Abstract
This study investigated the ability of substance P (Sub P) to induce dendritic varicosities (DVs) or beads in neurons of the rostral ventromedial medulla (RVM) of the rat. Microinjection of 5–200 pmol Sub P in the RVM produced a concentration-dependent increase in the number of DVs in distal dendrites of RVM neurons that were immunoreactive for the neurokinin-1 receptor, but not serotonin. The effect was reversible, as DVs were essentially absent two and four hrs after microinjection. Fluoro-jade B labeled neurons were not evident in the RVM four days after microinjection of Sub P, although such neurons were present four days after microinjection of a neurotoxic dose of kainate. Bath application of Sub P to brainstem slices for a period as brief as 30 sec also produced DVs in neurokinin-1 immunoreactive RVM neurons. Prior exposure to L-703,606 prevented the formation of DVs by Sub P, implicating the neurokinin-1 receptor, a Gq type of G-protein coupled receptor, in the formation of DVs by Sub P. Finally, stabilization of microtubules by prior exposure to taxol also prevented the formation of DVs consistent with the idea that increases in intracellular Ca2+ lead to the formation of DVs secondary to a disruption of the linear arrays of microtubules in dendrites. These data establish a mechanistic basis for the formation of DVs by Sub P and support further studies to test the hypothesis that the formation of DVs is a morphological mechanism by which neurons can regulate their responses to inhibitory or excitatory inputs.
Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
Keywords: Rostral ventromedial medulla, Dendritic varicosity, Dendritic beading, Substance P, Neurokinin-1 receptor
1. Introduction
Internalization of the neurokinin-1 receptor (NK-1R) has been extensively used as a measure of the release or action of substance P (Sub P) in the central nervous system (Adelson et al., 2009; Allen et al., 1997; Marvizon et al., 1999; Marvizon et al., 2003). In the course of these studies, several authors have commented on changes that occur in the dendrites of these neurons. For example, the dendrites of dorsal horn neurons that exhibit internalization of NK-1R after intradermal injection of capsaicin exhibit a highly varicose or tortuous appearance (Mantyh et al., 1995b). The formation of dendritic beading or varicosities (DVs) has also been documented after microinjection of Sub P in the striatum (Mantyh et al., 1995a), and after prolonged application to cultured dorsal horn neurons (Marvizon et al., 1998).
Other than documentation of their occurrence, little is known about the mechanisms that underlie the formation of DVs induced by Sub P. Yet, DVs may have functional consequences for neuronal function. The formation of DVs is accompanied by a reduction in the diameter of the intervening segments of the dendrites (McNeil et al., 1999), which radically alters the geometry of the dendrites. In theory, such changes will reduce the passive electrical properties of dendrites because internal resistance is increased in the constricted regions between varicosities (Ellias and Stevens, 1980). Thus, synaptic potentials initiated in varicose dendrites could attenuate with distance. Dendritic varicosities could potentially function to isolate synaptic currents, reduce the electrotonic conduction of synaptic potentials along the dendrite, and effectively insulate the soma from excitatory or inhibitory inputs. The purpose of this study was to conduct an in vitro and in vivo analysis of DVs and the mechanisms that are responsible for their formation after application of Sub P. This study focused on the rostral ventromedial medulla (RVM) because this region contains high densities of NK-1R and Sub P (Ljungdahl et al., 1978; Nakaya et al., 1994; Saffroy et al., 2003). Moreover, there is strong evidence that endogenously released Sub P in the RVM may play a role in the maintenance of thermal hyperalgesia and mechanical allodynia after peripheral inflammatory injury (Hamity et al., 2010; Pacharinsak et al., 2008).
2. Results
2.1. Microinjection of Sub P produces DVs in vivo
The first set of experiments established that microinjection of Sub P, but not saline produced DVs in NK-1R immunoreactive RVM neurons. The total number of DVs was determined 10 to 240 min after microinjection of saline or Sub P (5 to 200 pmol in 0.5 μl). Counts were made in serial sections through the RVM. As illustrated in Fig. 1A,B, the DVs were approximately 2 microns in diameter, and were often regularly spaced along a dendrite giving the appearance of beads on a string. Dendritic varicosities were more common on distal dendrites, than on proximal dendrites. Dendritic varicosities were infrequently observed after microinjection of saline (Fig. 1C). Figure 2 illustrates the total number of DVs counted in serial sections through the RVM after microinjection of Sub P. The dose-effect curve for Sub P was U-shaped, with the maximum number of DVs observed after microinjection of 100 pmol Sub P (Fig. 2A). Dendritic varicosities persisted for up to 60 min after microinjection, but were essentially absent by 120 min (Fig. 2B). Substance P did not produce DVs in distal or proximal dendrites of serotonergic RVM neurons (Fig. 1D), which is consistent with its inability to produce inward currents in serotonergic neurons and absence of colocalization of NK-1R to serotonergic neurons in the RVM (Leger et al., 2002; Zhang and Hammond, 2009).
Figure 1.
(A) Representative photomicrograph of dendritic varicosities (DVs) in neurokinin-1 receptor expressing neurons of the rostral ventromedial medulla (RVM) demonstrating the clarity with which DVs could be viewed with a 60× N.A. 1.4 objective and 10× eyepiece objective. The vertical line drawn perpendicular to the dendrite’s trajectory illustrates how the diameter of a DV was determined. Note that the segments between the DVs are constricted. (B) Representative photomicrographs of DVs in neurokinin-1 receptor expressing neurons of the rostral ventromedial medulla 1 hr after microinjection of 100 pmol substance P. (C) Few DVs were observed in neurokinin-1 receptor expressing neurons in the RVM after microinjection of saline. (D) Substance P did not produce DVs in serotonergic neurons in the RVM. Scale bar is 10 μm in panel A and 100 μm in panels B–D. Arrowheads indicate DVs. Where not visible in panels B–D, the microinjection site was within 250 – 500 microns.
Figure 2.
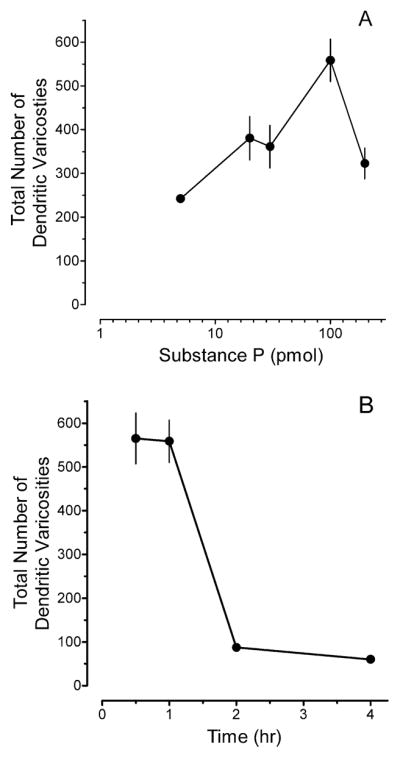
(A) Microinjection of Substance P (Sub P) in the rostral ventromedial medulla (RVM) of adult rats produced dendritic varicosities (DVs) in a dose-dependent manner. Values are the mean ± S.E.M. of the total number of DVs counted in serial sections through the RVM of 3–9 rats. (B) Time course of DV formation in the RVM after microinjection of 100 pmol Sub P. Values are the mean and S.E.M. of the total number of DVs counted in serial sections through the RVM of 3–6 rats.
2.2. Sub P produces DVs in a concentration- and time-dependent manner
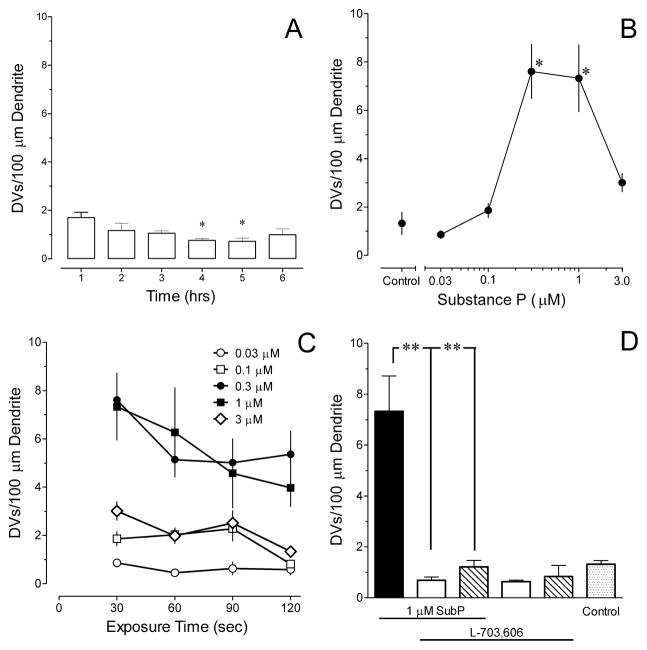
Subsequent experiments used the in vitro brainstem slice preparation to further probe the action of Sub P. Initial experiments determined that negligible numbers of DVs were observed in the dendrites of NK-1R RVM neurons in brainstem slices that incubated for 1 to 6 hrs before placement in the recording chamber and perfusion with ACSF for an additional 10 min (Fig. 3A). With prolonged periods of incubation, there was a slight decrease in the average density of DVs (P < 0.02) (Fig. 3A). The mean diameter of the DVs ranged from 2.1 to 2.6 μm. Slices from the first three hrs of incubation were pooled to generate a control group for statistical comparisons.
Figure 3.
The effects of substance P (Sub P) are concentration- and time-dependent and mediated by neurokinin-1 receptors (NK-1R) as determined in brainstem slices from juvenile rats. (A) Negligible numbers of dendritic varicosities (DVs) are present in brainstem slices incubated for as long as 6 hrs before transfer to the recording chamber. The induction of DVs in NK-1R immunoreactive neurons in the RVM is highly dependent on (B) the concentration (illustrated for a 30 s duration of exposure) and (C) duration of exposure to Sub P. (D) Pretreatment with 0.1 (open bars) or 1.0 μM (hatched bars) L703,606 prevents the formation of DVs by 30 sec exposure to 1 μM Sub P, but does not decrease the number of DVs by itself. The stippled bar is ACSF only (control). * P< 0.05, ** P < 0.01 compared to control. † P < 0.05, ‡ P < 0.01 compared to Sub P. Data are mean and S.E.M. of determinations in 4–9 slices obtained from 3–6 rats.
The time-course and concentration-dependence of the induction of DVs by Sub P was determined by applying concentrations ranging from 0.03 – 3 μM for periods ranging from 30 to 120 sec. Only one concentration and period of exposure was tested in a slice. Figure 3B illustrates that the concentration-response curve for the induction of DVs by Sub P was U-shaped with the maximum number of DVs induced by exposure to 0.3 or 1 μM Sub P. The mean diameter of DVs measured in NK-1R immunoreactive RVM neurons ranged from 1.8 to 2.2 μm with no systematic difference among the different treatment groups. The induction of DVs was also time-dependent, with maximal numbers of DVs observed after just 30 sec exposure to Sub P (Fig. 3C). Therefore, for subsequent experiments that examined the mechanism for Sub P induction of DVs, slices were exposed to 1 μM Sub P for 30 sec.
2.3. Induction of DVs by Sub P is mediated by NK-1R
Pretreatment of brainstem slices with 0.1 or 1.0 μM L-703,606, a selective NK-1R antagonist (Cascieri et al., 1992), for 10 min before and during exposure to Sub P prevented the formation of DVs by Sub P (Fig. 3D). Exposure to the antagonist alone did not decrease the number of DVs (Fig. 3D), indicating that DVs observed under basal conditions are not due to endogenously released Sub P.
2.4. Induction of DVs by Sub P involves microtubules
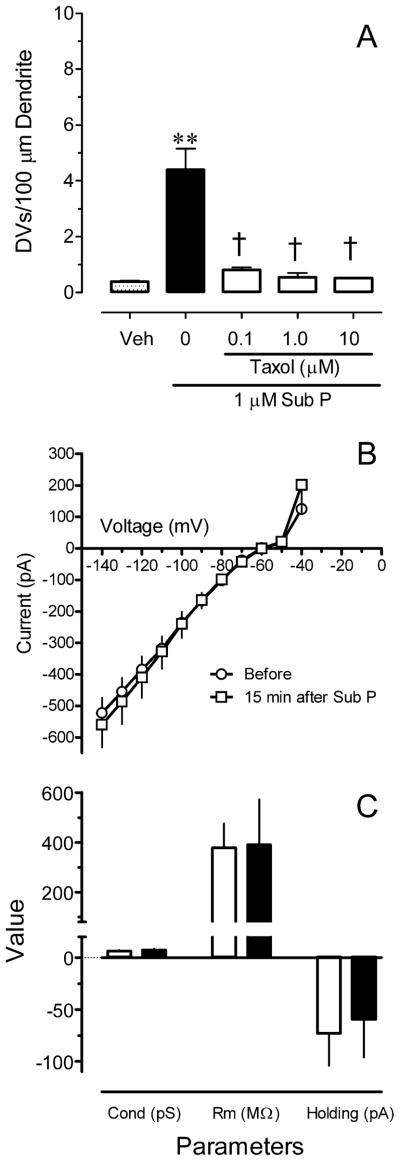
Pretreatment with 10 μM taxol, a microtubule stabilizer, 10 min before and during application of Sub P significantly inhibited the formation of DVs by Sub P (Fig. 4A; P < 0.01). The number of DVs produced by the 1 μM Sub P in this experiment, in which a small concentration of DMSO (0.17%) was present in the vehicle, was not significantly different from that determined in ACSF (P > 0.15).
Figure 4.
The production of dendritic varicosities (DVs) involves microtubules, and does not alter passive membrane properties of neurokinin-1 receptor (NK-1R) expressing neurons in the RVM. (A) Pretreatment with the microtubule stabilizer taxol (10 μM) prevents the formation of DVs by 30 sec exposure to 1 μM substance P. Data are the mean ± S.E.M of determinations in 3–4 slices. (B) Current-voltage relationship for three NK-1R expressing RVM neurons before (circles) and 15 min after a 30 sec exposure to 1 μM Sub P (squares). (C) Brief exposure to Sub P did not alter passive membrane properties of NK-1R-expressing RVM neurons. Data are mean ± S.E.M. for three neurons.
2.5. The formation of DVs does not adversely affect membrane properties or apparent viability of neurons
To determine whether the formation of DVs by Sub P altered the passive or action potential properties of RVM neurons, whole cell patch clamp recordings were made from NK-1R positive neurons after exposure to 1 μM Sub P for 30 sec. Figure 4B illustrates that the formation of DVs did not alter the current-voltage relationship for NK-1R-positive neurons; application of Sub P produced an inward current in each of these neurons. The formation of DVs did not alter the conductance, resting membrane potential, capacitance, access resistance or holding current (Figure 4C and data not shown).
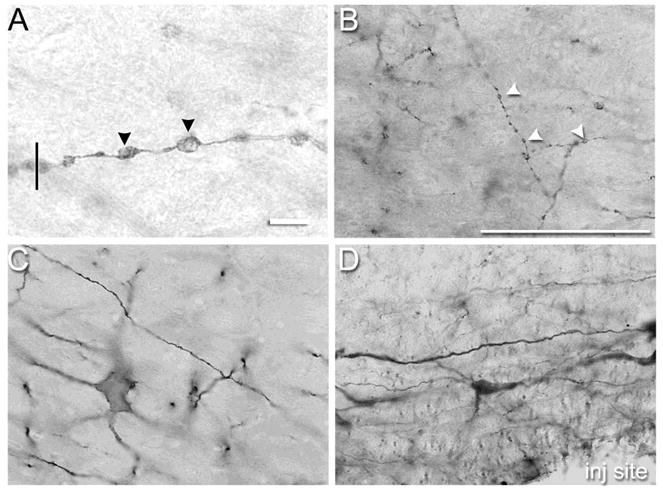
Fluoro-jade B staining can be used to visualize degenerating neurons (Schmued and Hopkins, 2000). The validity of the method was first confirmed by systemic administration of 10 mg/kg kainate, which produces modest seizures and resulted in labeling of hippocampal neurons. It was further probed by microinjection of 1.2 nmol of kainate in the RVM, which resulted in large numbers of labeled neurons in the RVM (Fig. 5A,B). In contrast, no Fluoro-jade B staining of RVM neurons was evident either one or four days after microinjection of 100 pmol Sub P or saline (Fig. 5C,D).
Figure 5.
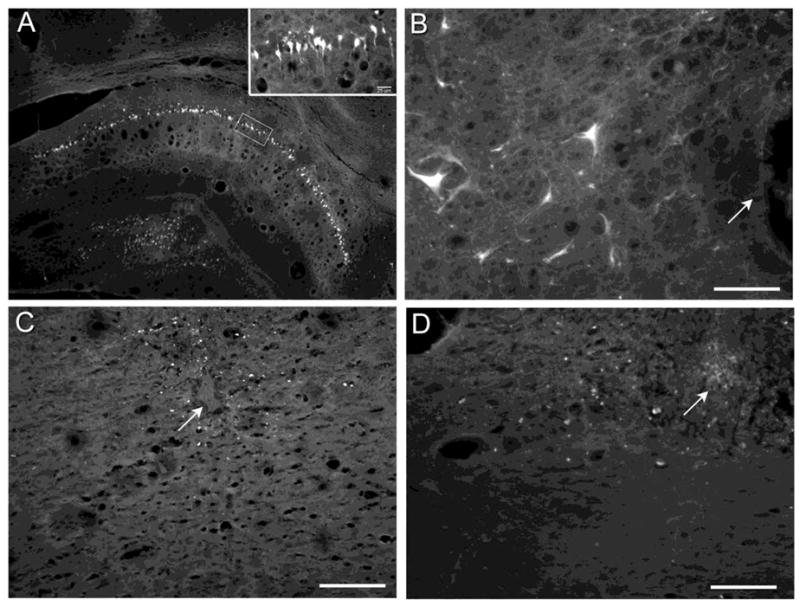
Representative micrographs of Fluoro-jade B staining of (A) hippocampal neurons 4 days after i.p. injection of 10 mg/kg kainate or (B) RVM neurons four days after microinjection of 1.2 nmol kainate into the RVM. Insert in panel A is a high magnification image of the boxed area. Fluoro-jade B staining was not observed in the RVM four days after microinjection of (C) 100 pmol Sub P, a dose known to produce DVs in RVM neurons, or (D) saline. Arrows identify the microinjection site. Scale bars are 100 μm.
3. Discussion
3.1. Sub P induces the formation of DVs
The principal finding of this study is that Sub P produced morphological changes in the dendrites of NK-1R immunoreactive neurons in the RVM through a mechanism that involved activation of the NK-1R and disruption of microtubules. The effect of Sub P was highly dependent on both concentration and duration of exposure. Similar U-shaped dose-effect curves for the effects of Sub P have been reported by others (Reid et al., 1990). Substance P is not alone in its ability to produce DVs. The NK-1R receptor is a G protein-coupled receptor (GPCR) of the Gq type. Other Gq-coupled receptor agonists such as platelet activating factor or the mGluR1/5 receptor agonist (S)-3,5-dihydroxyphenylglycine produce DVs in cultured hippocampal neurons (Clark et al., 2000; McNeil et al., 1999) and cultured striatal neurons, respectively (Mao and Wang, 2002). That antagonism of the NK-1R receptor alone did not decrease DVs suggests that the few DVs that form under basal conditions are not due to endogenous release of Sub P in the RVM.
It is unlikely that the formation of DVs is indicative of neuronal injury. First, the process of cutting brainstem slices did not elicit more than a negligible number of DVs. Were formation of DVs a response to neuronal injury, one might have expected the number of DVs to increase with longer incubation times. Rather, a slight decrease in the number of DVs occurred as incubation time increased. Second, microinjection of 100 pmol Sub P in the RVM, which produced the maximal number of DVs, did not result in any labeling of RVM neurons by Fluoro-Jade B, whereas labeling was evident after microinjection of a neurotoxic dose of kainate. Although the absence of labeling by Fluoro-Jade B is not definitive proof of an absence of injury, the fact that DVs did not alter the passive membrane properties of neurons or IV relationships is additional evidence that the formation of DVs is not necessarily injurious to the neuron.
3.2. Potential mechanisms
Gq coupled receptors activate phospholipase C, increase the levels of diacylglycerol and inositol 1,4,5-trisphosphate, and result in the intracellular release of Ca2+ from the endoplasmic reticulum. Indeed, the Ca2+ ionophore A23187 also induces DVs in cultured spinal cord neurons (Emery and Lucas, 1995). There is abundant evidence that mobilization of intracellular Ca2+ causes depolymerization of microtubules (Jones et al., 1980; Mattson, 1990; Mattson et al., 1991; Ringel and Horwitz, 1991; Schliwa et al., 1981). For example, high cytosolic Ca2+ may activate the proteolytic enzyme calpain I, which depolymerizes microtubules (Siman and Noszek, 1988). Alternatively, moderate Ca2+ mobilization may activate kinases that can phosphorylate microtubule-associated proteins (MAPs) such as MAP2 that is present in dendrites. Phosphorylation of MAP2 leads to its dissociation from microtubules, which become unstable and depolymerize (Sanchez et al., 2000).
Depolymerization of microtubules results in the formation of DVs. Electron microscopic examination of varicose dendrites in retinal amacrine cells (Ellias and Stevens, 1980; Sasaki-Sherrington et al., 1984), and cultured hippocampal neurons exposed to platelet activating factor indicates that microtubules are disrupted in the varicosities (Gache et al., 1994; McNeil et al., 1999). Although the microtubules continue to form normal linear arrays in the intervening constrictions (McNeil et al., 1999), the intervening segments can undergo constriction (McNeil et al., Fig. 1 this study). Nocodazole, which selectively disrupts microtubule structure (Gache et al., 1994; McNeil et al., 1999; Solomon, 1980) also produces DVs in cultured neurons (Gache et al., 1994; Jacobs and Stevens, 1986; McNeil et al., 1999). Treatment of hippocampal cultures with taxol, which promotes tubulin polymerization and stabilizes the microtubule structure (Horwitz, 1992; Ringel and Horwitz, 1991), prevents the formation of DVs by nocodazole (McNeil et al., 1999). Taxol also prevents the formation of DVs by platelet-activating factor in cultured hippocampal neurons (McNeil et al., 1999). In the present study, pretreatment with taxol also prevented the formation of DVs by Sub P. Finally, there is strong evidence that SubP can also disrupt microtubules (Maccioni et al., 1986; Manolides et al., 1988). These results indicate that mobilization of intracellular Ca2+ following activation of Gq-coupled GPCRs, as modeled here with Sub P and NK-1R, disrupts microtubules leading to the formation of DVs.
3.3. Sub P–induced DVs differ from those induced by excitatory amino acids
Excitatory amino acids also produce DVs in cultured neurons (Bindokas and Miller, 1995; Emery and Lucas, 1995; Hasbani et al., 1998; Park et al., 1996; Sloviter and Dempster, 1985; Stewart et al., 1991). However, the varicosities produced by excitatory amino acids do not appear to be produced by Ca2+ influx, as both N-methyl-D-aspartate and kainate induce varicosities in Ca2+-free buffer. Rather, the ionic mechanism involves the influx of Na+ and Cl−, which produce osmotic swelling of dendrites (Al-Noori and Swann, 2000; Hasbani et al., 1998). Activation of Na+ channels by veratridine also produces varicosities of the same shape and size as those produced by kainate. Varicosities induced by excitatory amino acids also vary in appearance; they are as large as 15 μm and filled with vacuoles (Emery and Lucas, 1995). In contrast, varicosities produced by agonists acting at GPCRs of the Gq type, such as Sub P and neurotensin, are much smaller and not vacuolated. Gq-coupled GPCRs and excitatory amino acids therefore appear to produce DVs by different mechanisms. Excitatory amino acids produce osmotic swelling of dendrites, whereas agonists of Gq-coupled GPCRs appear to cause disruption of microtubules that leads to the formation of DVs.
3.4. Possible ramifications of DV formation
As illustrated in Figure 6, the electrotonic conduction of synaptic potentials can be estimated by the product of the initial membrane potential (V0) and e−(d/λ), where d is distance along the dendrite and λ is the space constant (the square root of the ratio of membrane resistance Rm to the internal resistance Ri) or the distance at which the initial voltage V falls to 37% of its initial value (V0) (Koch, 1999). The internal resistance of the dendrite is primarily determined by the diameter of the narrowest constricted regions (Tanelian and Markin, 1997), and will be directly proportional to the number of constricted regions since the total resistivity of resistances in series is the sum of the individual resistances. Varicose dendrites have a shorter space constant because of the high internal resistance produced by the thin intervening constrictions. Consequently, synaptic potentials decrement over a shorter distance in varicose dendrites. As such, DVs may represent a morphological means to isolate neurons from inhibitory or excitatory inputs. Direct confirmation of this proposal will require quantitative measurement of dendrite diameter using electron microscopic methods, as well as measurement of evoked postsynaptic currents in RVM neurons.
Figure 6.
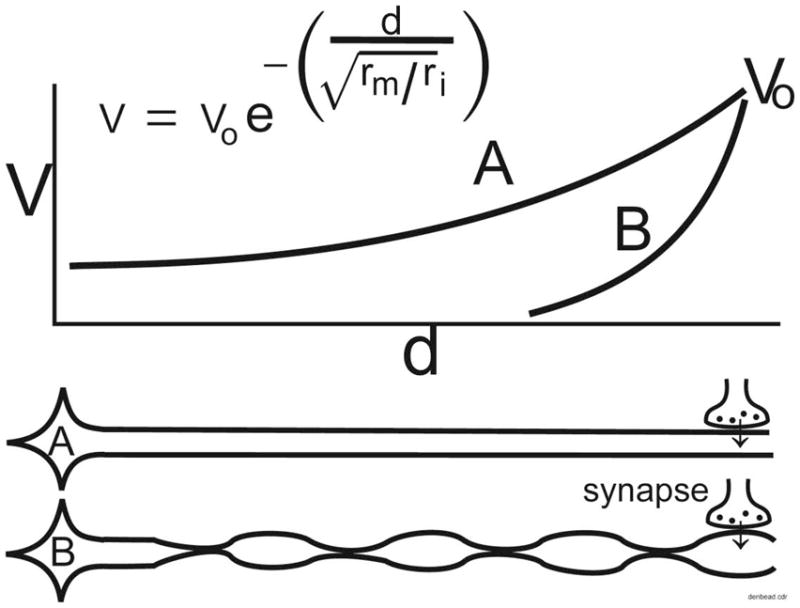
Schematic illustrating how dendritic varicosities (DVs) and the intervening constrictions can alter the electrotonic spread of potentials from dendrites to the soma, effectively isolating the soma from excitatory or inhibitory drive. This figure does not represent actual data, but is a graphic illustration of the known passive electrical properties of dendrites. Vo: initial membrane potential. Vd: voltage at distance d from the source. d: distance from the source on the dendrite. rm: membrane resistance. ri: internal resistance. The space constant, also known as λ, is the square root of the ratio of rm and ri.
As noted earlier, the passive membrane characteristics of NK-1R expressing RVM neurons did not change despite the formation of DVs. This finding was not unexpected because the largest numbers of DVs appeared on distal dendrites, with few numbers observed on larger, proximal dendrites of NK-1R immunoreactive RVM neurons (Fig. 1). The formation of DVs on distal dendrites would not be expected to appreciably alter the space clamp characteristic of these neurons.
3.5. Conclusions
The present findings suggest that the actions of Sub P go beyond simple activation of NK-1R receptors and mobilization of intracellular Ca2+ stores and extend to the disruption of microtubules and the formation of DVs. Under conditions of sustained or high intensity activation, the release of endogenous Sub P may result in the formation of DVs that serve to isolate neurons from incoming inputs. In the case of excitatory inputs, the formation of DVs may serve a protective function. In the case of inhibitory inputs, the formation of DVs may represent a morphological mechanism to maintain neural activity. The present findings provide the first systematic analysis of the formation of DVs by Sub P, provide insight into the mechanisms by which these occur and set the stage for future studies of their possible role in regulating afferent input to neurons.
4. Experimental Procedures
4.1. Animals
These experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with the guidelines of the International Association for the Study of Pain and the National Resource Council Guide for the Care and Use of Laboratory Animals. Rats were housed in cages with free access to food and water on a 12 hr light/dark cycle with lights on at 6:00 a.m. Every effort was made to minimize stress and the number of animals used.
4.2. Microinjection Studies
Female Sprague Dawley rats (250–350 g) were anesthetized with 50 mg/kg i.p. pentobarbital and placed in a stereotaxic unit. A 27 ga injection cannula was positioned in the RVM for injection of drug. At designated times thereafter, rats were administered an overdose of pentobarbital (100 mg/kg i.p.) and perfused intracardially with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS). The brain was removed and placed in 30% sucrose-0.1 M PBS to cryoprotect. Serial coronal sections of 25 μm thickness were cut through the RVM and processed for NK-1R or serotonin immunoreactivity or Fluoro-jade B staining as described below. Sections were cut in the coronal plane because the dendrites of neurons in the RVM are predominantly oriented in the mediolateral plane and are more restricted in the rostrocaudal plane (Gao and Mason, 1997; Potrebic and Mason, 1993).
4.3. Immunohistochemistry
4.3.1. Neurokinin-1 receptor
After washing several times with 0.01 M PBS (pH 7.4) at room temperature, sections were incubated for 20 min in 1.67 % H2O2 in 100% methanol. After further washes with 0.01 M PBS, sections were incubated for 30 min in 1% sodium borohydride and washed extensively for an hour with 0.01 M PBS. Sections were then transferred to 2% normal goat serum and 0.3 % Triton X-100 in 0.01 M PBS (PBS-N-T) for two hrs after which they were transferred to 0.01 M PBS containing 0.8 % bovine serum albumin, 0.2% gelatin and 0.3% Triton X-100 for another 45 min. After blocking was complete, sections were transferred to guinea pig anti-NK-1R [1:8,000, Chemicon AB5800; (Song and Marvizon, 2003)] in PBS-N-T for 72 hr at 4°C. The sections were then washed with 0.01 M PBS and incubated for 1 hr at room temperature with biotinylated goat anti-guinea pig IgG (6 μg/ml; Vector Labs) and then incubated in the ABC Elite solution (1:125; Vector Laboratories) for 1 hr. After further washes, the sections were reacted with 3,3′-diaminobenzidine (0.5 mg/ml) and the reaction terminated by extensive washing with 0.01 M PBS. The sections were mounted out of distilled water onto slides, dehydrated with graded series of alcohols, cleared with xylenes and coverslipped. Omission of the primary antibodies or preabsorption with the epitope resulted in a loss of labeling.
4.3.2. Serotonin immunoreactivity or Fluoro-jade B staining
Sections were processed as detailed above except that sections were incubated in rabbit anti-serotonin antibody (1:64,000; Immunostar, catalog 20080, lot# 051007; Hudson, WI) (Carrera et al., 2008) for 72 hrs, and after washing with 0.01 M PBS were incubated for 1 hr at room temperature with biotinylated goat anti-rabbit IgG (6 μg/ml; Vector Labs) followed by incubation in the ABC Elite solution (1:125; Vector Laboratories) for 1 hr. The sections were reacted with 3,3′-diaminobenzidine (0.5 mg/ml) as above. Fluoro-Jade B staining was performed to detect degenerating neurons as described (Schmued and Hopkins, 2000).
4.4. Slice preparation
Ten- to 14-day-old male Sprague-Dawley rats (Charles River, Raleigh, NC) were killed by decapitation. The brain was rapidly removed into ice-cold ACSF and the brainstem was dissected free. Coronal slices of 160 μm thickness containing the RVM were cut with a Leica vibratome equipped with a sapphire blade as previously described (Zhang et al., 2006; Zhang and Hammond, 2009). Sections were equilibrated for at least 1 hr at 34 °C in ACSF bubbled with 95% CO2/O2. They were then transferred to the recording chamber, perfused continuously with bubbled ACSF (4 ml/min at 32–34°C) and allowed to equilibrate for an additional 10–15 min before an experiment was begun. At the end of the experiment, the brain stem slice was fixed in 4% paraformaldehyde-0.1 M phosphate buffer for 1 hr and then transferred to 0.1 M phosphate buffered saline (PBS) for storage at 4°C until processing.
4.5. Electrophysiological preparation
Brainstem slices were prepared for whole cell patch-clamp recordings and measurements of passive membrane were made as previously described (Zhang et al., 2006; Zhang and Hammond, 2009). Current-voltage relationships were obtained before and 15 min after a one-minute exposure to 1 μM Sub P by a stepwise change in voltage from −140 mV to −40 mV. A Digidata 1322A board and pClamp 9.0 software (Molecular Devices, Sunnyvale, CA) were used for data acquisition and analysis.
4.6. Analysis of DV formation
The RVM was defined as the triangular region bounded ventrally by the pyramids, extending laterally to the edge of each pyramid and dorsally no further than the dorsal edge of the motor nucleus of the seventh nerve with the apex of the triangle on the midline. These anatomical boundaries are readily apparent under brightfield and epifluroescent illumination conditions, permitting reliable and consistent demarcation of the region of interest. For the in vivo microinjection study, serial sections through the RVM were viewed using a Nikon microscope equipped with a 60X objective (N.A. 1.4) and a 10X eyepiece objective, which provided an ultimate magnification of 600X. Using Neurolucida software (Microbrightfield, Colchester, VT), the location of each DV was marked and then summed across the serial sections to yield a single total value for each rats (N = 3–9 rats/dose). For studies in the in vitro slice preparation, a different approach was used. One or two slices were randomly selected from each rat for analysis. Three to nine slices, which had been obtained from 3 to 6 rats, were analyzed for each concentration and duration of exposure to Sub P or other agents. Neurolucida was used to trace the length of NK-1R immunoreactive dendrites, as well as the diameter of the varicosity, and to plot the location of the DVs in the RVM. The number of DVs was normalized to the length of dendrite and expressed as the number of DVs per 100 μm of dendrite. These values were averaged to yield a single value for that slice. Values for each slice were then averaged and the data expressed as the mean ± S.E.M of all the slices in that treatment group. A subanalysis of the data revealed that the length of the NK-1R immunoreactive dendrites traced for this analysis did not vary among the different treatment conditions, ranging from as short as 1.6 μm for those cut tangentially in a section to as long as 385 μm for those coursing in the plane of the section.
4.7. Drugs
Substance P acetate salt and L-703,606 hydrate oxalate salt were purchased from Sigma (St. Louis, MO). Taxol was purchased from Tocris (Ellisville, MO). Taxol was dissolved in DMSO and diluted so that the final concentration of DMSO in the ACSF was 0.17% (v/v). This concentration of DMSO served as the vehicle control in experiments involving Taxol. All other drugs were dissolved in distilled water, aliquoted, and stored at −20 or −80°C until use.
4.8 Statistical analysis
Data were expressed as the mean ± S.E.M. A one way-ANOVA was used to compare the number of DVs over time in the absence of Sub P. Newman-Keul’s test was used to make posthoc comparisons between mean values of the different treatment groups. A two-way ANOVA was used to compare the effects of the different concentrations and exposure times of Sub P on DV formation followed by Newman-Keul’s test. A Student’s t-test for paired samples was used to compare membrane properties before and after drug treatment group. A P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by the Korea Research Foundation grant to E.H. funded by the Korean Government (MOEHRD) (KRF-2006-214-E00002) and by the National Institute on Drug Abuse (R21DA020622 to H.K.P.) We thank Dr. Liang Zhang, Matthew Severidt, and Shirley Knapp for their assistance with this work.
Abbreviations
- ACSF
Artificial cerebrospinal fluid
- DV
Dendritic varicosity
- GPCR
G protein coupled receptor
- NK-1R
Neurokinin-1 receptor
- PBS
Phosphate buffered saline
- RVM
Rostral ventromedial medulla
- Sub P
Substance P
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson D, Lao L, Zhang G, Kim W, Marvizon JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increase with the firing frequency of C-fibers. Neuroscience. 2009;161:538–553. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Noori S, Swann JW. A role for sodium and chloride in kainic acid-induced beading of inhibitory interneuron dendrites. Neuroscience. 2000;101:337–348. doi: 10.1016/s0306-4522(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindokas VP, Miller RJ. Excitotoxic degeneration is initiated at non-random sites in cultured rat cerebellar neurons. J Neurosci. 1995;15:6999–7011. doi: 10.1523/JNEUROSCI.15-11-06999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera I, Molist P, Anadon R, Rodriguez-Moldes I. Development of the serotoninergic system in the central nervous system of a shark, the lesser spotted dogfish Scyliorhinus canicula. J Comp Neurol. 2008;511:804–831. doi: 10.1002/cne.21857. [DOI] [PubMed] [Google Scholar]
- Cascieri MA, Ber E, Fong TM, Sadowski S, Bansal A, Swain C, Seward E, Frances B, Burns D, Strader CD. Characterization of the binding of a potent, selective, radioiodinated antagonist to the human neurokinin-1 receptor. Mol Pharmacol. 1992;42:458–463. [PubMed] [Google Scholar]
- Clark GD, Zorumski CF, McNeil RS, Happel LT, Ovella T, McGuire S, Bix GJ, Swann JW. Neuronal platelet-activating factor receptor signal transduction involves a pertussis toxin-sensitive G-protein. Neurochem Res. 2000;25:603–611. doi: 10.1023/a:1007598617374. [DOI] [PubMed] [Google Scholar]
- Ellias SA, Stevens JK. The dendritic varicosity: a mechanism for electrically isolating the dendrites of cat retinal amacrine cells? Brain Res. 1980;196:365–372. doi: 10.1016/0006-8993(80)90401-1. [DOI] [PubMed] [Google Scholar]
- Emery DG, Lucas JH. Ultrastructural damage and neuritic beading in cold-stressed spinal neurons with comparisons to NMDA and A23187 toxicity. Brain Res. 1995;692:161–173. doi: 10.1016/0006-8993(95)00726-7. [DOI] [PubMed] [Google Scholar]
- Gache Y, Guilleminot J, Nunez J. High molecular weight tau distribution and microtubule stability in neuroblastoma N115 cells. Exp Brain Res. 1994;100:267–275. doi: 10.1007/BF00227196. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Somatodendritic and axonal anatomy of intracellularly labeled serotonergic neurons in the rat medulla. The Journal of Comparative Neurology. 1997;389:309–328. [PubMed] [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct Roles for Sodium, Chloride, and Calcium in Excitotoxic Dendritic Injury and Recovery. Experimental Neurology. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci. 1992;13:134–136. doi: 10.1016/0165-6147(92)90048-b. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Stevens JK. Experimental modification of PC12 neurite shape with the microtubule-depolymerizing drug Nocodazole: a serial electron microscopic study of neurite shape control. J Cell Biol. 1986;103:907–915. doi: 10.1083/jcb.103.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Gray EG, Barron J. Cold stable microtubules in brain studied in fractions and slices. J Neurocytol. 1980;9:493–504. doi: 10.1007/BF01204838. [DOI] [PubMed] [Google Scholar]
- Koch C. Biophysics of Computation. Oxford University Press; New York: 1999. [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Maccioni RB, Cann JR, Stewart JM. Interaction of substance P with tubulin. Eur J Biochem. 1986;154:427–435. doi: 10.1111/j.1432-1033.1986.tb09415.x. [DOI] [PubMed] [Google Scholar]
- Manolides L, Baloyannis S, Manolides S. Substance P increases the polymorphism of the synaptic vesicles in the temporal isocortex cultured in vitro. Acta Otolaryngol. 1988;105:500–506. doi: 10.3109/00016488809119509. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995a;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995b;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Glutamate cascade to cAMP response element-binding protein phosphorylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors. Mol Pharmacol. 2002;62:473–484. doi: 10.1124/mol.62.3.473. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Eskandari S, Ennes HS, Mayer EA. Substance P induces brief, localized increase in [Ca2+]i in dorsal horn neurons. Neuroreport. 1998;9:3369–3374. doi: 10.1097/00001756-199810260-00006. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Engle MG, Rychlik B. Effects of elevated intracellular calcium levels on the cytoskeleton and tau in cultured human cortical neurons. Mol Chem Neuropathol. 1991;15:117–142. doi: 10.1007/BF03159951. [DOI] [PubMed] [Google Scholar]
- McNeil RS, Swann JW, Brinkley BR, Clark GD. Neuronal cytoskeletal alterations evoked by a platelet-activating factor (PAF) analogue. Cell Motil Cytoskeleton. 1999;43:99–113. doi: 10.1002/(SICI)1097-0169(1999)43:2<99::AID-CM2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Potrebic SB, Mason P. Three-dimensional analysis of the dendritic domains of on- and off-cells in the rostral ventromedial medulla. The Journal of Comparative Neurology. 1993;337:83–93. doi: 10.1002/cne.903370106. [DOI] [PubMed] [Google Scholar]
- Reid MS, Herrera-Marschitz M, Hokfelt T, Ohlin M, Valentino KL, Ungerstedt U. Effects of intranigral substance P and neurokinin A on striatal dopamine release--I. Interactions with substance P antagonists. Neuroscience. 1990;36:643–658. doi: 10.1016/0306-4522(90)90007-q. [DOI] [PubMed] [Google Scholar]
- Ringel I, Horwitz SB. Effect of alkaline pH on taxol-microtubule interactions. J Pharmacol Exp Ther. 1991;259:855–860. [PubMed] [Google Scholar]
- Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116:761–773. doi: 10.1016/s0306-4522(02)00748-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61:133–168. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sherrington SE, Jacobs JR, Stevens JK. Intracellular control of axial shape in non-uniform neurites: a serial electron microscopic analysis of organelles and microtubules in AI and AII retinal amacrine neurites. J Cell Biol. 1984;98:1279–1290. doi: 10.1083/jcb.98.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Euteneuer U, Bulinski JC, Izant JG. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981;78:1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dempster DW. “Epileptic” brain damage is replicated qualitatively in the rat hippocampus by central injection of glutamate or aspartate but not by GABA or acetylcholine. Brain Res Bull. 1985;15:39–60. doi: 10.1016/0361-9230(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Solomon F. Neuroblastoma cells recapitulate their detailed neurite morphologies after reversible microtubule disassembly. Cell. 1980;21:333–338. doi: 10.1016/0092-8674(80)90469-9. [DOI] [PubMed] [Google Scholar]
- Song B, Marvizon JC. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce micro-opioid receptor internalization in the rat spinal cord. J Neurosci. 2003;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GR, Olney JW, Pathikonda M, Snider WD. Excitotoxicity in the embryonic chick spinal cord. Ann Neurol. 1991;30:758–766. doi: 10.1002/ana.410300604. [DOI] [PubMed] [Google Scholar]
- Tanelian DL, Markin VS. Biophysical and functional consequences of receptor-mediated nerve fiber transformation. Biophysical Journal. 1997;72:1092–1108. doi: 10.1016/S0006-3495(97)78759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sykes KT, Buhler AV, Hammond DL. Electrophysiological heterogeneity of spinally projecting serotonergic and nonserotonergic neurons in the rostral ventromedial medulla. J Neurophysiol. 2006;95:1853–1863. doi: 10.1152/jn.00883.2005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol. 2009;102:1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]