Abstract
Our laboratory has been investigating the participation of striatal neurokinin-1 receptors in the methamphetamine (METH)-induced loss of striatal neurons. Signaling through these receptors exacerbates the METH-induced striatal apoptosis. METH induces the synthesis of nitric oxide (NO) and the latter has been linked to the activation of neurodegenerative cascades. In the present study, we assessed the role of the neurokinin-1 receptor in the production of striatal 3-nitrotyrosine (3-NT) and L-citrulline (indirect indices of NO production). To that end, we injected male mice with a bolus of METH (30 mg/kg, ip) and visualized striatal neuronal nitric oxide synthase (NOS)-positive cells by immunohistochemistry and protein levels by Western blot. The expression of neuronal NOS or protein levels at 2, 4 & 8 hours post-METH was unchanged. Next, we assessed 3-NT and L-citrulline by immunohistochemistry. At 4 hours post-METH, striatal 3-NT and L-citrulline levels were increased 30- and 5-fold, respectively, relative to controls and the selective neurokinin-1 receptor antagonist WIN-51,708 attenuated these increases. Intrastriatal infusion of the neurokinin-1 receptor agonist GR-73632 induced striatal 3-NT production that was attenuated with systemic injection of WIN-51,708 or 7-nitroindazole (7-NI, an inhibitor of neuronal NOS). Moreover, infusion of calmidazolium (calmodulin inhibitor) with GR-73632 prevented the production of 3-NT. These data are consistent the hypothesis that METH-induced production of NO is modulated by the striatal neurokinin-1 receptors and that this receptor may participate in the biochemical activation of neuronal NOS.
Keywords: methamphetamine, neurokinin-1 receptor, substance P, striatum, nitric oxide, SOM/NPY/NOS interneuron
1. INTRODUCTION
METH is a psychostimulant drug that stimulates excessive dopaminergic transmission in the brain excessively enhancing motor performance, promoting alertness and producing euphoria, accounting for the high addictive potential and popularity for this club drug. METH enters the dopamine terminals of the striatum through the dopamine transporter via an exchange diffusion mechanism (Fischer and Cho, 1979) causing the leakage of vesicular dopamine and outward transport to the synaptic space (Sulzer et al., 1995; Jones et al., 1998). The excessively high levels of extracellular dopamine induced by METH initiate a neurotoxic cascade producing free radicals and quinones that are damaging to proteins (Fornstedt and Carlsson, 1989; Hastings et al., 1996). The protracted overflow of dopamine induced by METH promotes the overflow of the excitotoxic transmitter glutamate (Nash and Yamamoto, 1992), nitric oxide production (Taraska and Finnegan, 1997; Zhu et al., 2009), microglial activation (LaVoie et al., 2004; Thomas et al., 2004), and bioenergetic compromise due to mitochondrial dysfunction (Chan et al., 1994; Brown and Yamamoto, 2003). The neurotoxic state induced by METH causes pre-synaptic neural damage including reduction of dopamine transporter function (Fleckenstein et al., 1997), oligomerization of the transporter (Baucum et al., 2004), decreased vesicular transporter function (Fleckenstein et al., 2003), inhibition of tyrosine hydroxylase activity (Hotchkiss et al., 1979), and protein levels (Yu et al., 2004), decreased tissue dopamine content (Wagner et al., 1980) and degeneration of striatal dopamine terminals (Ricaurte et al., 1982). Moreover, METH causes neuronal cell loss in the striatum (Deng et al., 2001; Zhu et al., 2006), cortex (Eisch and Marshall, 1998) and olfactory bulb (Deng et al., 2007) of rodent brain as well as grey matter deficits in humans (Thompson et al., 2004). It is clear that METH is damaging to brain tissue but the precise cellular and biochemical mechanisms by which it brings about the damage remain largely unknown.
The METH-induced overflow of dopamine and glutamate has as a consequence the excessive and sustained production of striatal nitric oxide and reactive nitrogen species, the latter inducing neurotoxic damage (Cerruti et al., 1995; Boje, 2004). Evidence implicating nitric oxide production in the striatal METH-induced neural damage accrues from pharmacological studies demonstrating that antagonists of neuronal nitric oxide synthase protect the striatum from METH (Itzhak and Ali, 1996; Itzhak et al., 2000), moreover, mice lacking the neuronal nitric oxide synthase gene show partial resistance to METH (Imam et al., 2001). Our laboratory has shown that the METH-induced elevation of striatal 3-nitrotyrosine (an indirect index of nitric oxide production) was significantly attenuated by pretreatment with a neurokinin-1 receptor antagonist (Zhu et al., 2009). The striatal neurokinin-1 receptors are expressed by cholinergic and somatostatin/NPY/NOS interneurons. The latter population is comprised of approximately 1% of striatal neurons and is the only striatal neuron expressing the neuronal nitric oxide synthase (Kawaguchi et al., 1995). Interestingly, ablation of the striatal somatostatin/NPY/NOS interneurons with the selective neurotoxin substance P-saporin completely abrogated the METH-induced apoptosis of some striatal neurons (Zhu et al., 2009). Evidence implicating the neurokinin-1 receptor and nitric oxide production comes from studies involving cultured synoviocytes that respond to substance P (natural ligand of the neurokinin-1 receptor) by augmenting nitric oxide production (O’Shaughnessy et al., 2006) and also in rat skin microvasculature (Ralevic et al., 1995). In the light of the above, it becomes important to investigate the role of the neuropeptide substance P in the production of striatal nitric oxide. In the present study we show pharmacological evidence in support of the hypothesis that the neurokinin-1 receptor modulates the METH-induced elevation of striatal 3-nitrotyrosine.
2. RESULTS
We have shown that METH increases striatal NO production from 2–24 hours after a bolus injection of the psychostimulant (Zhu et al., 2009). To further characterize this effect, we assessed the impact of METH on the number of cells expressing neuronal NOS and protein levels. The impact of METH on the number of striatal cells expressing neuronal NOS in a coronal section of tissue was assessed by immunohistochemistry. Cells expressing neuronal NOS stained darkly and appeared scattered throughout the striatum. The number of neuronal NOS-positive cells was unchanged by a bolus of METH (30 mg/kg, ip) at 8 hours post-injection (Figure 1). The level of striatal neuronal NOS was assessed by Western blot. Striatal protein levels for neuronal NOS were unchanged by METH at 2, 4 or 8 hours post-injection (Figure 1). We confirmed the colocalization of the neurokinin-1 receptor on the SOM/NPY/NOS striatal interneurons by double label immunohistofluorescence with confocal microscopy. All striatal neurons staining positive for SOM also co-stained with the neurokinin-1 receptor (Figure 2).
Figure 1.
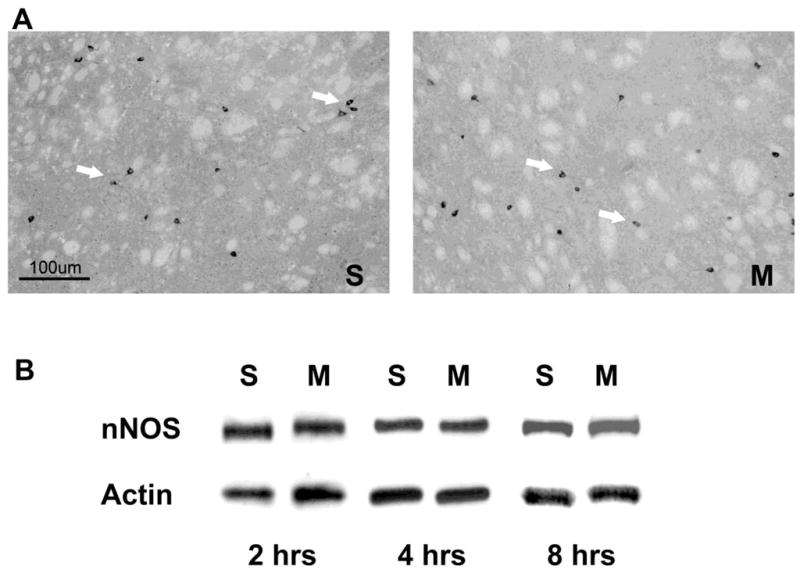
Visualization of neuronal NOS by immunohistochemistry and Western blot in animals treated with saline (S) or METH (M). A) neuronal NOS-positive cells were visualized with a polyclonal antibody to NOS in striatal tissue. Mice (n=7) were received one injection of METH (30 mg/kg, ip) and sacrificed 8 hours post-injection. Arrows denote cells staining positive for neuronal NOS. B) Western blot analysis of striatal neuronal NOS protein levels at 2, 4 and 8 hours after a bolus injection of METH (30 mg/kg, ip).
Figure 2.
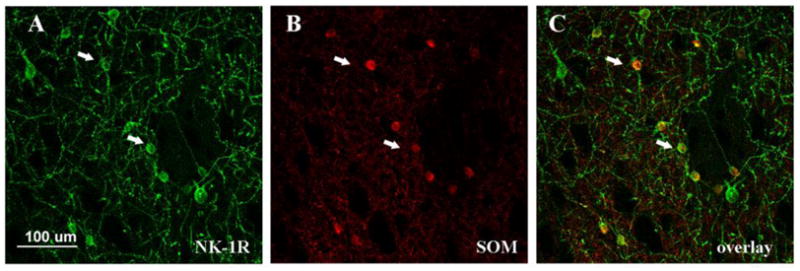
Colocalization of the neurokinin-1 receptor (NK-1R) with somatostatin (SOM) in striatal neurons by confocal microscopy. Neurokinin-1 receptor-expressing neurons were visualized by immunohistofluorescence with FITC (A) and somatostatin with Cy3 (B). All striatal neurons staining positive for somatostatin co-stained with neurokinin-1 receptors. Arrows indicate the position of two representative double-positive neurons.
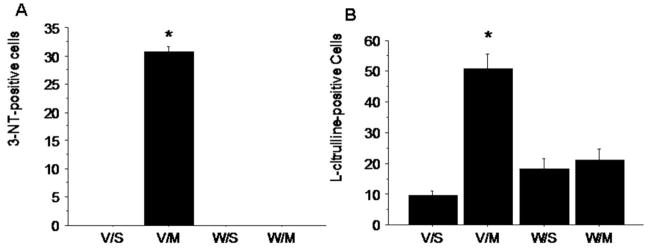
To investigate the role of the neurokinin-1 receptor on the METH-induced production of striatal NO, we injected mice with METH (30 mg/kg, ip) and assessed with immunohistochemistry the levels of the indirect indices of NO production, 3-NT and L-citrulline. We counted the number of 3-NT and L-citrulline-positive cells in four striatal quadrants as previously described (Zhu et al., 2006). The level of staining for 3-NT in saline-treated controls was equivalent to background staining. METH-induced a robust increase of 3-NT in striatal cells presumably the same cells expressing neuronal NOS. Interestingly, pre-treatment 30 minutes before METH with the neurokinin-1 receptor antagonist WIN-51,708 (5 mg/kg, ip) abrogated the METH-induced augmentation of striatal 3-NT (Figure 3A). METH also induced a robust increase of striatal L-citrulline that was inhibited by pre-treatment with WIN-51,708 (Figure 3B).
Figure 3.
Effect of METH on the indirect indices of NO striatal production 3-nitrotyrosine (A) and L-citrulline (B). Mice (n=7) received METH (30 mg/kg, ip) and were sacrificed 4 hours post-injection. A group a mice received the neurokinin-1 receptor antagonist WIN-51,708 (5 mg/kg, ip) 30 minutes before METH. V/S, vehicle/saline; V/M, vehicle/METH; W/S, WIN-51,708/saline; W/M, WIN-51,708/METH. The number of cells staining positive for 3-NT or L-citrulline were counted manually in bright field as described. *P<0.05 (ANOVA).
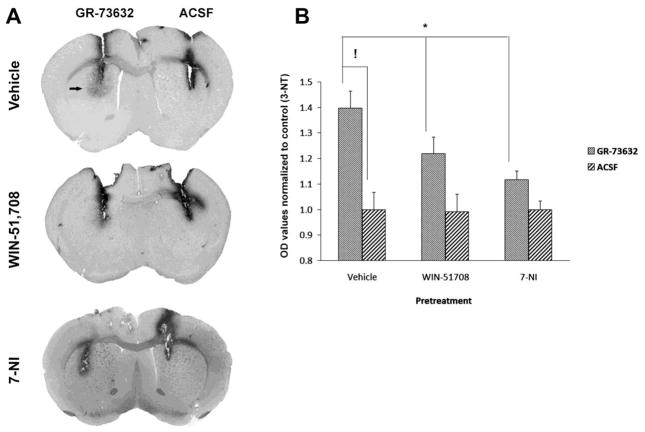
In order to further characterize the role of the neurokinin-1 receptor on the METH-induced production of striatal NO, we tested the effect of the long-lasting substance P (natural ligand of the neurokinin-1 receptor) analogue GR-73632 on 3-NT production by injecting it directly into the striatum because this compound does not cross the blood-brain barrier. One side of the striatum received a microinjection of GR-73632 (20 nmol, 1 μl). and the contralateral striatum received an equivalent volume of artificial cerebrospinal fluid and served as control (Figure 4A). 3-NT production was assessed by immunohistochemistry in an area 1.5 mm surrounding the injection site 30 minutes after the intrastriatal injection of GR-73632. The substance P analogue GR-73632 significantly increased striatal 3-NT production relative to the contralateral control. This increase was attenuated by WIN-51,708 (5 mg/kg, ip) given 30 minutes prior to the GR-73632 (Figure 4A and B). In addition, the GR-73632 increase of striatal 3-NT was attenuated by pre-treatment with the selective neuronal NOS inhibitor 7-nitroindazole (25 mg/kg, ip) given 30 minutes before GR-73632 (Figure 4A and B).
Figure 4.
Effect of the substance P agonist GR-73632 on the striatal production of 3-nitrotyrosine (3-NT). Mice (n=7) received intrastriatal injections of artifical cerebrospinal fluid (ACSF, 1 μl) in one striatum or GR-73632 (20 nM, 1 μl) in the contralateral striatum (A). Some mice (n=7) received WIN-51,708 (5 mg/kg, ip) or 7-nitroindazole (7-NI, 25 mg/kg, ip) 30 minutes prior to GR-73632 (B). Animals were sacrificed 30 minutes after the intrastriatal injections. 3-NT was visualized by immunohistochemistry as described. Note that the neurokinin-1 receptor antagonist WIN-51,708 attenuated the GR-73632-induced production of 3-NT. *P<0.05, !P<0.001 (two-way ANOVA, F = 31.6).
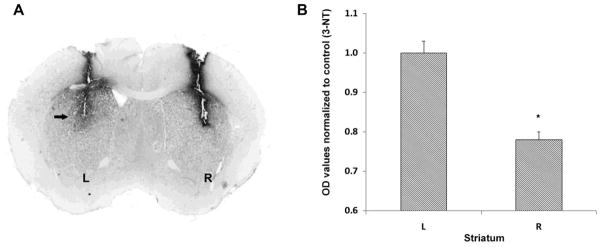
Lastly, we investigated the role of calcium-calmodulin on the GR-73632-induced elevation of striatal 3-NT because calmodulin is a calcium signaling protein that is known to active nitric oxide synthases (Spratt et al., 2007) and calmidazolium is a selective antagonist of calmodulin (Khan et al., 2001). Mice received an injection of GR-73632 (20 nM, 1 μl) into the left striatum and the right striatum received a mixture of GR-73632 and the calcium-calmodulin inhibitor calmidazolium (100 nM, 1 μl; Figure 5A). Calmidazolium significantly attenuated the GR-73632-induced elevation of striatal 3-NT (Figure 5B). Infusion of calmidazolium had no effect on striatal 3-NT (data not shown).
Figure 5.
Effect of inhibition of calcium-calmodulin on GR-73632-induced striatal 3-nitrotyrosine (3-NT) production. Mice (n=7) received GR-73632 (20 nM, 1 μl) in the left striatum (L) and GR-73632 plus the calcium-calmodulin inhibitor calmidazolium (100nM, 1 μl) in the right striatum (R). Animals were sacrificed 30 minutes after the intrastriatal injections. 3-NT was visualized by immunohistochemistry (A) and the level of staining was quantified as described and expressed as OD values (B). Note that the calmidazolium attenuated the GR-73632-induced production of 3-NT. *P<0.05 (ANOVA).
3. DISCUSSION
Our results are consistent with the hypothesis that the neuropeptide substance P signaling through the neurokinin-1 receptor modulates the METH-induced production of striatal NO. Our data demonstrated that the METH-induced production of striatal 3-NT and L-citrulline, indirect indices of NO production, was attenuated by the systemic injection of the neurokinin-1 receptor antagonist WIN-51,708. Moreover, the intrastriatal microinjection of the substance P agonist GR-73632 stimulated NO production that was attenuated by WIN-51,708 or the neuronal NOS inhibitor 7-nitroindazole. The GR-73632-induced production of striatal NO was calcium-dependent.
Recently we reported that a bolus injection of METH (30 mg/kg, ip) induced the production of striatal NO for up to 24 hours post-injection and this effect of METH on NO was attenuated by pharmacological antagonism of the neurokinin-1 receptor (Zhu et al., 2009). Our results demonstrate that the METH-induced production of NO is a consequence of the biochemical activation of neuronal NOS because we did not observe either an increase in the number of striatal neurons expressing this enzyme or higher levels of expression of this enzyme in striatal tissue. In the striatum, neuronal NOS is expressed by the SOM/NPY interneurons (Kawaguchi et al., 1995) and is expressed constitutively while the inducible NOS is expressed primarily by activated microglia and astrocytes express all three isoforms of the enzyme (Boje, 2004). The striatal interneurons expressing NOS also express the neurokinin-1 receptor (Gerfen, 1992) thus establishing a neuroanatomical basis connecting NO production with the neuropeptide substance P. That this connection is functional in the presence of METH is supported by our published results demonstrating increased neurokinin-1 receptor signaling in the presence of METH in the striatal SOM/NPY/NOS interneurons (Wang and Angulo, 2010). The above represents one mechanism accounting in part for the METH-induced production of striatal NO. Another mechanism operating in parallel may involve the microglia and astrocytes both of which express the neurokinin-1 receptor (Martin et al., 1993; Rasley et al., 2002; Too et al., 1994; Torrens et al., 1986). The role of neuroglial neurokinin-1 receptors on the METH-induced production of NO is under investigation in our laboratory.
The GR-73632 compound is a selective neurokinin-1 receptor agonist that mimics the behavioral and biochemical properties of substance P in vivo and in vitro (Hagan et al., 1991; Hall et al., 1991; Hall and Morton, 1991; Andoh et al., 1998; Tang et al., 2007). Intra-ventral tegmental area infusion of GR-73632 increased locomotor activity and terminal dopamine turnover in the nucleus accumbens (Elliott et al., 1991; Elliott et al., 1992). Intrathecal injection of GR-73632 in the spinal cord of mice caused dose-dependent behavioral responses such as scratching, biting and licking that were inhibited by the co-administration of a neurokinin-1 receptor antagonist (Sakurada et al., 1999). Moreover, in similar fashion to substance P, the infusion of GR-73632 elicited dopamine and acetylcholine turnover in the striatum (Galarraga et al., 1999). Our data show that the intrastriatal injection of GR-73632 induced the production of NO and this induction was attenuated by the co-administration of WIN-51,708. Moreover, the NO production emanated in part from the biochemical activation of neuronal NOS because it was sensitive to 7-nitroindazole, a selective inhibitor of this enzyme. This observation, together with the finding that METH induces neurokinin-1 receptor trafficking in SOM/NPY/NOS striatal interneurons (Wang and Angulo, 2010), suggest that substance P plays a role in the METH-induced production of striatal NO. It would be interesting to determine if the GR-73632 compound induces striatal neural damage.
The neuronal NOS is regulated in a calcium-calmodulin-dependent manner in which calmodulin plays a regulatory role through the process of electron transfer from the flavin center to the heme group in the enzyme. Calcium-calmodulin binds to the neuronal NOS transforming it to the active state for NO production (Abu-Soud and Stuehr, 1993; Matsuoka et al., 1994). Glutamate signaling increases the intracellular concentration of calcium leading to the activation of neuronal NOS via a calcium-calmodulin-dependent mechanism (Bredt et al., 1992; Hirata et al., 1995; Hayashi et al., 1999). Substance P has been shown to elevate the intracellular calcium level in rat pancreatic acinar cell line and spinal cord (Womack et al., 1985, 1988). Moreover, in rat skin microvasculature substance P mediated inflammation by augmenting NO (Ralevic et al., 1995). Similarly, in cultured synoviocytes substance P acting via the neurokinin-1 receptor increased NO production (O’Shaughnessy et al., 2006). In this study we report that in striatal tissue the GR-73632-induced production of NO was attenuated by inhibition of calmodulin. In the light of this and the above observations, we hypothesize that striatal substance P signaling through the neurokinin-1 receptor leads to the biochemical activation of neuronal NOS via a mechanism involving calcium. More work is needed to elucidate the mechanism of substance P activation of striatal neuronal NOS in the presence of METH.
In summary, the data demonstrate that up to the first eight hours after a bolus high dose of METH, neither the number of striatal cells expressing neuronal NOS nor the protein levels for the enzyme are changed suggesting that in part the METH-induced elevation of NO is due to the biochemical activation of the enzyme. Moreover, all the striatal interneurons expressing SOM also express the neurokinin-1 receptor. One possible activator is the neuropeptide substance P. Pharmacological activation of the neurokinin-1 receptor with a selective substance P agonist induced the production of NO and this biochemical activation is dependent on calcium-calmodulin. Work in our laboratory is investigating the mechanistic role of neuropeptides on the biochemical activation of striatal neuronal NOS in the presence of METH.
4. EXPERIMENTAL PROCEDURES
4.1 Animals
Male ICR mice (Taconic, Germantown, NY) between 10 to 13 weeks of age were housed individually on a 12-h light/dark cycle with food and water available ad libitum. The mice were habituated for two weeks prior to commencement of intraperitoneal (i.p.) drug administration. All procedures regarding animal use were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee at Hunter College of the City University of New York.
4.2 Drug preparation and treatment
(+)-Methamphetamine hydrochloride (Sigma, St. Louis, MO) was dissolved in PBS and injected intraperitoneal (i.p.) at a dose of 30 mg/kg of body weight in a volume of 200 μL. The non-peptide neurokinin-1 receptor antagonist WIN-51,708 (17-hydroxy-17-ethynyl-5—androstano [3,2-b] pyrimido [1,2-a] benzimidazole) (RBI/Sigma, Natick, MA) was dissolved in vehicle (45%(w/v) 2-Hydroxypropyl-β-Cyclodextrin) (RBI/Sigma, Natick, MA). Vehicle or WIN-51,708 (5mg/kg) was given intraperitoneally 30 minutes prior to the injection of METH. GR-73632 (Sigma, MO) was dissolved in artificial cerebrospinal fluid at a concentration of 20 nM and injected intrastriatal in a volume of 1 μL. 7-nitroindazole (Sigma, MO) was dissolved in peanut oil and injected intraperitoneal at 25 mg/kg. Calmidazolium chloride (Sigma, MO) was dissolved in DMSO at a concentration of 100 nM and injected intrastriatal in a volume of 1 μl.
4.3 Intrastriatal microinjections
Mice were anesthetized with inhaled isoflurane (2.5%) and their heads were placed in a stereotaxic frame (Model 5000, David Kopf Instruments, CA). A hole was drilled in the skull and a 25 gauge 2μl Hamilton microinjection syringe was lowered into the striatum. Distance of injection sites (bregma 0.5mm lateral ±2.0 mm; ventral +2.5mm) was determined using a mouse brain atlas (Franklin and Paxinos, 1997). The microinjection needle was left in position for 5 minutes prior to drug injection. Drugs were injected over a 10-minute period at a rate of 0.1 μl/minute and the needle was left in place for an additional 5 minutes before removal from the striatum. Animals received bilateral injections: one striatum received vehicle and served as control and the contralateral striatum received drug treatment.
4.4 Immunohistochemistry for the neurokinin-1 receptor, neuronal NOS, 3-nitrotyrosine, L-citrulline and double label of neurokinin-1 receptor with somatostatin
The animals were fully anesthetized with Ketamine (100 mg/kg) and Acepromazine (3 mg/kg). The animals were perfused through the heart with 20 ml of PBS followed by 20 ml of 4% paraformaldehyde and 0.2% glutaraldehyde. The brains were post-fixed overnight in the fixative at 4°C followed by 20% sucrose solution over 24 hours at 4°C for cryo-protection. The brains were frozen at −80ºC until used. Coronal sections 30μm in thickness were cut in a microtome at −20ºC and stored in anti-freezing solution (30% glycerin solution in ethylene glycol) at −20ºC until used.
Sections of striatal tissue were washed 3x for 5 minutes each in PBS with 0.3% Triton X-100 followed by a 30-minute incubation at room temperature with 10% Normal Sheep Serum (NSS) to block nonspecific binding. The sections were incubated overnight at 4°C with polyclonal rabbit anti-neurokinin-1 receptor antibody (1:1000, Chemicon, CA) or anti-neuronal NOS (1:500, Chemicon, CA) diluted in the same blocking buffer. After rinsing 3x for 5 minutes each with PBS, the sections were incubated with sheep anti-rabbit secondary antibody conjugated with FITC (1:1000, Chemicon, CA) for 2 hours at room temperature. The sections were washed 3x for 10 minutes each with PBS and mounted onto glass slides.
For double labeling, the primary antibodies used were rabbit anti-neurokinin-1 receptor (1:1000, Chemicon, Temecula, CA) followed by goat anti-somatostatin (1:200, Santa Cruz, CA) diluted in 10% Normal Donkey Serum in blocking buffer with 0.3% Triton X-100. The sections were incubated overnight at 4°C. Secondary antibodies were donkey anti-rabbit conjugated with FITC (1:1000, Novus Biologicals, CO) and donkey anti-goat conjugated with Cy3 (1:100, Chemicon, CA). After 2 hours of incubation at room temperature, the sections were washed 3x for 10 minutes each with PBS and mounted onto glass slides.
For immunostaining of 3-nitrotyrosine, floating sections were washed with PBS 3x 10 minutes each followed by incubation for 10 minutes in citric acid (10 mM, 65°C). The sections were rinsed with PBS 3x for 10 minutes each and incubated for 30 minutes with 3% H2O2 to block endogenous enzyme activity. The sections were then incubated with M.O.M Blocking buffer (Vector Laboratories, CA) for 1 hour at room temperature followed by 3 washes of 10 minutes each in working solution of M.O.M diluents buffer. The sections were incubated with a monoclonal mouse anti-mouse antibody against 3-nitrotyrosine (1:500, SCBT, CA) in diluents buffer at 4°C overnight. Next day, the sections were rinsed with PBS 3x for 10 minutes each and incubated with diluted biotinylated secondary antibody in diluents buffer for one hour at room temperature. The sections were rinsed with PBS 3x for 10 minutes each and incubated with VECTASTAIN ABC kit (Vector Laboratories, CA) for 30 minutes at room temperature. The sections were rinsed with PBS for 5 minutes and incubated with DAB Substrate Kit (Vector Laboratories, CA) according to the manufacturer’s instructions. The sections were rinsed with PBS for 5 minutes and mounted onto glass slides with Vector H-5000 (Vector Laboratories, CA). L-citrulline immunohistochemistry was performed as described above for 3-nitrotyrosine using a rabbit antibody against L-citrulline (1:200, Chemicon, CA) followed by VECTASTAIN ABC and DAB Substrate kits (Vector Laboratories, CA).
4.5 Western blot of neuronal NOS
After drug administration, mice were decapitated at different time points. Striata were immediately dissected and frozen on dry ice. Tissues were homogenized with lysis buffer (50 mM Tris-HCL pH 7.4, 150 mM NaCl, 320 mM sucrose, 5 mM HEPES, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DTT, 1% Inhibitor Cocktail [1.04 mM AEBSF, 0.8 μM aprotinin, 0.02 mM leupeptin, 0.04 mM bestatin, 0.015 mM pepstatin A, 0.014 mM E-64 (Sigma, St. Louis, MO)]. Homogenates were centrifuged at 5000 rpm for 20 minutes at 4°C. The supernatants were used for Western blot analysis. Protein concentration was determined by the Bradford assay. Samples were subjected to 7.5% SDS-PAGE gel, and proteins were transferred onto Nitrocellulose paper. Membranes were blocked with 5% nonfat dry milk and probed with rabbit anti-nNOS (1:2500, Chemicon, Temecula, CA) overnight at 4°C with gentle shaking. Membranes were washed with TBS and incubated with HRP-conjugated goat anti-rabbit immunoglobulin (1:5000, Chemicon, Temecula, CA) for 1 hour at room temperature. After rinsing with Tris buffer/saline, proteins were detected with the SuperSignal West Pico Chemilumescent Substrate (Pierce, Rockford, IL) and exposed on Kodak Biomax film (Kodak, Rochester, NY). For internal standards, membranes were stripped and reprobed with rabbit anti-β-actin (1:5000, RBI/Sigma, Natick, MA). Densitometry was performed and analyzed with Quantum software. The density of each band was normalized against that of β-actin.
4.6 Quantification of 3-nitrotyrosine immunostaining
The densitometry was performed to assess the amount of 3-NT staining after immunohistochemistry using Quantum software. The area of analysis consisted of a 1.5 mm region adjacent to the track made by the injection needle in the dorsal striatum. The first 0.2 mm from the needle track was excluded from the analysis. Background levels from control side (contralateral striatum) were subtracted from the values obtained for the experimental side (ipsilateral striatum).
4.7 Statistical analysis
Statistical comparisons were performed from mean ± SEM. Differences between groups were analyzed by ANOVA followed by post hoc comparison using Fisher’s protected least significance test. Differences between two groups were analyzed by Student’s t-test. The significance criterion was set at p=0.05.
RESEARCH HIGHLIGHTS.
Wang and Angulo
Methamphetamine activates striatal nitric oxide synthesis.
Substance P modulates nitric oxide synthesis in the presence of methamphetamine.
Activation of the striatal neurokinin-1 receptor induces nitric oxide production.
Acknowledgments
This work was supported by R01 DA020142 from the National Institute on Drug Abuse to JAA. Support for infrastructure came from the Research Centers in Minority Institutions grant number RR003037 awarded to Hunter College by NCRR/NIH.
Abbreviations used
- FITC
fluorescein isothiocyanate
- ICR
Institute for Cancer Research
- ip
intraperitoneal
- METH
(+)-methamphetamine hydrochloride
- 7-NI
nitroindazole
- 3-NT
nitrotyrosine
- NPY
neuropeptide Y
- NO
nitric oxide
- NOS
nitric oxide synthase
- PBS
phosphate-buffered saline, pH 7.4
- SOM
somatostatin
- WIN-51
708, 17-β-Hydroxy-17-a-ethynyl-5-a-androstano[3,2-β]pyrimido[1,2-a]benzimidazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci USA. 1993;90:10769–72. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–45. [PubMed] [Google Scholar]
- Baucum AJ, II, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine-and hyperthermia-associated mechanism. J Neurosci. 2004;24:3436–43. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje KMK. Nitric oxide neurotoxicity in neurodegenerative diseases. Frontiers Biosci. 2004;9:763–76. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Ferris DL, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium-calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976–81. [PubMed] [Google Scholar]
- Brown JM, Yamamoto BK. Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther. 2003;99:45–53. doi: 10.1016/s0163-7258(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Cerruti C, Sheng P, Ladenheim B, Epstein CJ, Cadet JL. Involvement of oxidative and L-arginine-NO pathways in the neurotoxicity of drugs of abuse in vitro. Clin Exp Pharmacol Physiol. 1995;22:381–2. doi: 10.1111/j.1440-1681.1995.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW. Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem. 1994;62:2484–87. doi: 10.1046/j.1471-4159.1994.62062484.x. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine adminstration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–43. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–45. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Mason GS, Stephen-Smith M, Hagan RM. Behavioral and biochemical responses following activation of midbrain dopamine pathways by receptor selective neurokinin agonists. Neuropeptides. 1991;19:119–26. doi: 10.1016/0143-4179(91)90141-5. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Mason GS, Graham EA, Turpin MP, Hagan RM. Modulation of the rat mesolimbic dopamine pathway by neurokinins. Behav Brain Res. 1992;51:77–82. doi: 10.1016/s0166-4328(05)80314-6. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–09. [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum, Eur. J Pharmacol. 1997;334:111–4. doi: 10.1016/s0014-2999(97)01175-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol. 2003;479:283–9. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Fornstedt B, Carlsson A. A marked rise in 5-S-cysteinyl-dopamine levels in guinea-pig striatum following reserpine treatment. J Neural Transm. 1989;76:155–61. doi: 10.1007/BF01578755. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: The Aademic Press; 1997. [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Tapia D, Reyes A, Bargas J. Action of substance P (neurokinin-1) receptor activation on rat neostriatal projection neurons. Synapse. 1999;33:26–35. doi: 10.1002/(SICI)1098-2396(199907)33:1<26::AID-SYN3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The meostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Hagan RM, Ireland SJ, Jordan CC, Beresford IJ, Deal MJ, Ward P. Receptor-selective, peptidase-resistant agonists at neurokinin NK-1 and NK-2 receptors: new tools for investigating neurokinin function. Neuropeptides. 1991;19:127–35. doi: 10.1016/0143-4179(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Hall JM, Morton IK. Novel selective agonists and antagonists confirm neurokinin NK1 receptors in guinea-pig vas deferens. Br J Pharmacol. 1991;102:511–17. doi: 10.1111/j.1476-5381.1991.tb12202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Mitchell D, Morton IK. Neurokinin receptors in the rabbit iris sphincter characterized by novel agonist ligands. Eur J Pharmacol. 1991;199:9–14. doi: 10.1016/0014-2999(91)90630-9. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996;93:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nishio M, Naito Y, Yokokura H, Nimura Y, Hidaka H, Watanabe Y. Regulation of neuronal nitric oxide synthase by calmodulin kinases. Biol Chem. 1999;274:20597–602. doi: 10.1074/jbc.274.29.20597. [DOI] [PubMed] [Google Scholar]
- Hirata K, Kuroda R, Sakoda T, Katayama M, Inoue N, Suematsu M, Kawashima S, Yokoyama M. Inhibition of endothelial nitric oxide synthase activity by protein kinase C. Hypertension. 1995;25:180–85. doi: 10.1161/01.hyp.25.2.180. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–78. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Jr, Ali SF. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem. 2001;76:745–49. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–73. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JJ, Ali SF. nNOS inhibitors attenuate methamphetamine-induced dopaminergic neurotoxicity but not hyperthermia in mice. NeuroReport. 2000;11:2943–46. doi: 10.1097/00001756-200009110-00022. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–35. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Khan SZ, Dyer JL, Michelangeli F. Inhibition of the type 1 inositol 1,4,5-triphosphate-sensitive Ca2+ channel by calmodulin antagonists. Cel Signaling. 2001;13:57–63. doi: 10.1016/s0898-6568(00)00140-6. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exptl Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Martin FC, Anton PA, Gornbein JA, Shanahan F, Merrill JE. Production of interleukin-1 by microglia in response to substance P: role for a non-classical NK-1 receptor. J Immunol. 1993;42:53–60. doi: 10.1016/0165-5728(93)90212-h. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Stuehr DJ, Olson JS, Clark P, Ikeda-Saito M. L-arginine and calmodulin regulation of the heme iron reactivity in neuronal nitric oxide synthase. J Biol Chem. 1994;269:20335–39. [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxy-methamphetamine. Brain Res. 1992;581:237–43. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy MC, Vetsika EK, Inglis JJ, Carleson J, Haigh R, Kidd BL, Winyard PG. The effect of substance P on nitric oxide release in a rheumatoid arthritis model. Inflamm Res. 2006;55:263–40. doi: 10.1007/s00011-006-0079-8. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Khalil Z, Helme RD, Dusting GJ. Role of nitric oxide in the actions of substance P and other mediators of inflammation in rat skin microvasculature. Eur J Pharmacol. 1995;284:231–39. doi: 10.1016/0014-2999(95)00321-b. [DOI] [PubMed] [Google Scholar]
- Rasley A, Bost KL, Olson JK, Miller SD, Marriott I. Expression of functional NK-1 receptors in murine microglia. Glia. 2002;37:258–67. doi: 10.1002/glia.10034. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Sakurada C, Watanabe C, Inoue M, Tan-No K, Ando R, Kisara K, Sakurada T. Spinal actions of GR73632, a novel tachykinin NK1 receptor agonist. Peptides. 1999;20:301–304. doi: 10.1016/s0196-9781(98)00171-5. [DOI] [PubMed] [Google Scholar]
- Spratt DE, Taiakina V, Palmer M, Guillemette JG. Differential binding of calmodulin domains to constitutive and inducible nitric oxide synthase enzymes. Biochem. 2007;46:8288–300. doi: 10.1021/bi062130b. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–08. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HB, Li YS, Arihiro K, Nakata Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons. Mol Pain. 2007;3:42–51. doi: 10.1186/1744-8069-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraska T, Finnegan KT. Nitric oxide and the neurotoxic effects of methamphetamine and 3,4-methylenedioxymethamphetamine. J Pharm Exp Ther. 1997;280:941–7. [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharm Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Too HP, Marriott DR, Wilkin GP. Preprotachykinin-A and substance P receptor (NK1) gene expression in rat astrocytes in vitro. Neurosci Lett. 1994;182:185–87. doi: 10.1016/0304-3940(94)90793-5. [DOI] [PubMed] [Google Scholar]
- Torrens Y, Beaujouan JC, Saffroy M, Daguet de Montety MC, Bergstrom L, Glowinski J. Substance P receptors in primary cultures of cortical astrocytes from mouse. Proc Natl Acad Sci USA. 1986;83:9216–20. doi: 10.1073/pnas.83.23.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–60. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Angulo JA. Methamphetamine induces striatal neurokinin-1 receptor endocytosis primarily in somatostatin/NPY/NOS interneurons and the role of dopamine receptors in mice. Synapse. 2010 doi: 10.1002/syn.20848. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Hanley MR, Jessell TM. Functional substance P receptors on a rat pancreatic acinar cell line. J Neurosci. 1985;5:3370–78. doi: 10.1523/JNEUROSCI.05-12-03370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, MacDermott AB, Jessell TM. Sensory transmitters regulate intracellular calcium in dorsal horn neurons. Nature. 1988;334:351–53. doi: 10.1038/334351a0. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–31. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neurosci. 2006;140:607–22. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu W, Wang J, Ali SF, Angulo JA. The neurokinin-1 receptor modulates the methamphetamine-induced striatal apoptosis and nitric oxide formation in mice. J Neurochem. 2009;111:656–68. doi: 10.1111/j.1471-4159.2009.06330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]