Abstract
Monocyte-derived macrophages are critical in the host foreign body response to biomaterials and have been studied extensively in various culture conditions in vitro such as medium supplemented with fetal bovine serum (FBS) or autologous human serum (AHS). Since monocyte maturation into macrophages is highly plastic and may vary considerably depending on the surface, isolation procedures, and in vitro culture conditions, we hypothesize that variations in protein adsorption and serum type will greatly impact monocyte behavior in a surface-dependent manner. The impact of xenoproteins on monocyte-surface interaction is not well studied methodically and the use of AHS rather than FBS for macrophage-biomaterials studies in vitro is far from universal. The commonly used reference materials: tissue culture polystyrene (TCPS), polyethylene glycol (PEG), and poly-dimethylsiloxane (PDMS) were employed in this study and we found a 3-fold higher adherent monocyte density on TCPS when AHS was used versus FBS-supplemented medium. On PEG hydrogels, an 8-10 fold higher adhesion density was observed when AHS was employed versus FBS, while on PDMS no difference in adhesion density was observed between the two sera conditions. Additionally, the presence of lipopolysaccharide abrogated the serum-dependent effect on cell adhesion on TCPS. Significant differential variations in protein release were observed between the serum conditions on these surfaces, in particular there was a 100-fold higher concentration of growth-related oncogene for the AHS condition on PDMS even though the adhesion levels were comparable between the two serum conditions. These results emphasize the combined impact of the surface type and FBS xenoproteins in mediating the observed monocyte response to biomaterials in vitro.
Keywords: monocyte, macrophage, fetal bovine serum, polyethylene glycol, PDMS, human serum
Introduction
Monocyte-derived macrophages function in multiple phases of the host response, both promoting pro-inflammatory and anti-inflammatory activities through the release of numerous chemical mediators and influencing the adaptive immune response through the presentation of antigens [1]. Thus, monocyte-dervied macrophages are a significant cell type in the investigation of material-mediated inflammation and wound healing. Multiple differences between transformed macrophage cell lines and primary macrophages matured from peripheral blood monocytes have been observed, consequently primary macrophages are likely more clinically-relevant to biomaterials research [2-4]. Monocytes may be isolated via a variety of means including flow cytometry, density gradients, and surface adhesion; however, due to the plasticity of monocytes each of these methods may lead to variation in the final isolated monocyte phenotype [5,6]. Much like the plasticity observed in vivo with organ-specialized monocyte-derived macrophages, variable protein adsorption to biomaterial surfaces may lead to surface-specific macrophage phenotypes [7,8].
Similarly to the effects of the surface on monocyte maturation into macrophages, culture conditions can dramatically affect the maturation process. The phenotype can be modified in vitro by exposure to various cytokines or exogenous stimuli such as lipopolysaccharide (LPS) during the maturation process [9-11]. Both fetal bovine serum (FBS) and autologous human serum (AHS) are in use as additions in in vitro culture conditions in biomaterials research [12-16]. The role of AHS and FBS in modifying monocyte maturation (via surface antigens and protein release) has been studied extensively in suspended monocyte-derived dendritic cells (DC) for clinical applications [17-21]; however, the impact of FBS xenoproteins on human non-DC monocytes, and more specifically on biomaterial-monocyte interactions, is not limited [22,23]. Depending on the surface properties, biomaterials promote differential adsorption of certain serum proteins, and thus we hypothesized that the effect of serum type may vary by surface [1]. For example, polyethylene glycol (PEG) hydrogels limit adsorption of common serum proteins but may sustain significant complement component 3 (C3) activity comparable to other surfaces, potentially allowing this protein to take on a more influential role in mediating monocyte/macrophage responses [24]. FBS high abundance proteins include: bovine serum albumin (BSA), alpha-1-antiproteinase, plasminogen, lactoperoxidas, kniogen (LMW II), alpha-2-HS-glycoprotein, hemiferrin, prothrombin, apolipoprotein A-I, integrin beta-1, IGFBP2, IGF II, TGF-beta1 [25]. AHS high abundance proteins include: albumin, complement factor H, angiotensinogen, prostate-specific antigen [26]. Thus it is likely that the surface concentration and protein identity would differ significantly between AHS and FBS. Furthermore, there is a lack of extensive proteomic analysis of AHS and FBS with a specific focus on adhesion proteins for monocytes.
In this study, human blood-derived monocytes were obtained from multiple donors and cultured in FBS- or AHS-supplemented medium on PEG hydrogels, tissue culture polystyrene (TCPS), and polydimethylsiloxane (PDMS). TCPS and PDMS were selected because they are both commonly used reference materials with differing surface properties, and PEG hydrogels were selected because they have been shown to support monocyte adhesion while limiting protein adsorption along with having substantially differing surface properties from TCPS and PDMS [1,16,24, 27,28]. PEG hydrogels are highly hydrophilic swollen polymer networks, while the PDMS discs are highly hydrophobic films, and TCPS is a rigid, slightly hydrophobic surface. Exogenous lipopolysaccharide (LPS) was employed as a positive control for the innately activated highly pro-inflammatory macrophage maturation state, which bear similarities to the classically activated M1 macrophage cell type but do not require concurrent interferon-gamma (IFN-γ) [29]. To initially assess the extent of cell activation, selected critical proteins were quantified: broadly acting inflammatory cytokines IL-1β, interluekin-1 alpha (IL-1α), and tumor necrosis factor alpha (TNF-α); growth factors including granulocyte macrophage-colony stimulating factor (GM-CSF) and transforming growth factor-alpha (TGF-α); and chemokines including induced protein-10 (IP-10) and monocyte chemoattractant protein-1 (MCP-1). To further assess the polarization state of the adherent cells, both surface CD86 and CD163 markers and soluble pro-inflammatory and anti-inflammatory protein markers were quantified: interleukin-12 (IL-12(p40) and IL-12(p70)), growth-related oncogene-2 (GRO-2 or CXCL2), TNF-α, IFN-γ, IL-10, and IL-1β [29]. These molecules are among the many biomarkers for the pro-inflammatory M1 and more diverse / anti-inflammatory M2 states and thus may be used for comparison to these molecularly induced states [29]. The alternatively activated M2 phenotype is characterized by high levels of the anti-inflammatory signaling factors IL-1ra, IL-10 and IGF-1. While adhering to strict human subject safety standards, multiple donors were used for each portion of this study to garner statistical significance since large donor variability has been reported when using primary monocytes [30-34].
Materials and methods
Material preparation
PEG diacrylate (PEGdA) was synthesized from PEG-diol (MW 3400; Sigma) following previously established methods [16]. Briefly, PEG-diol was dissolved in dried THF at a 1g : 5ml ratio of PEG : THF. Acryloyl chloride (ACl) and triethylamine (TEA) were added at a 1:4:6 ratio of PEG : ACl : TEA, stirred in the dark for 3 hours, filtered twice, precipitated dropwise in a large volume of cold hexane (at least 100ml hexane per 1g starting PEG-diol), filtered, and dried in a vacuum oven. The PEGdA product was characterized via high performance liquid chromatography with purity typically >97%. To obtain PEG hydrogels, a mixture of 10 wt% PEGdA and 0.1 wt% UV photoinitiator (Irgacure 2959®) was dissolved in PBS at 60°C. The heated solution was pipetted into a polytetraflouroethylene mold (7mm diameter by 0.75mm thick) then clamped between two glass coverslips that were first cleaned in a sulfuric acid and nitric acid wash (3:1 respectively; 1N:1N). The entire assembly was placed under a UV lamp (UVP Model B 100AP) and polymerized for 3 minutes per side to form the PEG hydrogel. Samples were cold sterilized twice for 30 minutes with 70% ethanol and stored in sterile PBS for at least 24 hours for equilibration. Immediately before cell culture, samples were washed at least five times in sterile PBS to remove any residual traces of ethanol.
Medical grade PDMS (Specialty Manufacturing Inc; non-reinforced vulcanized gloss/gloss) in 0.030in thickness (∼0.76mm) was punched into 5/16in-diameter discs (7.9mm diameter) using a die and hammer punch. The diameter of 7.9 was chosen for consistency with the increase in size of the PEG hydrogels due to swelling. The PEG hydrogel diameter was measured after 24h of swelling in PBS and found to be 7.9 mm, while no swelling was observed for the PDMS discs. PDMS discs were sonicated and vortexed in a 0.5% solution of Triton X-100 for cleaning then cold sterilized in 70% ethanol twice for 30 minutes. The PDMS discs were stored in sterile PBS for at least 24 hours for equilibration and washed at least five times in sterile PBS prior to use. 48-well culture plates were used as the TCPS surface (BD Falcon). TCPS well plates were placed at room temperature with sterile PBS prior to use in culture. Both the PEG hydrogels and the PDMS discs were placed in 48-well culture plates prior to cell seeding.
Monocyte culture and analyses
Human subject protocol guidelines were followed for blood collection; however, these guidelines strictly limit the amount of blood that can be safely taken and severely limit the number of different assays that can be performed. Consequently, the same culture wells are tracked continuous for the length of these studies rather than stopped at each timepoint. Additionally, the same three donors were not available for the two major portions of this study; however, three donors were used for each portion and no statistical comparisons are made between the two portions that use different donors. Further, the high level of similarity in the results between the two donor groups suggests good reproducibility of these findings.
In the initial portion of the study we evaluated the monocyte/macrophage response to AHS/FBS on PEG and TCPS. Primary monocytes were isolated from the citrated whole blood of three medication-refrained healthy adult human volunteers via a well-established non-adherent density gradient procedure [15,16]. At the time of each donation, additional non-citrated blood was collected to obtain AHS. Briefly, the blood was incubated at 37°C for 3h followed by centrifugation. Serum was collected from the top of the tube away from the thrombus and care was taken to avoid collecting any red blood cells or blood clot. AHS was immediately aliquoted into smaller volumes for replenishment of the cell culture at every timepoint and frozen at -20°C. Immediately after isolation, cells were re-suspended in either 10% FBS (Atlanta Biologicals, triple 0.1μm filtered, not heat-inactivated) or 10% AHS (not heat inactivated) in RPMI 1640 media, then statically seeded in 48-well TCPS plates with or without the PEG hydrogels at a concentration of (1,000,000 cells/ml). Cell cultures were maintained at 5% CO2 and 37°C. At each culture time point (2h, 24h, 4d, 7d, and 14d), culture supernatant was collected for protein analysis (described below). Following supernatant collection, the cells were washed twice and the media was replenished. Between the 7d and 14d time points, the cells were washed twice and the media replenished every 2 days. The cultures were continued for 14d, and thus the images used for calculating adhesion densities followed the same wells for the entire 14 days.
The wells were imaged using an inverted light microscope (Nikon, Eclipse TE300) coupled to a computer-assisted video analysis system (MetaMorph v4.1) at 2h, 24h, 4d, 7d, and 14d. A minimum of three images was taken of each well at 20× (0.36 mm2), from which the adherent cell density was determined. Protein analysis was performed on the culture supernatant via a microsphere-based multiplex assay (Bio-Rad, Millipore). Three samples were analyzed per donor at each time point. The microsphere assays were performed according to the manufacturers' instructions for all analytes: IL-1β, IL-1α, TNF-α, GM-CSF, TGF-α, IP-10, MCP-1 (Millipore Millplex Map). Standard curves were determined with 10% FBS and 10% AHS for each donor to ensure elimination of the background presence of the protein targets in the serum. The standard curve for the microsphere assay utilized standards up to 10,000 pg/ml and was a 5 parameter logistic (5PL) regression curve that accounts for asymmetry in the low and high concentration ends of the assay. Thus, it is S-shaped with linearity in the mid concentration levels and non-linearity at the high and low ends of the curve. All extrapolated data is above the highest standard concentration of 10,000 pg/ml, but is still below the upper asymptote of the regression curve equation. While the suppliers' Technical documents make no claim on a measure of confidence for extrapolated values below the maximum asymptote of the curve, the confidence of those values is in line with the confidence of the interpolated data (within the standard range). In this study, all values above 20,000 pg/ml were reported as “20,000 pg/ml” to limit reliance on extrapolated data beyond two times the upper limit of the standard curve.
Following the differential adhesion results on PEG and TCPS, we expanded the study to include an additional surface and utilized different biomarkers to better characterize differences in the macrophage responses to serum type and surface. Further, LPS was used as a positive control for pro-inflammatory innately activated macrophages. Cells were isolated as described above and suspended in either 10% AHS in RPMI 1640, 10% FBS in RPMI 1640, 10% AHS with 100 ng/ml LPS, and 10% FBS with 100 ng/ml LPS. The suspended cells were then statically seeded at (1,000,000 cells/ml) on TCPS, PDMS, and PEG hydrogels. The cells were imaged as in part one at 2h, 24h, 4d, and 7d. Surfaces were also washed and the media replenished as before. Supernatant was collected at each time point to quantify IL-1β, IL-10, IL-12(p40), IL-12(p70), TNF-α, GRO-2, and IFN-γ as described above. At day 7 after imaging and supernatant collection, the adherent cells were fixed in 1% paraformaledhyde (PFA, Polysciences, Inc.) and stained with antibodies directed against CD86 conjugated to Alexa Fluor 488 (Biolegend) and CD163 conjugated to Alexa Fluor 647 (Biolegend) at the manufacturer's recommended dilution. Cells were imaged via fluorescent confocal microscope (Bio-Rad MRC-1024, Kr/Ar: 41, iris: 4.0, gain: 1284, offset: -5.0, room temperature, 0.30 numerical aperature for 10× and 0.50 for 20×, immersion medium is air, Lasersharp 5.2 software). Autofluorescence was minimal as was background staining with control antibodies (BioLegend). The brightness of the images was enhanced to improve image quality, however no quantitative conclusions were drawn based on differential brightness. Autofluorescence and background staining with control antibodies was found to be negligible and these black images are not shown.
Statistical analysis
Cell density and protein concentration data were analyzed via two-way analysis of variance and Tukey post testing (SigmaStat v2.03) with p values < 0.05 considered as significant.
Results
Initial monocyte culture with AHS or FBS on PEG or TCPS
The morphologies of the adherent cells were very different, with enhanced spreading observed for the AHS supplemented cultures on both TCPS and PEG (Fig. 1). When cultured in AHS but not with FBS, the cells took on more of a spindle shape on TCPS in contrast to a more rounded shape on PEG. This difference in shape became evident at the 4d timepoint and beyond but was not observable at 24h. The adherent cell density was vastly different on TCPS and PEG hydrogels when either AHS or FBS supplemented medium was used. For instance, at 2h on TCPS, the adherent cell density was 2-3 fold higher for the AHS condition than for the FBS condition, while on the PEG hydrogels the difference was nearly 10-fold higher (Fig. 2). This trend continued at 24h for both surfaces and remained consistent and statistically significant on PEG up to day 7, after which there was no longer a statistical difference in adhesion between the FBS and AHS conditions. IL-1β and TNF-α concentrations were significantly different between the AHS and FBS conditions in the presence of PEG hydrogels particularly at 24h (Fig. 3) while no difference was observed for IL-1α, GM-CSF, TGF-α, and IP-10 (not shown). The chemokine MCP-1 was generally high for both surfaces and serum conditions from 24 hr thereafter (Fig. 4). This is consistent with other reported studies where TCPS produced increases in pro-inflammatory markers over PDMS [45]. Taken together, these results indicate that selected monocyte/macrophage responses (i.e., adhesion, IL-1β and TNF-α release) were modulated by both the serum proteins present and the substrate; while other monocyte/macrophage responses (i.e., GM-CSF release) were not.
Figure 1.
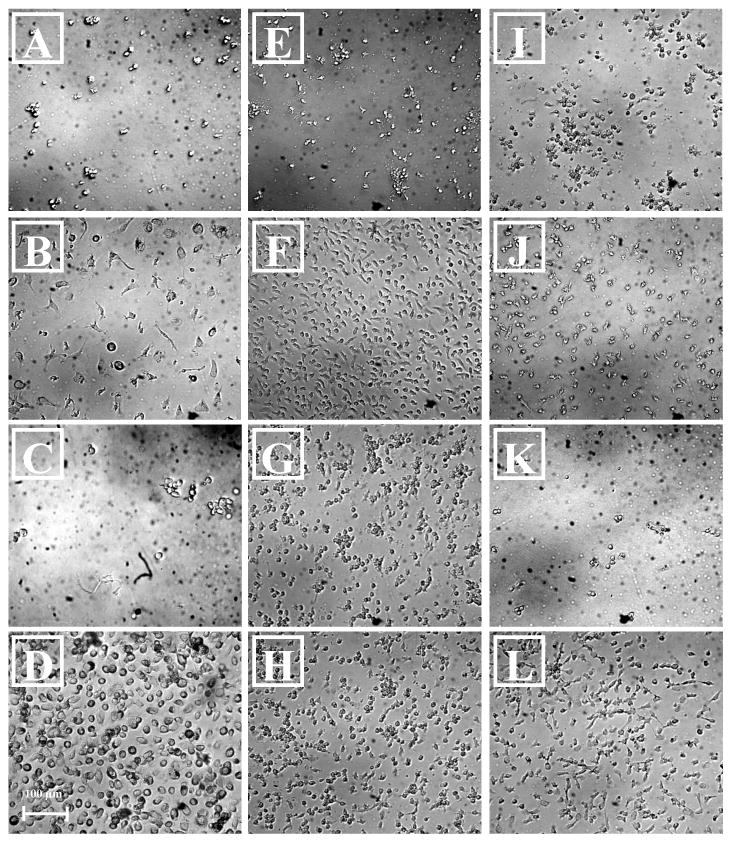
Light microscopy photomicrograph of adherent cells on TCPS and PEG hydrogels: (20× magnification) 4d in 10% FBS supplemented media on TCPS (A), 4d in 10% AHS supplemented media on TCPS (B), 4d in 10% FBS supplemented media on PEG hydrogel (C), 4d in 10% AHS supplemented media on PEG hydrogel (D), 24h in 10% FBS supplemented media on TCPS (E), 24h in 10% AHS supplemented media on TCPS (F), 24h in 10% FBS and 100ng/ml LPS supplemented media on TCPS (G), 24h in 10% AHS and 100 ng/ml LPS supplemented media on TCPS (H), and on PDMS and PEG: 24h in 10% FBS supplemented media on PDMS (I), 24h in 10% AHS supplemented media on PDMS (J), 24h in 10% FBS on PEG (K), 24h in 10% AHS on PEG (L).
Figure 2.
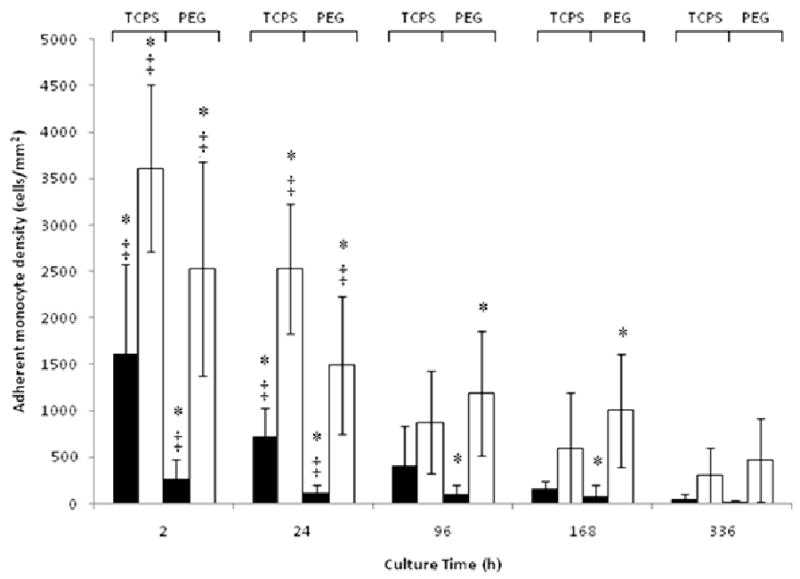
Adherent cell density on TCPS or PEG hydrogels cultured with FBS (▬) or AHS (▭). All data is presented as average ± standard deviation (n = 3 human blood donors).
*: Differs significantly from other serum type on same surface at same time point (p < 0.05)
‡: Differs significantly from other surface with same serum type at same time point (p < 0.05)
Figure 3.
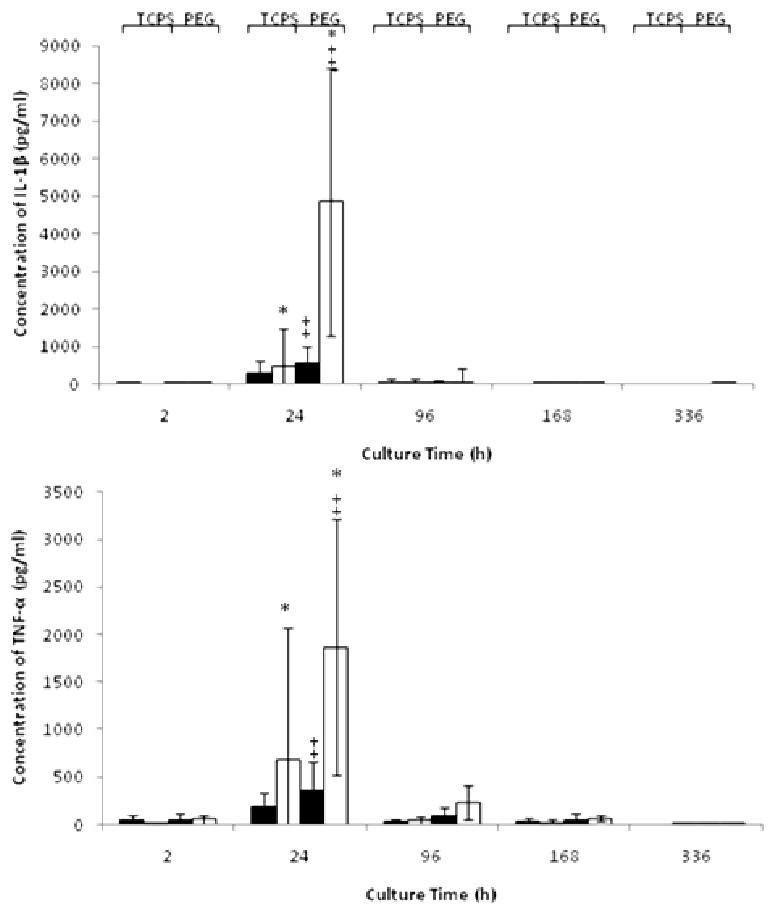
Concentration (pg/ml) of IL-1β and TNF-α in the presence of TCPS or PEG cultured with FBS (▬) or AHS (▭). All data is presented as average ± standard deviation (n = 3 human blood donors).
*: Differs significantly from other serum type on same surface at same time point (p < 0.05)
‡: Differs significantly from other surface with same serum type at same time point (p < 0.05)
Figure 4.
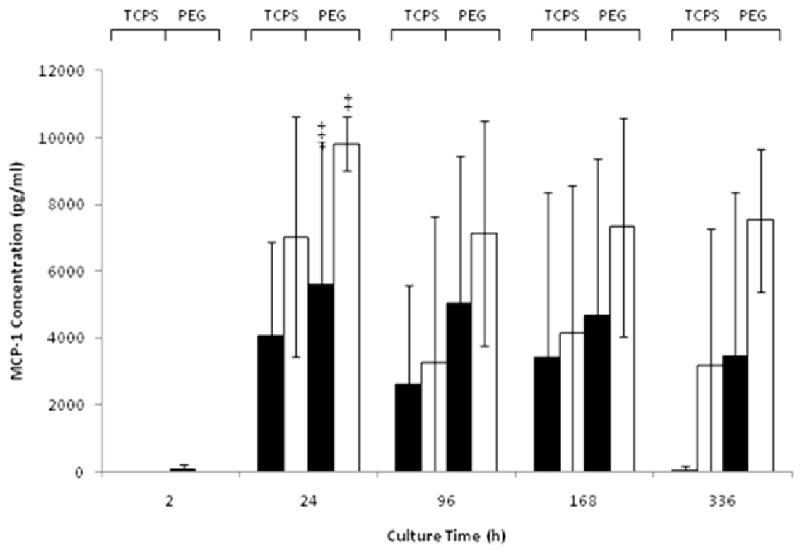
Concentration (pg/ml) of MCP-1 in the presence of TCPS or PEG cultured with FBS (▬) or AHS (▭). All data is presented as average ± standard deviation (n = 3 human blood donors).
*: Differs significantly from other serum type on same surface at same time point (p < 0.05)
‡: Differs significantly from other surface with same serum type at same time point (p < 0.05)
Monocyte maturation modulated by material and xenoproteins
Primary monocytes were statically seeded on TCPS with or without exogenous LPS, PDMS, and PEG hydrogels using AHS or FBS-supplement medium. At 2h and 24h, the adherent cell density on TCPS was significantly higher when cultured with AHS than FBS (Fig. 5). Further, for the entire 7d culture, the adherent monocyte density on PEG was significantly higher (approximately 10-fold) when cultured with AHS than with FBS. The adherent monocyte density on PEG for the AHS condition was also higher than that on PDMS or TCPS with or without LPS at 4d. In contrast to TCPS without LPS and PEG, monocyte adhesion to PDMS and TCPS cultured with LPS did not differ between the two serum conditions for the entire 7d culture. The morphology of the adherent monocytes on the various surfaces differed depending on the serum condition (Fig 1). Greater cell spreading was observed for PEG and TCPS surfaces when the culture medium was supplemented with AHS instead of FBS, but not for PDMS and TCPS cultured with LPS. Adherent monocytes were stained for CD86 and CD163 at 7d (Figure 6) when a mature macrophage phenotype would be expected [35]. Adherent monocytes/macrophages on all surfaces and cultured with either AHS or FBS supplementation stained for CD86, in contrast, little to no staining was observed for CD163, a surface antigen involved in endocytosis [29].
Figure 5.
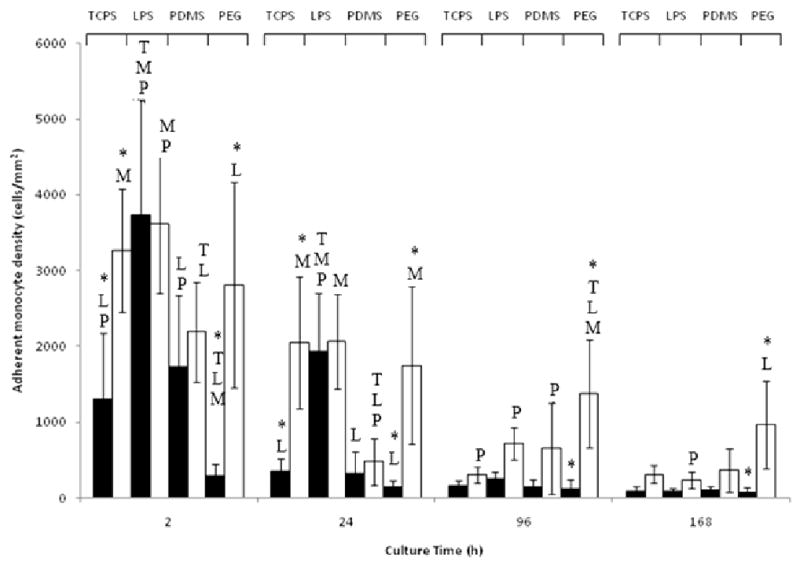
Adherent cell density on TCPS, TCPS supplemented with 100 ng/ml LPS (LPS), PDMS, or PEG hydrogels (PEG) cultured with FBS (▬) or AHS (▭). All data is presented as average ± standard deviation (n = 3 human blood donors).
T: Differs significantly from TCPS (p < 0.05)
L: Differs significantly from TCPS with added LPS (p < 0.05)
M: Differs significantly from PDMS (p < 0.05)
P: Differs significantly from PEG (p < 0.05)
*: Differs significantly from other serum type on same surface at same timepoint (p < 0.05)
Figure 6.
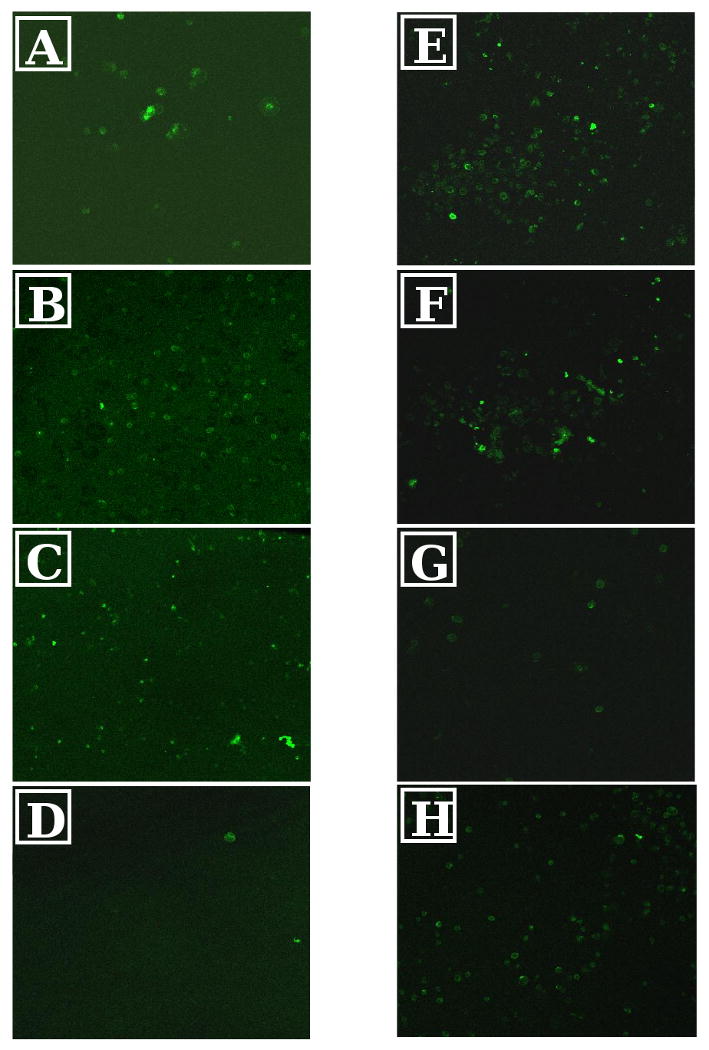
Confocal microscopy photomicrograph of adherent cells on (20× magnification): 7d in 10% FBS supplemented media on TCPS (A), 7d in 10% AHS supplemented media on TCPS (B), 7d in 10% FBS and 100ng/ml LPS supplemented media on TCPS (C), 7d in 10% AHS and 100 ng/ml LPS supplemented media on TCPS (D), 24h in 10% FBS supplemented media on PDMS (E), 24h in 10% AHS supplemented media on PDMS (F), 24h in 10% FBS on PEG (G), 24d in 10% AHS on PEG (H). Green staining is of CD86 while red staining is of CD163.
Various protein expressions showed dependency on serum type and surface type (Fig 7). Specifically, the GRO-2 concentration was significantly higher by nearly 100-fold at 24h for monocytes adherent on PDMS with AHS supplemented medium as compared to that of FBS. The GRO-2 concentration was also significantly higher for monocytes adherent on PEG with AHS supplemented medium as compared to FBS at 24h and 4d. The IFN-γ concentration for monocytes cultured with FBS was significantly higher than that with AHS. The IL-1β concentration was significantly higher for monocytes cultured on TCPS with LPS and AHS at 24h than all other surfaces supplemented with AHS. The IL-1β concentration was also significantly higher for monocytes on PEG cultured with AHS than with FBS at 24h. The IL-12 p40 fraction concentration was significantly higher for monocytes adherent on PEG supplemented with AHS compared to TCPS or PDMS. The p70 fraction of IL-12 was virtually undetected on all surfaces under all conditions. The TNF-α concentration was significantly higher for monocytes adherent on PEG with culture supplemented with AHS as compared to FBS. Lastly, as expected, the protein concentrations for the LPS positive control were significantly higher at various time points for GRO-2, TNF- α and IL-10. Additionally, IFN-γ was measurable at 24h for the LPS conditions and not for the others (Fig. 7).
Figure 7.
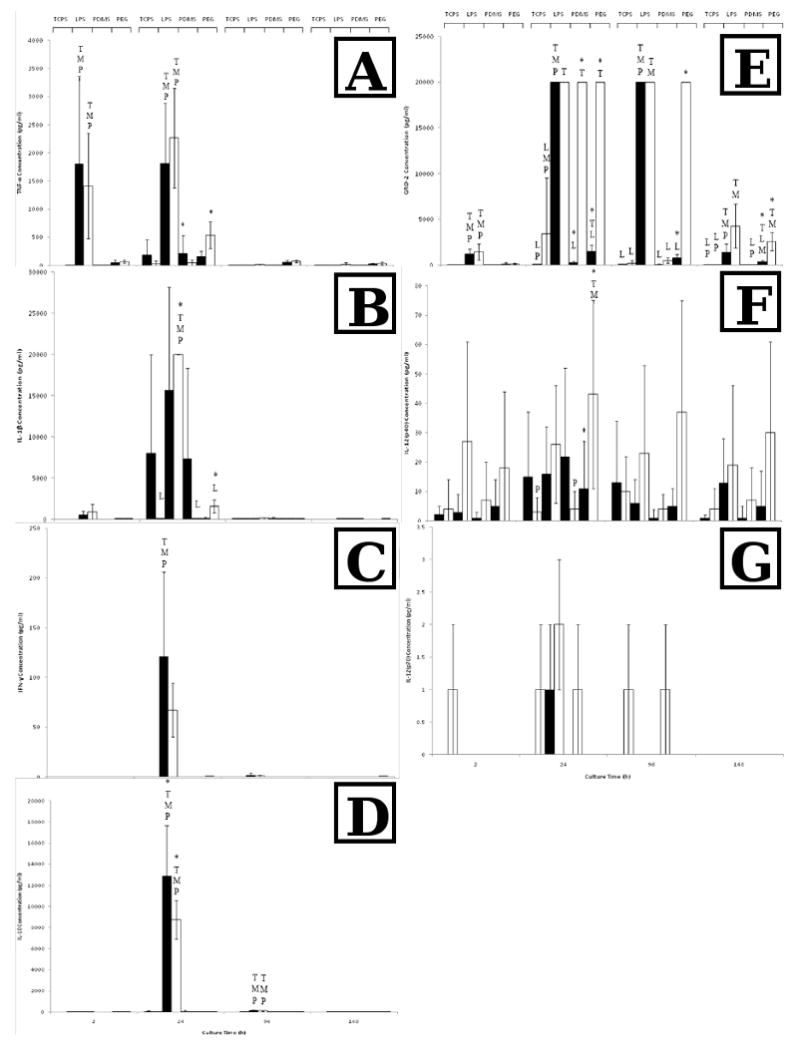
TNF-α (A), IL-1β (B), IFN-γ (C), IL-10 (D), GRO-2 (E), IL-12(p40) (F) and IL-12(p70) (G) concentrations for TCPS, TCPS supplemented with 100 ng/ml LPS (LPS), PDMS, or PEG hydrogels (PEG) cultured with FBS (▬) or AHS (▭). All data is presented as average ± standard deviation (n = 3 human blood donors).
T: Differs significantly from TCPS (p < 0.05)
L: Differs significantly from TCPS with added LPS (p < 0.05)
M: Differs significantly from PDMS (p < 0.05)
P: Differs significantly from PEG (p < 0.05)
*: Differs significantly from other serum type on same surface at same timepoint (p < 0.05)
Discussion
The increased spreading of the cells for the AHS conditions on PEG and TCPS suggests that the combination of soluble and adsorbed AHS proteins may have better allowed for greater cytoplasmic extension in comparison to the xenogenic bovine proteins. Greater cell spreading on TCPS for the AHS condition has previously been observed in monocytes being driven to form foam cells, and our observations align with these published images [36]. Cells adhere via interaction with adsorbed proteins, thus the difference in both cell spreading and in the adhesion levels may indicate that the human serum proteins were more bioavailable for cell interaction and that the AHS proteins were likely more recognizable to receptors on the cell surface compared to proteins found in FBS [37]. The differential adhesion levels between the AHS and FBS (3-fold for TCPS, 10-fold for PEG) suggest that the material surface can greatly influence the serum effect on monocyte adhesion. Given that the only difference between the FBS and AHS conditions are the proteins present, this phenomenon of differential adhesion may have an explanation rooted in protein adsorption to the biomaterials. For example, PEG hydrogels are well known as resistant to protein adsorption; however, a study on the PEG-like surface tetraglyme indicated that complement protein C3 is the primary mediator of monocyte adhesion to tetraglyme [38]. Additionally, C3 activity on PEG hydrogels is comparable to TCPS, and monocyte adhesion to PEG hydrogels is lowered when using C3-depleted serum [24]. C3 is present only at very low, nearly undetectable levels in FBS, while adult humans have a C3 concentration of approximately 75-135 mg/dl [39,40]. Thus, if more C3 were present in the AHS condition this could potentially have promoted more cell adhesion once C3 was adsorbed/absorbed to the PEG hydrogel, although C3 was likely not the only factor as the differential adhesion in this case is 10-fold and in the C3-depleted study reduced cell adhesion at 24h was closer to half [24]. Monocyte adhesion to TCPS is mediated by a number of proteins, including C3, and thus is not subjected to such substantial differences in cell adhesion between FBS and AHS due to a dependence on a single protein for adhesion [15,33]. To further corroborate this, activated C3 (C3b and iC3b) has been shown to complex with IgG thereby masking IgGs surface-bound presence or releasing it from a materials surface [41] and immobilized, not soluble, IgG is a potent inducer of the anti-inflammatory cytokine IL-1ra [42], thus the highly activated C3 on PEG in the AHS condition could partially explain the observed inflammatory response. These findings raise important concerns when making a comparison between monocyte biomaterial studies that utilize different types of serum. The study also indicate the potential role of PEG in influencing monocyte binding to a single adsorbed protein (i.e. C3).
Differences in protein concentrations were also observed between AHS- versus FBS-treated groups. The higher concentrations of IL-1β and TNF-α on the PEG surface compared to TCPS suggest a heightened inflammatory response to PEG, as has been demonstrated previously and may be due to adsorbed C3 [16,43,44]. However, the increased amount of inflammatory protein secretion for the AHS condition on PEG compared to the FBS condition may also be the result of the substantial difference in cell adhesion. While not statistically significant, differences in the GM-CSF, TGF-α, and IP-10 concentration between serum and surface types may have been masked by the high level of deviations that are commonly associated with donor variability, while the concentration of the chemokine MCP-1 detected in the cultures may simply be an overall response to the stress of cell culture where the MCP-1 concentrations on PDMS and TCPS were both comparably high. The stress on cells induced by the culture condition has been previously reported by another group [45].
As with the first portion of the study, the difference in adhesion between AHS/FBS was much greater for PEG than TCPS, showing that this finding is consistent. However, the lack of observed differences in the adhesion level on TCPS between serum types in the presence of LPS is likely due to LPS activation. LPS has been shown previously to enhance monocyte adhesion, and this was true for the FBS condition at 2h and 24h [46]. This enhanced monocyte adhesion was likely not observed for the AHS condition because the adherent monocyte density on TCPS with AHS supplementation was already very high. Thus, any increase in adhesion due to LPS activation may not have been observed due to the unavailability of space on the surface for additional cells to adhere. Similarly, no difference in cell adhesion was observed for all time points between AHS and FBS supplementation on PDMS. PDMS is a hydrophobic surface that can mediate a high level of protein adsorption, which may have negated any possible contribution by the functionality of the adsorbed proteins (i.e., AHS vs FBS) in promoting cell adhesion [47,48]. This explanation further aligns with the observed lack of a difference in cell spreading between the AHS and FBS conditions on PDMS and TCPS cultures with LPS.
The adherent cells on all surfaces for both serum conditions stained for CD86, a surface antigen involved in co-stimulation of T cell activation and proliferation that is indicative of a pro-inflammatory M1 macrophage phenotype [31, 49]. In contrast, even on extremely high laser settings, little to no staining was observed for CD163, a scavenger receptor more indicative of a deactivated, pro-healing maturation state [31]. The consistent CD86 expression suggests that the cells are maturing and also that a basal level of stress from cell culture is plausible [45]. Additionally, it is also plausible that the culture system was lacking a specific signal to trigger the cells to shift form an M1 to an M2 phenotype.
We further quantified the concentration of specific proteins associated with pro-inflammatory and anti-inflammatory states to better characterize the state of the adherent cells. The extremely high concentrations of the chemokine GRO-2 and the pro-inflammatory cytokine IL-1β under the +LPS condition indicates an expected highly pro-inflammatory response from the cells [31,50,51]. In sharp contrast, the 100-fold higher GRO-2 protein concentration for the AHS culture condition on PDMS over the FBS culture was unexpected, especially considering that the adhesion levels were very comparable. Macrophages have previously been shown to respond in a somewhat inflammatory manner to PDMS and PDMS is a hydrophobic polymer thus promoting protein adsorption [45]. Consequently, it is possible that the adsorbed AHS proteins, in contrast to the adsorbed FBS proteins, were in a conformation that better stimulated a large release of GRO-2. However, GRO-2 proteins can also be implicated in M2 activities and when considered with the low concentration of other inflammatory proteins at 24h for the AHS culture on PDMS may indicate similarities with macrophages that are not strongly M1 activated [29]. There are very few, if any, examples of monocyte studies on biomaterials that measure GRO-2 and thus this finding may suggest the importance of GRO-2 as a marker for monocyte/macrophage responses to biomaterials. Lastly, the higher GRO-2 concentration for the AHS condition on PEG is likely explained by the higher adherent cell density for the AHS condition and not a novel phenomena as observed on PDMS. However, the higher GRO-2 may be indicative of pro-inflammatory cellular response to the PEG hydrogels for both the AHS and FBS conditions.
IFN-γ is a pro-inflammatory cytokine that is rarely produced by macrophages and only under certain pro-inflammatory conditions, such as LPS supplementation [52]. Additionally, IL-10 is released in response to high concentrations of LPS to aid in the prevention of endotoxic shock, indicating that the innately activated macrophage control cells responded fairly predictably [53,54]. Conversely, the concentration of IL-10 was fairly low for all other conditions, indicating a lack of this type of anti-inflammatory/regulatory activity. This high IL-10 concentration for the LPS condition may at first appear to be counterintuitive as IL-10 is a potent anti-inflammatory molecule [29]. However, IL-10 is also released in response to high concentrations of LPS to aid in the prevention of endotoxic shock [53,54]. Thus, the concurrent upregulation of pro- and anti-inflammatory cytokines, especially under LPS-challenged condition, would not be unexpected. Further, although the concentration of IL-12(p40) tended to be higher for the +LPS condition, high levels of error rendered this result not statistically significant.
In contrast to the initial portion of the study, high levels of IL-1β were observed on TCPS for the FBS condition although not statistically significant. This was also observed for the FBS condition on PDMS. However, the standard deviation was very large. This was because the extremely high levels of IL-1β came from one blood donor and were not observed in the other donors, potentially indicating that this donor's macrophages may have some sensitivity to the bovine proteins in FBS. Contamination of the FBS is unlikely as FBS from the same bottle was used for the other donors in the study without similar response. Additionally, we have observed this phenomenon previously in our lab (unpublished data). Colli et al. previously demonstrated higher IL-1β production for macrophages cultured on TCPS with FBS compared to those cultured with AHS; however, when considering the present results the higher level of IL-1β may be a donor-specific response [36]. Donor variability can be substantial in primary monocyte cultures but is not always explicitly documented in the biomaterials literature. Surprisingly, a higher IL-1β concentration for the FBS condition on PEG was not observed, suggesting that the surface also plays a role in mediating release of IL-1β from FBS sensitive macrophages. The lack of many surface proteins to bind to for the FBS condition on PEG may have influenced this low IL-1β release. The TNF-α concentration paralleled the results for IL-1β, and again were primarily due to one blood donor with a strong response.
Conclusions
The impact of xenoproteins in FBS on the interaction between material and primary human cells is not well documented, and thus this study raises important considerations for the culture medium when comparing literature on monocyte adhesion and inflammatory responses to biomaterials. Our study implicates the critical role of the biomaterial surface in tandem with the culture serum in mediating the adhesion and protein release of human blood-derived monocytes. For example, monocyte adhesion was approximately 3-fold higher on TCPS in the presence of AHS as compared to FBS, while on PEG hydrogels this difference was much greater (10-fold). Further, the addition of LPS or the use of PDMS eliminated any differential cell adhesion between the two serum types. Through various markers, we also observed that most of the cells exhibited pro-inflammatory markers although the response on PEG tended to be higher, and further GRO-2 protein was secreted at a 100-fold higher concentration for the AHS condition on PDMS compared to FBS, and this phenomena was unique to this surface.
Acknowledgments
This study was supported in part by NIH grants HL77825 and EB6613.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JM. Inflammation, wound healing, and the foreign-body response. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. San Diego: Elsevier Academic Press; 2004. pp. 296–304. [Google Scholar]
- 2.Katsikis J, Yu H, Maurer F, Medcalf R. The molecular basis for the aberrant production of plasminogen activator inhibitor type 2 in THP-1 monocytes. Thromb Haemost. 2000;84:468–73. [PubMed] [Google Scholar]
- 3.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line. Int J cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 4.Rothlein R, Springer TA. The requirements for lymphocyte function-associated antigen in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986;163:1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generate dendritic cells. Immunology. 2005;144:204–212. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehner M, Holter W. Endotoxin-free purification of monocytes for dendritic cell generation via discontinous density gradient centrifugation based on diluted Ficoll-Paque Plus. Int Arch Allergy Immunol. 2002;128:73–76. doi: 10.1159/000058006. [DOI] [PubMed] [Google Scholar]
- 7.Labow RS, Meek E, Santerre JP. Hydrolytic degradation of poly(carbonate)-urethanes by monocyte-derived macrophages. Biomaterials. 2001;22:3025–3033. doi: 10.1016/s0142-9612(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 8.Collier TO, Anderson JM. Protein and surface effects on monocyte and macrophage adhesion, maturation, and survival. J Biomed Mater Res. 2002;60:487–496. doi: 10.1002/jbm.10043. [DOI] [PubMed] [Google Scholar]
- 9.Shi C, Simon DI. Integrin signals, transcription factors, and monocyte differentiation. Trends Cardiovasc Med. 2006;16:146–152. doi: 10.1016/j.tcm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Watson RWG, Redmond HP, Bouchierhayes D. Role of endotoxin in mononuclear phagocyte-mediated inflammatory responses. J Leukocyte Biol. 1994;56(1):95–103. doi: 10.1002/jlb.56.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo MD, Salter CE, Hurley DJ, Moore JN. A comparison of equine and bovine sera as sources of lipopolysaccharide-binding protein activity in equine monocytes incubated with lipopolysaccharide. Vet Imunol Immunopathol. 2008;121:275–280. doi: 10.1016/j.vetimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.McBane JE, Matheson LA, Sharifpoor, Santerre, Labow RS. Effect of polyurethane chemisrtry and protein coating on monocyte differentian towards a wound healing phenotype macrophage. Biomaterials. 2009;30:5497–5504. doi: 10.1016/j.biomaterials.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Boynton EL, Waddell J, Meek E, Labow RS, Edwards V, Santerre JP. The effect of polyethylene particle chemistry on human monocye-macrophage function in vitro. J Biomed Mater Res. 2000;52:239–245. doi: 10.1002/1097-4636(200011)52:2<239::aid-jbm1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Labow RS, Meek E, Santerre JP. Hydrolytic degradation of poly(carbonate)-urethanes by monocyte-derived macrophages. Biomaterials. 2001;22:3025–3033. doi: 10.1016/s0142-9612(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 15.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Am J Pathol. 1995;147(5):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt DR, Kao WJ. Monocyte activation in response to PEG hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths. J Biomed Mater Res. 2007;83A(3):617–625. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- 17.Moldenhauer A, Nociari MM, Dias S, Lalezari P, Moore MAS. Optimized culture conditions for the generation of dendritic cells from peripheral blood monocytes. Vox Sanguinis. 2003;84:228–236. doi: 10.1046/j.1423-0410.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 18.Pietschmann P, Stockl J, Draxler S, Majdic O, Knapp W. Functional and phenotypic characteristics of dendritic cells generated in human plasma supplemented medium. Scand J Immunol. 2000;51:377–383. doi: 10.1046/j.1365-3083.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 19.Royer PJ, Tanguy-Royer S, Ebstein F, Sapede C, Simon T, Barbieux I, Oger R, Gregoire M. Culture medium and protein supplementation in the generation and maturation of dendritic cells. Scand J Immunol. 2005;63:401–409. doi: 10.1111/j.1365-3083.2006.001757.x. [DOI] [PubMed] [Google Scholar]
- 20.Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K, Schmitt D, Gebuhrer L, Rigal D. Distinct subsets of dendritic cells resembling DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Meth. 2000;238:119–131. doi: 10.1016/s0022-1759(00)00147-2. [DOI] [PubMed] [Google Scholar]
- 21.Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Schmitt D, Gebuhrer L, Rigal D. Human serum or autologous plasma, allows generation of more mature dendritic cells than fetal calf serum, or serum-free medium. Exp Hematol. 1999;27(7):46–46. [Google Scholar]
- 22.Mazlyzam AL, Aminuddin BS, Saim L, Ruszymah BH. Human serum is an advantageous supplement for human dermal fibroblast expansion: clinical implications for tissue engineering of skin. Arch Med Res. 2008;39:743–752. doi: 10.1016/j.arcmed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Shahdadfar A, Fronsdal K, Haug T, et al. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Schmidt DR, Joyce EJ, Kao WJ. Application of MS-based proteomics to study serum protein adsorption/absorption and complement C3 activation on polyethylene glycol hydrogels. Accepted by J Biomater Sci, Polym Ed. doi: 10.1163/092050610X508400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiaoyang Z, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E. Proteomic Analysis for the Assessment of Different Lots of Fetal Bovine Serum as a Raw Material for Cell Culture. Part IV. Application of Proteomics to Manufacture of Biological Drugs. Biotechnol Prog. 2006;22:1294–1300. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- 26.Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a Human Blood Serum Proteome. Molec & Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- 27.Dewez JL, Doren A, Schneider YJ, Rouxhet PG. Competitive adsorption of proteins: key of the relationship between substratum surface properties and adhesion of epithelial cells. Biomaterials. 1999;20:547–559. doi: 10.1016/s0142-9612(98)00207-5. [DOI] [PubMed] [Google Scholar]
- 28.Naim JO, Van Oss CJ, Ippolito KML, Zhang JW, Jin LP, Fortuna R, Buehner NA. In vitro activation of human monocytes by silicones. Colloids Surf B Biointerfaces. 1998;11:79–86. [Google Scholar]
- 29.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot AEI, Buser R, Borlat F, Alouani S, Soler D, Offord RE, Schroder JM, Power CA, Wells TNC. Amino-terminally modified RANTES analogues demonstrate differential effects on RANTES receptors. J Biol Chem. 1999;274(45):32478–32485. doi: 10.1074/jbc.274.45.32478. [DOI] [PubMed] [Google Scholar]
- 31.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol. 1999;73(11):8966–8974. doi: 10.1128/jvi.73.11.8966-8974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardiner DL, Michinton R. The macrophage assay. Australian Journal of Medical Laboratory Science. 1987;8(2):19–24. [Google Scholar]
- 33.Atkinson S, Valadas E, Smith SM, Lukey PT, Dockrell HM. Monocyte derived macrophage cytokine responses induced by M-bovis. BCG. 2000;80(4-5):197–207. doi: 10.1054/tuld.2000.0247. [DOI] [PubMed] [Google Scholar]
- 34.Bol SM, Van Remmerden Y, Sietzema JG, Kootstra NA, Schuitemaker H, Van't Wout AB. Donor variation in in vitro HIV-1 susceptibility of monocyte-derived macrophages. Virology. 2009;390(2):205–211. doi: 10.1016/j.virol.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Dinnes DLM, Marcal H, Mahler SM, Santerre JP, Labow RS. Material surfaces affect the protein expression patterns of human macrophages: a proteomics approach. J Biomed Mater Res. 2007;80A:895–908. doi: 10.1002/jbm.a.30967. [DOI] [PubMed] [Google Scholar]
- 36.Colli S, Lalli M, Rise P, Mussoni L, Eliginia S, Galli C, Tremoli E. Increased thrombogenic potential of human monocyte-derived macrophages spontaneously transformed into foam cells. Thromb Haemost. 1999;81:576–781. [PubMed] [Google Scholar]
- 37.Horbett TA. The role of adsorbed proteins in tissue response to biomaterials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science An introduction to materials in medicine. San Diego: Elsevier Academic Press; 2004. pp. 237–246. [Google Scholar]
- 38.Mayorga L, Ratner BD, Horbett TA. The role of complement adsorption and activation in monocyte adhesion to ultralow protein adsorption surfaces made by RF plasma deposition and activation in monocyte adhesion to ultralow protein adsorption surfaces made by RF plasma deposition of PEO-like tetrathylene dimethyl ether (tetraglyme) World Biomaterials Congress. 2008;1162 [Google Scholar]
- 39.Linscott WD, Triglia RP. The bovine complement system. Adv Exp Med Biol. 1981;137:413–430. [PubMed] [Google Scholar]
- 40.Borigini MJ. Medical Encylopedia: Complement componenet 3 (C3) National Institutes of Health; [Google Scholar]
- 41.Nilsson UR. Deposition of C3b/iC3b leads to the concealment of antigens, immunoglobulin and bound C1q in complement-activating immune complexes. Mol Immunol. 2001;38:151–160. doi: 10.1016/s0161-5890(01)00039-6. [DOI] [PubMed] [Google Scholar]
- 42.Clinchy B, Gunneras M, Hakansson A, Hakansson L. Production of IL-1Ra by human mononuclear blood cells in vitro: Influence of serum factors. Cytokine. 2006;34:320–330. doi: 10.1016/j.cyto.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RJ. The complement system. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: An introduction to materials in medicine. San Diego: Elsevier Academic Press; 2004. pp. 318–328. [Google Scholar]
- 44.Griffin FM, Mullinax PJ. In vivo activation of macrophage C3 receptors for phagocytosis. J Exp Med. 1985;162:352–357. doi: 10.1084/jem.162.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matheson LA, McBane JE, Malowany JI, Santerre JP, Labow RS. Is cell culture stressful? Effects odegradable and nondegradable culture surfaces on U937 cell function. Biotechniques. 2007;42:744–750. doi: 10.2144/000112460. [DOI] [PubMed] [Google Scholar]
- 46.Kang YH, Lee CH, Brummel SE, Newball HH, Forrester J. Effects of endotoxin on expression of VLA integrins by human bronchoalveolar lavage macrophages. J Leukoc Biol. 1995;57:624–634. doi: 10.1002/jlb.57.4.624. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt DR, Waldeck H, Kao WJ. Protein adsorption to biomaterials. In: Bizios R, Puleo D, editors. Biological interactions on materials surfaces: understanding and controlling protein, cell, and tissue. New York: Springer; 2009. pp. 1–18. [Google Scholar]
- 48.Chen H, Brook MA, Sheardown H. Silicone elastomers for reduced protein adsorption. Biomaterials. 2004;25:2273–2282. doi: 10.1016/j.biomaterials.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 50.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- 51.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30(3):459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho-Pinto CE, Garcia MI, Mellado M, Rodriguez-Frade JM, Martin-Caballero J, Flores J, Martinez AC, Balomenos D. Autocrine production of IFN-gamma by macrophages controls their recruitment to the kidney and the development of glomerulonephritis in MRL/lpr mice. J Immunol. 2002;169:1058–1067. doi: 10.4049/jimmunol.169.2.1058. [DOI] [PubMed] [Google Scholar]
- 53.Kwan WH, Boix C, Gougelet N, Fridman WH, Mueller LPS induces rapid IL-10 release by M-CSF-conditioned, tolerogenic dendritic cell precursors. J Leukoc Biol. 2007;82:1–9. doi: 10.1189/jlb.0406267. [DOI] [PubMed] [Google Scholar]
- 54.Berg DJ, Kuhn R, Rajewsky, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]


