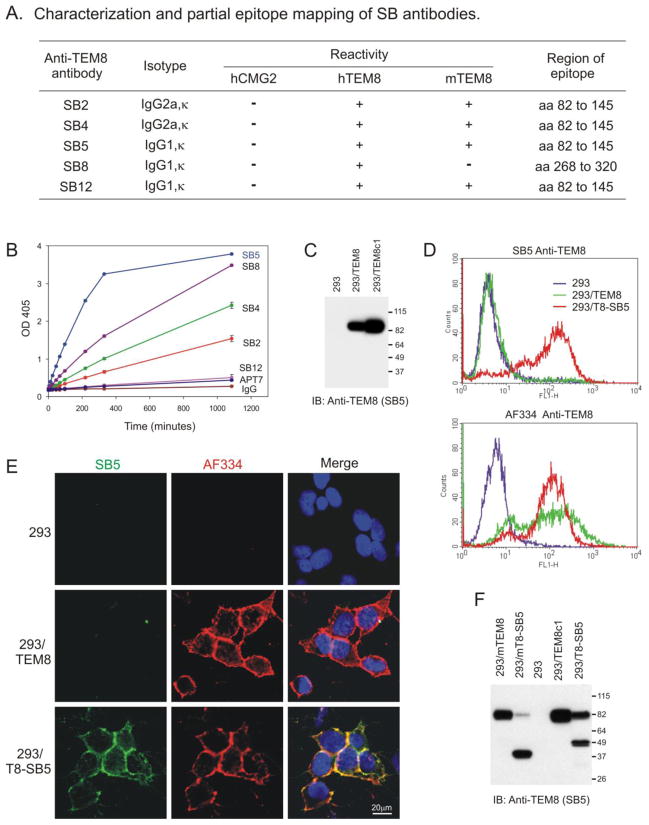
FIGURE 1.
SB antibodies recognize TEM8 at the cell surface following selection with SB5 antibodies. A, Table showing SB antibody isotypes, cross-reactivity with homologous proteins, and the amino acid (aa) region of TEM8 (Genbank No. AF279145) containing the SB epitopes. B, ELISA used to measure reactivity of SB mAbs with soluble AP-TEM8 fusion protein. C, Western blot showing strong reactivity of SB5 with an ~85kDa product from a stable TEM8 transfected pool (293/TEM8), or a high expressing clone (293/TEM8c1) derived from the pool. D, SB5 mAbs failed to detect TEM8 on the surface of 293 or 293/TEM8 cells by flow cytometry whereas 293/TEM8 cells selected with SB5-immunomagnetic beads (293/T8-SB5) were strongly labeled (D, upper panel). AF334 anti-TEM8 antibody was able to detect TEM8 on both 293/TEM8 and 293/T8-SB5 cells (D, lower panel). E, Co-immunofluorescence staining demonstrates lack of reactivity of SB5 with 293/TEM8 cells, but strong reactivity (green) with 293/T8-SB5 cells. AF334 anti-TEM8 antibody stained both 293/TEM8 and 293/T8-SB5 cells (red), and co-localized with the SB5 signal in 293/T8-SB5 cells (merged yellow stain). Co-immunofluorescence staining of nuclei with DAPI (blue) revealed similar cell numbers in each group. F, Smaller products of ~35 to 50 kDa were observed in SB5-selected 293 cells expressing exogenous mouse (293/mT8-SB5) or human (293/T8-SB5) TEM8.
Next, we developed a capture ELISA to determine if any of the SB mAbs could bind the native extracellular domain of TEM8 fused to alkaline phosphatase (AP-TEM8). Although several mAbs were able to bind soluble AP-TEM8 fusion protein, SB5 bound best and none of the mAbs reacted with the control proteins AP-TEM7 or AP alone (Fig. 1B). SB5 antibody also worked best for western blotting and immunoprecipitation of TEM8, without any apparent cross-reactivity to other proteins [see Fig. 1C and [2]]. However, each of the SB mAbs failed to detect significant levels of cell-surface TEM8 on 293 cells stably transfected with a full-length TEM8 expression vector (293/TEM8; Fig. 1D and E). We also tested several commercially available antibodies each of which failed to detect native TEM8 at the cell surface1. The lack of cell surface staining was not cell type specific because these antibodies also failed to detect TEM8 on the surface of TEM8-positive primary endothelial cells and TEM8 transfected CHO cells1. Despite this lack of staining, TEM8 was presumably present on the surface of 293/TEM8 cells based on cell surface labeling with non-permeable biotin (Fig. S1) and subsequent studies which employed a tagged version of the receptor and a newly developed antibody (see below). Based on this, we hypothesized that the epitope for SB5 and other currently available anti-TEM8 antibodies is normally masked on the surface of 293/TEM8 cells.
3.2. SB5 Antibodies Recognize a Cryptic Subpopulation of 293/TEM8 Cells
While performing immunofluorescence staining for cell surface TEM8 in 293/TEM8 cells using SB5 antibodies we noticed a very small fraction of the cells (<0.5%) were strongly positive, while 293 parent cells were completely negative. These positive cells were not apparent by flow cytometry analysis due to their low frequency. In order to determine if this rare fraction of the 293/TEM8 parent population could be enriched, we purified these cells using SB5-linked magnetic beads. After expanding the SB5-bead bound cells in culture and repeating the selection and expansion 3 more times, we were able to obtain a variant subline, called 293/hT8-SB5, that uniformly reacted with SB5 mAbs by both immunofluorescence and flow cytometry (Fig. 1D and E). Likewise, when we repeated the SB5-selection using 293 cells transfected with mouse TEM8 (293/mT8-SB5) again we were able to derive sublines, this time with mTEM8 detectable on the cell surface, while parallel control selections performed on parent 293 cells failed to result in any enrichment. Importantly, SB8 human-specific anti-TEM8 mAbs labeled the cell surface of 293/hT8-SB5 cells similar to SB5 (Fig. S2) but failed to detect mouse TEM8 on the surface of 293/mT8-SB5 cells1. Because SB5 and SB8 recognize independent epitopes, this result confirmed the specificity of these antibodies for TEM8. Preliminary mapping of the SB antibody binding sites using peptide deletions of the TEM8 extracellular domain revealed that SB5 and SB8 mAbs recognize distinct epitopes separated by at least 123 amino acids (Fig. 1A). Immunoblotting with several independent SB mAbs also revealed smaller 35 to 50kDa products in the SB5-selected cells (Fig. 1F) which presumably represent intracellular degradation products of the TEM8 extracellular domain because these fragments, unlike the full-length 85kDa product, were not labeled with biotin immediately following cell surface biotinylation (Fig. S1). Throughout these studies the small TEM8 fragments were always observed in cells that displayed surface-exposed SB5 binding sites, but never in cells with an SB5-masked form of TEM8, thus providing an index of SB5 accessibility.
3.3. SB5 Anti-TEM8 Antibodies are Internalized
Anthrax toxin proteins bind TEM8 or CMG2 and are taken up into cells through a highly regulated endocytosis-mediated process [23, 24], but internalization of anti-TEM8 antibodies has not yet been described. To determine if SB5 mAbs could be taken up by 293/T8-SB5 cells, we labeled SB5 with Cy5.5 and followed its uptake in live cells. Cy5.5-SB5 (red channel) could be readily observed inside cells as early as 30 minutes following treatment, and most was taken up into the cells by 3 hours (Fig. 2A). Some Cy5.5-SB5 signal (red) could also be detected on the surface 3 hours following internalization, which was verified by fixing the cells and amplifying the surface bound Cy5.5-SB5 signal with a FITC-labeled anti-mouse secondary antibody (green).
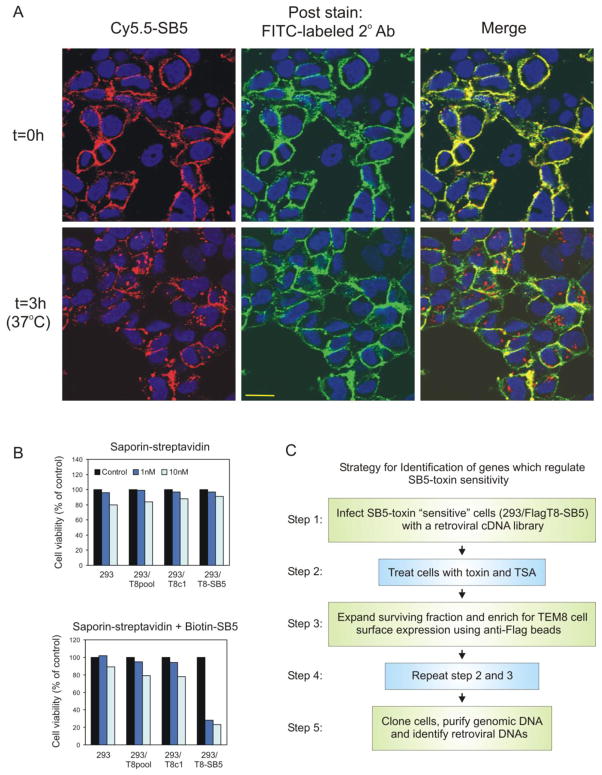
FIGURE 2.
SB5-saporin immunotoxins are internalized and selectively kill 293/T8-SB5 cells. A, Cy5.5-labeled SB5 (red) was taken up into 293/T8-SB5 cells after 3 hours at 37°C. Some of the Cy5.5-SB5 label was detected on the cell surface following the 3 hour incubation, which was confirmed by post-incubation staining with FITC-labeled secondary mAbs (green). The secondary antibody only recognizes primary Cy5.5-SB5 antibody that is present at the cell surface and provides extra sensitivity because of the added layer of amplification. Although some Cy5.5-SB5 antibody was detected at the cell surface, much of the primary antibody had clearly internalized (red, bottom right panel). B, 1 nM or 10 nM of saporin-streptavidin toxin combined with biotin-labeled SB5 selectively killed 293/T8-SB5 cells (bottom panel) compared to saporin-streptavidin alone (top panel). C, Strategy for identification of genes that regulate SB5-toxin sensitivity.
To determine if 293/T8-SB5 cells were sensitive to anti-TEM8 immunotoxin, we treated cells with biotinylated SB5 antibody and streptavidin-saporin. Saporin is a type I ribosome-inactivating protein that has no known specificity in mammalian cells and can be internalized only if conjugated to an appropriate antibody. 72 hours following treatment with 1 nM of SB5-saporin toxin, viability of 293/T8-SB5 was reduced by ~70% while both 293 and 293/TEM8 cells were unaffected at this concentration (Fig. 2B). Thus, saporin-conjugated SB5 mAbs are selectively toxic to 293/T8-SB5 cells following binding and internalization.
3.4. Development of a Genetic Screen to Identify Host-Cell Factors that Regulate TEM8 Structure
The above results suggested that the predominant form of TEM8 on the cell surface contains a masked SB5 binding site. Based on this, we hypothesized that additional factors may be present in 293/TEM8 cells, which are reduced or absent from SB5-selected 293/T8-SB5 cells and are required for maintenance of TEM8 in its SB5-masked state. For example, additional TEM8 binding proteins present in 293/TEM8 cells, which could be cytosolic, membrane or extracellular, may facilitate a conformational change in TEM8 which masks the SB5 binding site. Alternatively, a membrane spanning or extracellular binding partner may interact with TEM8 and directly block the SB5 antibody/epitope interaction. To identify dominant-acting factors which can regulate SB5 binding, we designed a phenotypic screen based on sensitivity of SB5-selected 293 cells to SB5-immunotoxins. The assay involved the transfer of a retroviral cDNA library derived from an SB5-toxin resistant (SB5-masked) cell line into a sensitive (SB5-exposed) cell line, selection with SB5-bound toxin, recovery of surviving colonies and the identification of individual cDNAs which can rescue cells from toxicity (Fig. 2C). We initially considered using the parental 293/T8-SB5 cell line, or clones of this cell line, as “sensitive” recipients for infection. However, when we exposed the parental 293/T8-SB5 stable pool or multiple independent clones derived from this pool to toxin, we consistently found that a small subpopulation of the treated cells were highly resistant to the toxin (Fig. 3A and B). When these rare surviving cells were expanded in culture for two weeks and then tested for TEM8 expression by flow cytometry and western blotting, TEM8 expression was near background levels (Fig. 3C and D). Although this result confirmed the remarkable specificity of the SB5 mAbs for TEM8, it also suggested that loss of TEM8 expression may lead to an excessive number of background colonies in our cDNA expression screen, and prompted us to explore possible mechanisms regulating the loss of TEM8 expression in SB5-toxin selected cells. Because TEM8 expression was lost in clones of 293/T8-SB5 which originated from single cells (Fig. 3D), we reasoned that TEM8 expression in these surviving cells may have been silenced epigenetically, as reported for other genes [25]. To test this, we treated the toxin-selected 293/T8-SB5 cells which had lost TEM8 expression with Trichostatin A (TSA), a histone deacetylase inhibitor. Indeed, TSA rescued TEM8 expression on the cell surface in a in a dose-dependent manner (Fig. 3E and F), suggesting that TEM8 expression is regulated by histone acetylation.
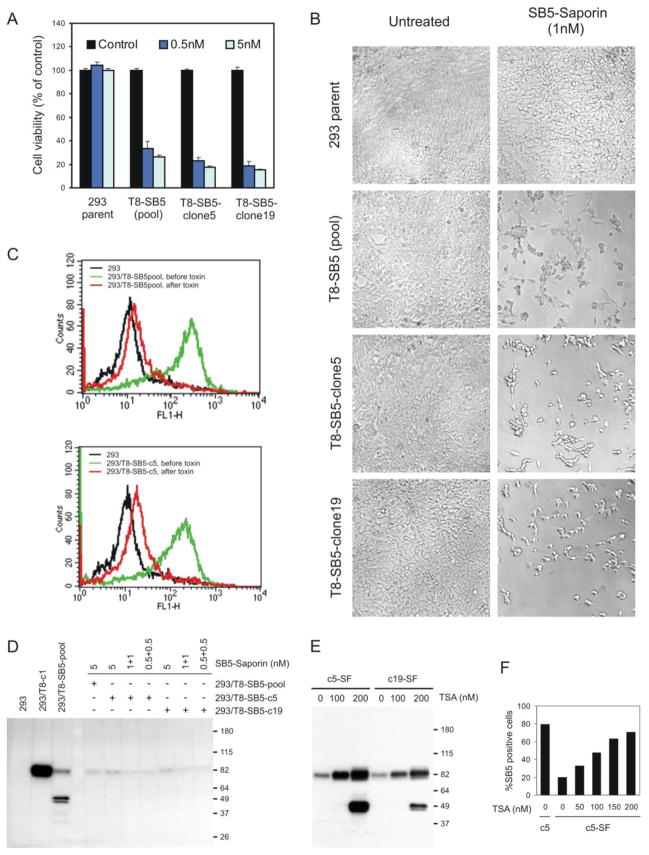
FIGURE 3.
A fraction of the 293/T8-SB5 cell line and its clonally-derived sublines survive saporin-SB5 immunotoxin treatment by epigenetic silencing of TEM8 expression. A, 293, 293/T8-SB5 (pool), 293/T8-SB5-clone5 or 293/T8-SB5-clone19 cells were untreated (control) or treated with biotinylated SB5 mAbs and 0.5 nM or 5 nM of saporin-streptavidin and cell viability was measured 72 hours later. The percent viability of the clones was similar to that of the pool. B, A similar number of surviving 293/T8-SB5 cells from the pool or its clonally derived sublines was observed by Brightfield microscopy 96 hours post-treatment with SB5 mAbs and 1 nM of saporin-streptavidin. C, Flow cytometry staining with SB5 mAbs revealed a significant reduction in TEM8 expression on the cell surface of the 293/T8-SB5 pool (top panel) or a clone derived from this pool (293/T8-SB5-c5; bottom panel) two weeks following treatment with 5 nM of SB5-saporin immunotoxin. D, Immunoblotting (IB) for TEM8 revealed a loss of TEM8 expression in toxin-treated surviving cells from the 293/T8-SB5 pool or its subclones (clone 5 and clone 19). In this experiment cells were treated either twice with 0.5 nM of toxin 3 days apart (0.5+0.5), twice with 1 nM of toxin 3 days apart (1+1), or once with 5 nM of SB5-saporin (5). Two weeks later, cells which had recovered were analyzed by western blotting. E and F, Trichostatin A (TSA) treatment for 24-hours resulted in a dose-dependent rescue of TEM8 expression in the toxin-treated surviving fraction of 293/T8-SB5 clone 5 (c5-SF) and clone 19 (c19-SF) as detected by western blotting (E) or flow cytometry (F).
Because TSA itself demonstrated some toxicity at the concentration needed to recover full TEM8 expression, we considered using positive selection with anti-TEM8 immunomagnetic beads as an additional method to ensure the maintenance of TEM8 expression on the surface of all recipient SB5-toxin treated cells. Because each of the SB mAbs and others commercially available were unable to detect the SB5-masked form of TEM8, we incorporated a 3x-FLAG tag into the N-terminus of TEM8. Immunofluorescence staining using anti-FLAG mAbs revealed FLAG-TEM8 on the cell surface of non-permeabilized 293/FlagT8 cells immediately following transfection (Fig. 4, bottom panel). As expected, however, SB5 staining remained undetectable by flow cytometry, and SB5 only labeled the occasional cell by immunofluorescence staining (Fig. 4, top panel). Next, we repeated our selection of 293/FlagT8 cells using SB5-linked magnetic beads, and then cloned the selected cells by limiting dilution. One of the clones, called 293/FlagT8-SB5, was chosen as the recipient for infection of our cDNA expression library.
FIGURE 4.
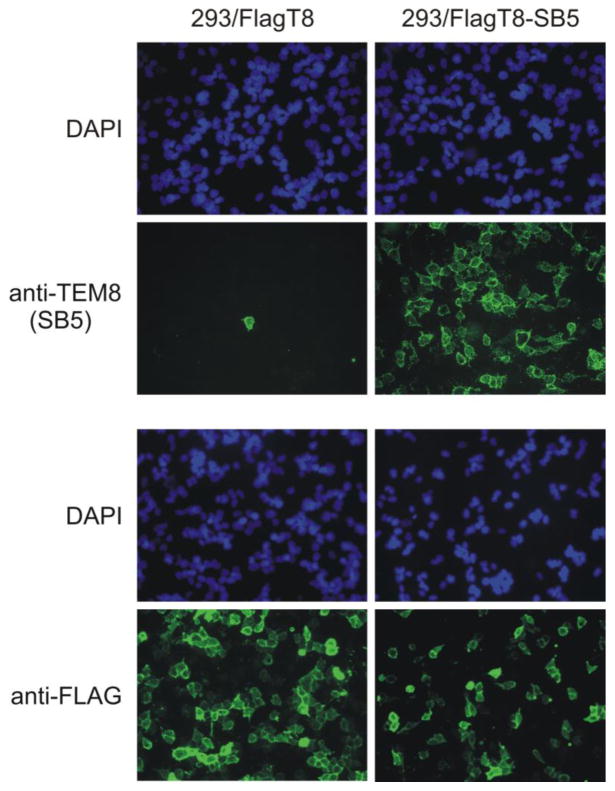
Anti-FLAG antibodies recognize N-terminal FLAG-tagged TEM8 on the cell surface without the need for cell surface enrichment. FLAG-tagged TEM8 could be detected in the majority of 293/FlagT8 cells using anti-FLAG mAbs immediately following transfection (green, left panel, bottom) whereas SB5 anti-TEM8 mAbs could only detect sporadic cells. However, both anti-FLAG and SB5 mAbs could detect TEM8 in 293/FlagT8-SB5 cells which had been selected with SB5 immunomagnetic beads (right panel). Nuclei from the same region were visualized with the DAPI (blue) filter.
To identify genes in our screen, 293/FlagT8-SB5 recipient cells were infected with a retroviral cDNA library derived from uterus, a tissue that expressed high levels of TEM8, and then treated with saporin-linked SB5 antibody along with 40 nM TSA, a concentration found in our earlier studies to be minimally-toxic to the cells. Importantly, the number of surviving colonies on each plate was 10-fold or higher in transduced versus non-transduced cells, suggesting a successful rescue by the cDNA library. After sorting cells with anti-FLAG-conjugated magnetic beads and repeating the toxin/TSA selection and sorting once more, cDNA was recovered from surviving clones and identified by DNA sequencing.
3.5. Genetic Screen Reveals Multiple Components of the Actin Cytoskeleton
A total of 99 cDNA inserts originating from 10 independent plates were recovered and sequenced, and 12 genes were identified two or more times (Table 1). Interestingly, 7 of the top 12 genes identified (~60%) are known to be directly or indirectly involved in regulation of the actin cytoskeleton. The most frequently observed genes were transgelin (TAGLN, SM22α) and alpha-smooth muscle actin (α-SMA, ACTA2), each identified 4 times from 4 independent plates. Transgelin is known to promote the cross-linking or “gelling” of actin and, similar to α-SMA, is thought to be important for contraction of smooth muscle cells [26–28].
TABLE 1.
Candidate genes recovered from transduced 293/FlagT8-SB5 cells following SB5-toxin treatment
| Gene | Name | Involved with actin | Plate of origin* |
|---|---|---|---|
| ACTA2 | Actin, alpha2, smooth muscle, aorta (α-SMA) | Y | 1, 2, 3, 4 |
| TAGLN | Transgelin | Y | 1, 3, 4, 8 |
| ARL1 | ADP-ribosylation factor-like 1 | N | 7, 8, 9 |
| ACTG2 | Actin, gamma 2, smooth muscle enteric | Y | 7, 10 |
| APOD | Apolipoprotein D | N | 1, 6 |
| COL6A2 | Collagen, type VI, alpha 2 | N | 6, 9 |
| GSN | Gelsolin | Y | 6, 7 |
| MYL9 | Myosin, light chain 9, regulatory | Y | 1, 4 |
| RPS6 | Ribosomal protein S5 | N | 1, 3 |
| SMARCB1 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin | Y | 7, 8 |
| TPM2 | Tropomyosin 2 (beta) | Y | 5, 10 |
| TPT1 | Tumor protein, translationally controlled 1 | N | 2, 9 |
Clones were isolated from 10 independent plates that were infected with virus separately.
Several of the identified genes, including smooth muscle actin and transgelin, are known to be expressed predominantly by vascular pericytes or smooth muscle cells. However, TEM8 was originally identified in a screen for genes overexpressed in tumor endothelial cells [1]. To determine if proliferative vascular smooth muscle cells also express TEM8 we evaluated TEM8 mRNA expression in cultured primary or immortalized endothelial or smooth muscle cells, and for comparison included three tumor cell lines of epithelial origin. TEM8 was found to be expressed in both vascular pericytes and endothelial cells, while expression was undetectable in the tumor cells analyzed (Fig. 5).
3.6. Transgelin and α-SMA Regulate TEM8 Cell Surface Structure
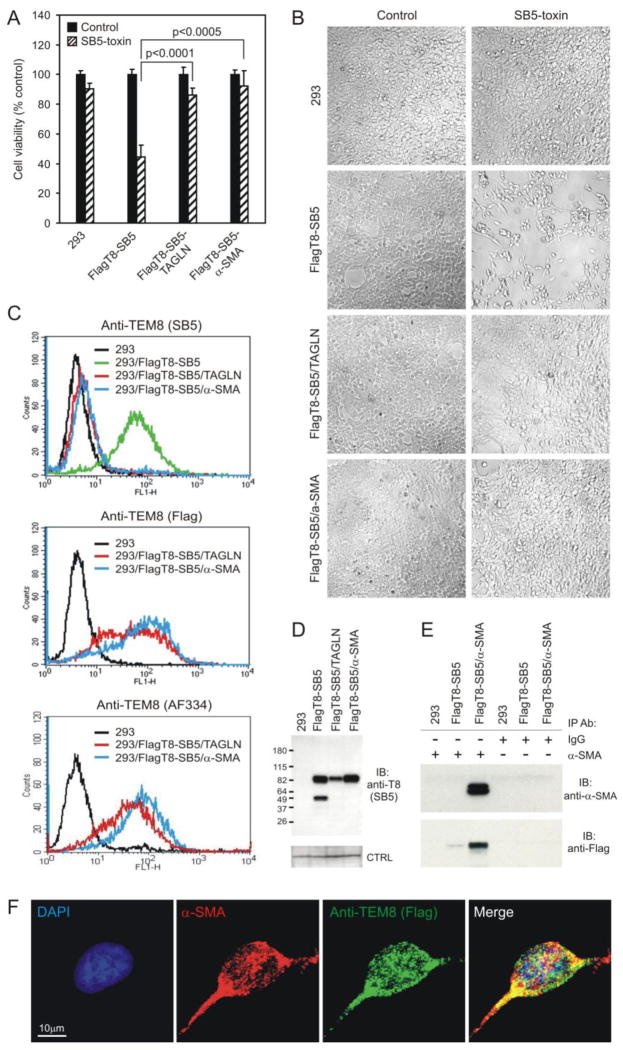
To determine if transgelin or α-SMA were responsible for mediating resistance to SB5-saporin, we transfected these genes into 293/FlagT8-SB5 cells and then treated cells with the SB5 antibody-toxin. Importantly, at 5 nM of SB5-saporin, expression of either transgelin or α-SMA rescued 293/FlagT8-SB5 cells from toxicity (Fig. 6A and B). An analysis of SB5 binding by flow cytometry revealed that SB5 was no longer able to bind TEM8 on the surface of 293/FlagT8-SB5 cells following transfection with either transgelin or α-SMA, even though the parent 293/FlagT8-SB5 cells labeled strongly (Fig. 6C, top panel). Importantly, however, TEM8 expression remained high in transgelin or α-SMA transfected 293/FlagT8-SB5 cells by immunoblotting (Fig 6D), and staining of cells with anti-FLAG mAbs by flow cytometry verified the expression of TEM8 on the cell surface of 293/FlagT8-SB5-TAGLN or 293/FlagT8-SB5-SMA cells (Fig. 6C, middle panel). These results suggest that an alteration in the structure of TEM8 at the cell surface is responsible for masking the SB5 epitope.
FIGURE 6.
Exogenous expression of transgelin or α-SMA rescues 293/FlagT8-SB5 cells from SB5-saporin toxicity. A, 293, 293/FlagT8-SB5, or 293/FlagT8-SB5 cells expressing transgelin (FlagT8-SB5/TAGLN) or α-SMA (FlagT8-SB5/α-SMA) were untreated (control) or treated with SB5-biotin and saporin-streptavidin (SB5-toxin) and cell viability was measured 72 hours later. B, Following toxin treatment, transgelin- or α-SMA-expressing cells from (A) appeared similar to the untreated controls. C, Flow cytometry revealed a lack of SB5 labeling in 293/FlagT8-SB5/TAGLN or FlagT8-SB5/α-SMA cells (top panel). However, cell-surface TEM8 was detected in the same cells using anti-FLAG mAbs (middle panel) or AF334 anti-TEM8 antibodies (bottom panel). D, TEM8 expression was maintained in 293/FlagT8-SB5 cells following transfection with TAGLN or α-SMA in cellular lysates analyzed by immunoblotting (IB) with SB5 antibodies. Non-specific bands were observed on the same blot upon re-probing with different antibodies and served as an internal loading control (CTRL). E, TEM8 was co-immunoprecipitated with α-SMA using anti-α-SMA mAbs but not with control IgG mAbs. F, Immunofluorescence staining of FlagT8-SB5/α-SMA cells with anti-α-SMA (red) and anti-FLAG antibody (green) revealed co-localization (merge, yellow) particularly in focal spots at the periphery of cells.
Next, we set out to determine if an alteration in TEM8 structure could be the consequence of a direct interaction between TEM8 and α-SMA or transgelin. Indeed, TEM8 co-immunoprecipitated with α-SMA (Fig. 6E) and these proteins were also found to co-localize at the cell surface by immunofluorescence staining (Fig. 6F). Although we have not yet been able to co-immunoprecipitate transgelin and TEM8, this could be due to a limitation of the mAbs currently available. Alternatively, TEM8 may bind transgelin indirectly.
Because the SB5-exposed form of TEM8 was derived from a cryptic population and was only observed on the majority of cells following cell surface enrichment with SB5-beads, these studies indicate that an SB5-masked form of TEM8 is generally present on the cell surface of TEM8-positive cells. To determine if the predominant SB5-masked form of TEM8 could also be internalized upon antibody binding, similar to the SB5-exposed form, we treated non-selected 293/FlagT8 cells with saporin-bound anti-FLAG antibodies. Saporin-anti-FLAG antibodies were selectively toxic to 293/FlagT8 cells compared to parental 293 cells1 suggesting that antibodies which can recognize the predominant SB5-masked form of TEM8 on the cell surface can also be internalized effectively.
3.7. Development of an Antibody that Recognizes the SB5-Masked Form of TEM8
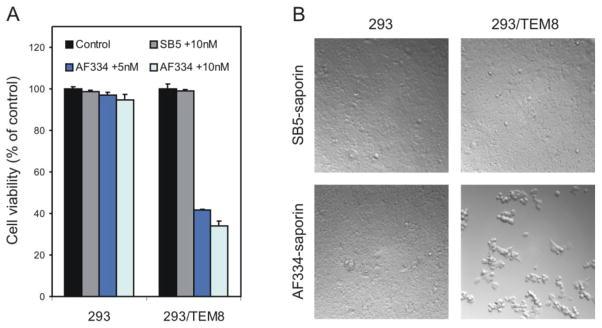
Encouraged by these results, we set out to develop a new anti-TEM8 monoclonal antibody which can recognize the predominant SB5-masked form of TEM8. Rather than using recombinant TEM8 protein as the immunogen, this time one of us (AF) employed an alternative method for antibody generation based on immunization of mice with TEM8-expressing cells followed by flow cytometry-based screening (see Materials and Methods for details). Using this strategy, a monoclonal antibody, called AF334, was identified that, like SB5, was able to bind both mouse and human TEM8 by flow cytometry. AF334 was also able to bind the SB5-exposed form of TEM8 on the surface of SB5-selected 293/hT8-SB5 cells (Fig. 1E and Fig. 1D, bottom panel, red). Importantly, however, AF334 differed from previously developed anti-TEM8 antibodies in that it also reacted strongly with the predominant SB5-masked form of TEM8 on the cell surface (Fig. 1D, bottom panel, green, Fig. 1E, and Fig. 6C, bottom panel). Next, we treated cells expressing the SB5-masked form of TEM8 with an AF334-saporin immunotoxin. Unlike SB5, AF334 was toxic towards 293/TEM8 cells but not 293 cells (Fig. 7A and B), demonstrating the ability of this antibody to selectively deliver toxin into cells expressing the predominant SB5-masked form of TEM8.
FIGURE 7.
293/FlagT8 cells (293/TEM8) are sensitive to AF334 anti-TEM8 saporin immunotoxins. A, Cell viability was measured in 293 or 293/TEM8 cells 72 hours following treatment with AF334 anti-TEM8 mAbs along with 5 nM or 10 nM of saporin-conjugated anti-mouse secondary mAbs. Note the AF334-specific toxicity observed in 293/TEM8 cells that had not undergone prior selection with SB5-immunomagnetic beads (p<0.001 at 5 nM, p<0.002 at 10 nM). B, Brightfield microscopy 72hour post-treatment with SB5 or AF334 followed by 10 nM of saporin revealed toxicity only in the AF334 treated 293/TEM8 cells. The relative level of TEM8 on the surface of the 293 or 293/TEM8 cells is shown in Figure 1D, bottom panel.
4. Discussion
The results of these studies suggest that SB5 recognizes a form of TEM8 that is normally masked, most likely due to binding of the TEM8 cytosolic domain with components of the actin cytoskeleton. Masking of the SB5 binding site could, in principle, be caused by oligomerization of TEM8 at the cell surface facilitated by interactions with the actin cytoskeleton. However, we favor the hypothesis that TEM8 exists in two alternative conformations, open and closed, based on its homology with integrins which also bind ECM and can change their conformation upon binding to components of the actin cytoskeleton.
Although the exact role of TEM8 remains unclear, binding of TEM8 to components of the actin cytoskeleton could be important biologically for several reasons. TEM8 has been shown to promote migration [6], a process known to depend on actin. Alpha-SMA and transgelin are both important for regulating smooth muscle contractility suggesting that TEM8 may also be involved in contraction. Finally, binding of collagen or other uncharacterized extracellular ligands to TEM8 may result in receptor internalization via an actin-dependent endocytotic pathway, similar to that exploited by anthrax toxin proteins.
These studies demonstrate that the potential therapeutic utility of SB mAbs is compromised by their inaccessibility to full-length TEM8 on the cell surface. Indeed, treatment of tumor bearing mice with an anti-TEM8/truncated tissue-factor fusion protein containing the variable domain of SB5 resulted in only a modest tumor growth delay [29], a small response considering the striking tumor regressions observed in earlier studies of tissue-factor induced vascular thrombosis [30]. However, by applying cell-based selection strategies that employ TEM8-expressing cells as the immunogen, we have been able to develop a new monoclonal antibody against TEM8, called AF334, which is able to recognize the predominant form of TEM8 on the cell surface. AF334-saporin immunotoxins were found to be selectively toxic to TEM8 expressing cells, similar to anthrax toxin proteins. However, unlike anthrax toxin proteins, AF334 does not react with CMG2. Because AF334 only recognizes TEM8, AF334-based immunotoxins could potentially have an improved specificity for tumor vessels compared to anthrax toxin proteins. Saporin is a plant-derived foreign product and its immunogenicity, like anthrax-based toxins, may ultimately hinder long term repeated dosing in mammals. Function-blocking anti-TEM8 antibodies or TEM8 antibodies conjugated to toxins that are less immunogenic may make more suitable agents for long term repeated use in vivo. Further studies are needed in order to determine the therapeutic potential of AF334 or AF334-based immunotoxins in preclinical tumor models.
In summary, by employing a genetic screen we provide direct evidence that the actin cytoskeleton is able to regulate TEM8 structure at the cell surface supporting the idea that TEM8, like integrins, may exist in both open and closed conformations. This study also demonstrates that TEM8 expression is regulated epigenetically by histone acetylation, providing a potential new avenue to modulate anthrax toxin susceptibility. These results have important implications for understanding the biological role of TEM8 and anthrax toxin pathogenicity, and should aid in efforts to develop the most effective anti-TEM8 diagnostic and therapeutic mAbs.
Research Highlights.
TEM8, like integrins, can exist in two different forms on the cell surface.
Binding of TEM8 to the actin cytoskeleton alters the structure of cell-surface TEM8.
TEM8 expression is regulated epigenetically by histone acetylation.
The first mAb that recognizes native TEM8 independent of its structure is described.
Supplementary Material
Acknowledgments
We thank Dr. Karlyne Reilly for helpful suggestions and Dr. Lino Tessarollo for critical evaluation of the manuscript. This research was supported by the intramural research program of the NIH, National Cancer Institute.
Abbreviations List
- TEM8
Tumor Endothelial Marker 8
- CMG2
capillary morphogenesis protein 2
- IgG
Immunoglobulin G
- mAbs
monoclonal antibodies
- α-SMA
alpha-smooth muscle actin
- PA
protective antigen
Footnotes
Mi Young Yang and Brad St. Croix, unpublished observations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 2.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 3.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 4.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higa GM, Abraham J. Biological mechanisms of bevacizumab-associated adverse events. Expert Rev Anticancer Ther. 2009;9:999–1007. doi: 10.1586/era.09.68. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- 8.Go MY, Chow EM, Mogridge J. The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun. 2009;77:52–59. doi: 10.1128/IAI.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 10.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 11.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanda A, St Croix B. Tumor endothelial markers: new targets for cancer therapy. Curr Opin Oncol. 2004;16:44–49. doi: 10.1097/00001622-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Duan HF, Hu XW, Chen JL, Gao LH, Xi YY, Lu Y, Li JF, Zhao SR, Xu JJ, Chen HP, Chen W, Wu CT. Antitumor activities of TEM8-Fc: an engineered antibody-like molecule targeting tumor endothelial marker 8. J Natl Cancer Inst. 2007;99:1551–1555. doi: 10.1093/jnci/djm132. [DOI] [PubMed] [Google Scholar]
- 14.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, Guo S, Han J, Liang H, Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 15.Felicetti P, Mennecozzi M, Barucca A, Montgomery S, Orlandi F, Manova K, Houghton AN, Gregor PD, Concetti A, Venanzi FM. Tumor endothelial marker 8 enhances tumor immunity in conjunction with immunization against differentiation Ag. Cytotherapy. 2007;9:23–34. doi: 10.1080/14653240601048369. [DOI] [PubMed] [Google Scholar]
- 16.Abi-Habib RJ, Singh R, Leppla SH, Greene JJ, Ding Y, Berghuis B, Duesbery NS, Frankel AE. Systemic anthrax lethal toxin therapy produces regressions of subcutaneous human melanoma tumors in athymic nude mice. Clin Cancer Res. 2006;12:7437–7443. doi: 10.1158/1078-0432.CCR-06-2019. [DOI] [PubMed] [Google Scholar]
- 17.Rouleau C, Menon K, Boutin P, Guyre C, Yoshida H, Kataoka S, Perricone M, Shankara S, Frankel AE, Duesbery NS, Vande Woude G, Biemann HP, Teicher BA. The systemic administration of lethal toxin achieves a growth delay of human melanoma and neuroblastoma xenografts: assessment of receptor contribution. Int J Oncol. 2008;32:739–748. [PubMed] [Google Scholar]
- 18.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc Natl Acad Sci U S A. 2003;100:657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, Moayeri M, Basile JR, Alfano RW, Gutkind JS, Frankel AE, Bugge TH, Leppla SH. Matrix Metalloproteinase-activated Anthrax Lethal Toxin Demonstrates High Potency in Targeting Tumor Vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duesbery NS, Resau J, Webb CP, Koochekpour S, Koo HM, Leppla SH, Vande Woude GF. Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple MEK pathways. Proc Natl Acad Sci U S A. 2001;98:4089–4094. doi: 10.1073/pnas.061031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KH, Liu S, Bankston LA, Liddington RC, Leppla SH. Selection of anthrax toxin protective antigen variants that discriminate between the cellular receptors TEM8 and CMG2 and achieve targeting of tumor cells. J Biol Chem. 2007;282:9834–9845. doi: 10.1074/jbc.M611142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One. 2007;2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh Y, Klimpel KR, Goel S, Swain PK, Leppla SH. Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect Immun. 1999;67:1853–1859. doi: 10.1128/iai.67.4.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeidan A, Sward K, Nordstrom I, Ekblad E, Zhang JC, Parmacek MS, Hellstrand P. Ablation of SM22alpha decreases contractility and actin contents of mouse vascular smooth muscle. FEBS Lett. 2004;562:141–146. doi: 10.1016/S0014-5793(04)00220-0. [DOI] [PubMed] [Google Scholar]
- 28.Je HD, Sohn UD. SM22alpha is required for agonist-induced regulation of contractility: evidence from SM22alpha knockout mice. Mol Cells. 2007;23:175–181. [PubMed] [Google Scholar]
- 29.Fernando S, Fletcher BS. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer Res. 2009;69:5126–5132. doi: 10.1158/0008-5472.CAN-09-0725. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Molema G, King S, Watkins L, Edgington TS, Thorpe PE. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.