Abstract
Prior clinical and preclinical studies suggest that omega-3 fatty acids negatively regulate pro-inflammatory signaling cascades, and that the atypical antipsychotic risperidone up-regulates omega-3 fatty acid biosynthesis. In the present study, we investigated the effects of chronic (40 d) risperidone treatment (3 mg/kg/day) on basal pro-inflammatory cytokine (interleukin-6, IL-6; tumor necrosis factor-alpha, TNFα) and C-reactive protein (CRP) production in control and n-3 fatty acid deficient rats. Relationships with erythrocyte polyunsaturated fatty acid composition were determined. Compared with untreated controls, untreated n-3-deficient rats exhibited significantly greater basal IL-6, TNFα, and CRP production. Following chronic risperidone treatment there were trends for greater IL-6, TNFα, and CRP production in controls, but these did not reach significance. In n-3-deficient rats, chronic risperidone normalized elevated IL-6, TNFα, and CRP levels. Erythrocyte arachidonic acid (20:4n-6) composition was positively correlated, and erythrocyte eicosapentenoic (20:5n-3) and docosahexaenoic acid (22:6n-3) inversely correlated, with plasma IL-6, TNFα, and CRP levels in untreated control and n-3-deficient rats, and these associations were not observed among risperidone-treated rats. The adrenic acid (22:4n-6)/arachidonic acid ratio, an index of elongase-mediated arachidonic acid biosynthesis, was reduced by risperidone in controls and elevated in n-3-deficient rats. These preclinical data demonstrate that chronic risperidone treatment normalizes constitutively elevated pro-inflammatory cytokine and CRP production in n-3 fatty acid deficient rats but not in controls, and that the mechanism is dissociable from n-3 fatty acid biosynthesis.
Keywords: Risperidone, Antipsychotic, Cytokine, Interleukin-6, Tumor necrosis factor-alpha, Inflammation, C-reactive protein, Eicosapentaenoic acid, Docosahexaenoic acid, Arachidonic acid, Erythrocyte, Rat
1. Introduction
Emerging evidence from case-control studies suggest that a dysregulation in the inflammatory immune response may be a pathophysiological feature associated with schizophrenia (Watanabe et al., 2010). Although some case-control studies have found that antipsychotic medications have immunosuppressive effects in schizophrenic patients, this has not been consistently observed across different studies (Drzyzga et al., 2006). Multiple factors that influence inflammatory immune activity, including diet, smoking, and medications, may contribute to discrepant findings in clinical studies (Drexhage et al., 2010). Preclinical studies have found that atypical antipsychotic medications, including risperidone, significantly attenuate elevated production of pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα), in microglia cells following interferon-γ exposure (Bian et al., 2008; Kato et al., 2007), and in mice following peripheral lipopolysaccharide administration (Sugino et al., 2009). These preclinical data demonstrate that atypical antipsychotic medications suppress inflammatory immune activation in response to exogenous stimuli. However, the mechanisms mediating the effects of atypical antipsychotics on constitutive inflammatory immune activity have not been systematically investigated.
Emerging data from clinical and preclinical studies suggest that atypical antipsychotic medications up-regulate long-chain polyunsaturated fatty acid biosynthesis (McNamara, 2009). Importantly, the long-chain omega-6 (n-6) fatty acid arachidonic acid (AA, 20:4n-6) is a primary substrate for cyclooxygenase-2 (COX-2)-mediated prostaglandin E2 (PGE2) synthesis, and PGE2 up-regulates IL-6 biosynthesis at the level of transcription via nuclear factor (NF)-κB (Portanova et al., 1996; Wang et al., 2010). In contrast, long-chain n-3 fatty acids, including eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3), and their lipid metabolites have robust immunosuppressive and anti-inflammatory properties (Calder, 2008; Groeger et al., 2010; Khalfoun et al., 1997; Lu et al., 2010; Mingam et al., 2008; Serhan, 2005). Importantly, we found that chronic risperidone treatment significantly increased erythrocyte membrane long-chain n-3 fatty acid composition in rats, and this effect was only observed in rats maintained on a diet fortified with the short-chain n-3 fatty acid α-linolenic acid (ALA, 18:3n-3)(McNamara et al., 2009). Together these data support the hypothesis that up-regulation of n-3 fatty acid biosynthesis may contribute to the anti-inflammatory and immunosuppressive effects of risperidone.
In a companion paper we reported that n-3 fatty acid deficient rats exhibit elevated basal production of pro-inflammatory cytokines (IL-6 and TNFα) and the acute phase protein C-reactive protein (CRP) compared with n-3 fatty acid adequate controls (McNamara et al., 2010). We additionally demonstrated that elevated basal cytokine and CRP production in n-3 fatty acid deficient rats was significantly attenuated by prior dietary-induced normalization of n-3 fatty acid status. In the present study, we determined the effect of chronic risperidone treatment on constitutive cytokine (IL-6 and TNFα) and CRP production in n-3 fatty acid adequate controls and n-3 fatty acid deficient rats. Our specific prediction was that risperidone would significantly reduce cytokine and CRP production in rats maintained on diet ALA-fortified diet in association with increased n-3 fatty acid biosynthesis, but not in rats maintained on diet ALA-free diet.
2. Materials and methods
2.1. Diets
Diets were either α-linolenic acid (ALA, 18:3n-3)-fortified (ALA+, TD.04285) or ALA-free (ALA−, TD.04286)(Harlan-TEKLAD, Madison, WI). Both diets were matched for all non-fat nutrients, and both diets contained n-3 fatty acid-free hydrogenated coconut (45 g/kg) and safflower (19 g/kg) oils. The ALA+ diet additionally contained ALA-containing flaxseed oil (6 g/kg). Analysis of diet fatty acid composition by gas chromatography confirmed that the ALA− diet did not contain ALA, but was matched with the ALA+ diet in saturated fatty acids (C8:0, C10:0, C12:0, C14:0, C16:0, C18:0), monounsaturated fatty acids (18:1n-9), and n-6 fatty acids (18:2n-6, linoleic acid, 22% of total fatty acid composition) (see Table 1 in McNamara et al., 2008). ALA represented 4.6% of total fatty acid composition in the ALA+ diet. Neither diet contained long-chain n-3 or n-6 fatty acids including DHA and arachidonic acid, respectively.
2.2. Animals
For perinatal ALA deficiency, nulliparous Long-Evans hooded dams (Harlan Farms, Indianapolis, IN) were fed the ALA− diet for 1 month prior to mating through weaning, and male offspring were maintained on the ALA− diet from P21-P100. Controls were born to nulliparous dams maintained on the ALA+ diet, and received the ALA+ diet from P21-P100. Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard non-barrier vivarium conditions (i.e., not specific pathogen-free) on a 12:12 h light:dark cycle. Rats were sacrificed by decapitation on P100 during the light portion of the cycle in a counterbalanced manner. Trunk blood was collected into EDTA-coated tubes, plasma was isolated by centrifugation (4° C), and erythrocytes washed 3× with 4° C 0.9% NaCl. All samples were immediately stored at −80°C.
2.3. Drug administration
On P60, one-half of rats in the control (n=10) and n-3-deficient (n=10) diet groups were randomly assigned to receive chronic treatment with drug vehicle (0.1 M acetic acid diluted in deionized water) or risperidone (3.0 mg/kg/day; Ortho-McNeil Janssen Pharmaceuticals) through their drinking water, as previously described (McNamara et al., 2009). This dose was selected based on our prior studies finding that it produces therapeutically-relevant plasma risperidone and 9-OH-RSP concentrations and significantly increases n-3 fatty acid biosynthesis in rats following chronic oral administration (McNamara et al., 2009). For three days prior to drug delivery, 24 h water consumption was determined for each cage using bottle weights (1 g water = 1 ml water), and ml water intake/mean kg body weight calculated. Risperidone was dissolved and diluted in 0.1 M acetic acid to prepare a stock solution (stored at 4 deg) which was added to tap water in a volume required to deliver the targeted daily dose. Fresh solutions were prepared, and drug concentrations adjusted to mean body weight, every 3 days. Red opaque drinking bottles were used to protect drug from light degradation. Rats were maintained on their respective drug and dose until being sacrificed on P100 (40 days of treatment).
2.4. Plasma cytokine and CRP levels
Plasma cytokine (pg/ml) and CRP (ng/ml) concentrations were determined with a multiplexing suspension array and flow-cytometry based analyzer Luminex™ 100 IS (MiraiBio, South San Francisco, CA) using a LINCOplex Cytokine/Chemokine Luminex® Bead immunoassay Kit according to manufacturer’s protocol (LINCO Research, St. Charles, MO). All analyses were performed by a technician blinded to group identity.
2.5. Erythrocyte fatty acid composition
The gas chromatography procedure used to determine erythrocyte composition (mg fatty acid/100 mg fatty acids) has been described in detail previously (McNamara et al., 2009). Briefly, total fatty acid composition was determined with a Shimadzu GC-2014 (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 4.3 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). We focused our analysis on fatty acids implicated in the regulation of immune and inflammatory signaling, including n-3 fatty acids, DHA (22:6n-3), EPA (20:5n-3), and EPA+DHA (‘omega-3 index’), and the n-6 fatty acids arachidonic acid (20:4n-6) and it’s elongase product adrenic acid (ADA, 22:4n-6).
2.6. Statistical analysis
Group differences in erythrocyte fatty acid composition and plasma inflammatory markers were evaluated with a two-factor ANOVA, with diet (ALA+, ALA−) and drug treatment (vehicle, RSP) as the main factors. Pairwise comparisons were made with unpaired t-tests (2-tail, α=0.05). Homogeneity of variance was first confirmed using Bartlett’s test. Parametric (Pearson) correlation analyses were performed to determine relationships between erythrocyte fatty acid composition and inflammatory markers (2-tail, α=0.05). Statistical analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Erythrocyte fatty acid composition
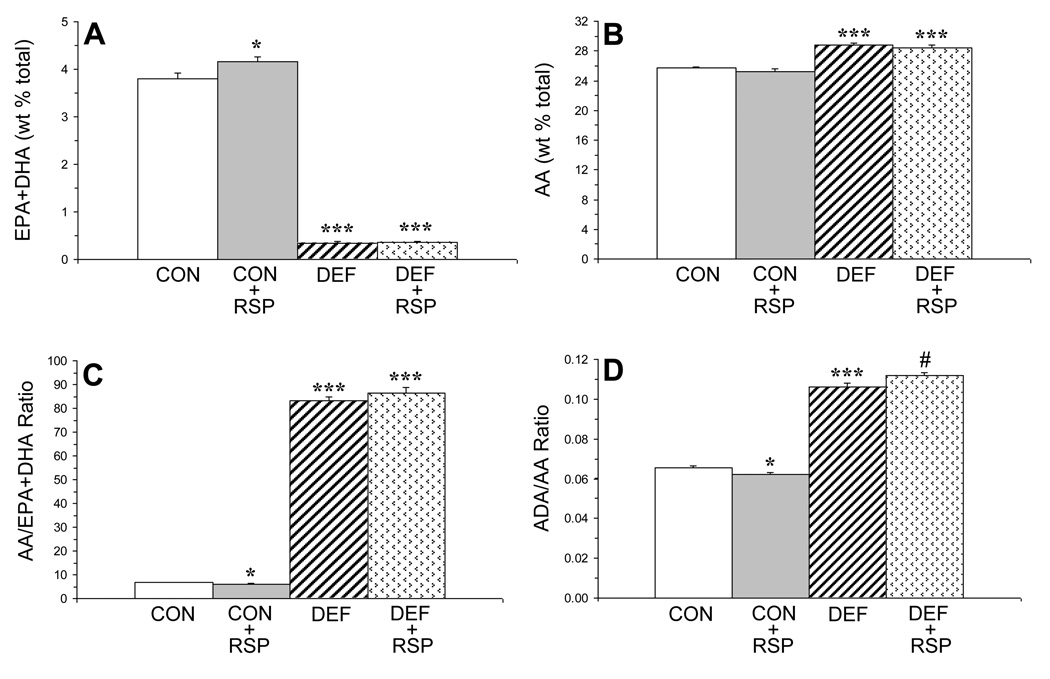
There was a significant main effect of Diet on erythrocyte EPA+DHA composition, F(1,39)=1859, P≤0.0001, and the main effect of Treatment and the Diet × Treatment interaction were not significant (Fig. 1A). For erythrocyte arachidonic acid composition, the main effect of Diet was significant, F(1,39)=98.6, P≤0.0001, and the main effect of Treatment and the Diet × Treatment interaction were not significant (Fig. 1B). For the erythrocyte AA/EPA+DHA ratio, the main effect of Diet was significant, F(1,39)=1202, P≤0.0001, and the main effect of Treatment and the Diet × Treatment interaction were not significant (Fig. 1C). For the erythrocyte ADA/AA ratio, the main effect of Diet, F(1,39)=1037, P≤0.0001, and the Diet × Treatment interaction, F(1,39)=9.3, P=0.004, were significant, and the main effect of Treatment was not significant (Fig. 1D).
Figure 1.
Erythrocyte composition (wt % total fatty acid composition) of combined long-chain n-3 fatty acids, eicosapentaenoic acid (EPA, 20:5n-3)+docosahexaenoic acid (DHA, 22:6n-3)(‘omega-3 index’)(A) and arachidonic acid (AA, 20:4n-6)(B), and the AA/EPA+DHA (C) and the adrenic acid (ADA, 22:4n-6)/AA (D) ratios in control (CON, n=10), control+risperidone (CON+RSP, n=10), n-3-deficient (DEF, n=10), and n-3-deficient+risperidone (DEF+RSP, n=10) rats. Values are group means ± S.E.M. *P≤0.05, ***P≤0.0001 vs. controls, #P≤0.05 vs. DEF rats.
3.2. Plasma inflammatory markers
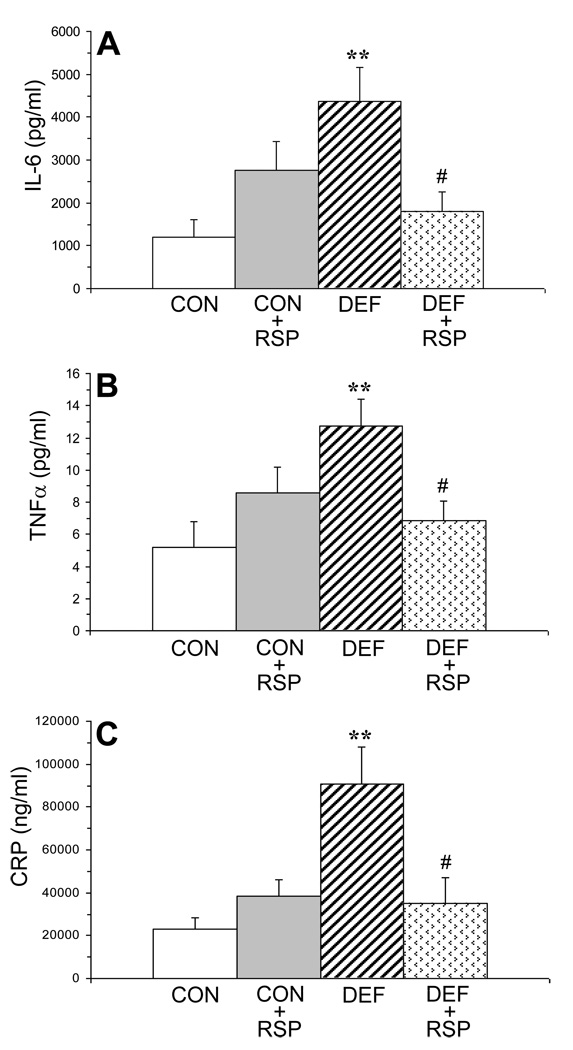
For plasma IL-6 concentrations (pg/ml), the Diet × Treatment interaction was significant, F(1,39)=8.8, P=0.006, and the main effects of Diet and Treatment were not significant (Fig. 2A). For plasma TNFα concentrations (pg/ml), the Diet × Treatment interaction was significant, F(1,39)=9.6, P=0.004, and the main effects of Diet and Treatment were not significant (Fig. 2B). For plasma CRP (ng/ml), the Diet × Treatment interaction, F(1,39)=11.5, P=0.002, and the main effects of Diet, F(1,39)=9.4, P=0.004, were significant, and the main effect of Treatment approached significance, F(1,39)=3.6, P=0.07 (Fig. 2C). Among all rats (n=40), IL-6 was positively correlated with CRP (r = +0.56, P=0.001) and TNFα (r = +0.53, P=0.002) concentrations.
Figure 2.
Plasma concentrations of interleukin-6 (IL-6, pg/mL)(A), tumor necrosis factor-alpha (TNFα, pg/ml)(B), and C-reactive protein (CRP, ng/ml)(C) in control (CON, n=10), control+risperidone (CON+RSP, n=10), n-3-deficient (DEF, n=10), and n-3-deficient+risperidone (DEF+RSP, n=10) rats. Values are group means ± S.E.M. **P≤0.01 vs. controls, #P≤0.05 vs. DEF rats.
3.3. Correlations with erythrocyte fatty acid composition
Among untreated control and n-3 fatty acid deficient rats (n=20), erythrocyte arachidonic acid composition was positively correlated with IL-6 (r = +0.54, P=0.01), TNFα (r = +0.69, P=0.003) and CRP (r = +0.54, P=0.02) concentrations, and erythrocyte EPA+DHA composition was inversely correlated with IL-6 (r = −0.66, P=0.003), TNFα (r = −0.65, P=0.006), and CRP (r = −0.62, P=0.008). The arachidonic acid/EPA+DHA ratio was positively correlated with IL-6 (r = +0.60, P=0.008), TNFα (r = +0.66, P=0.005), and CRP (r = +0.74, P=0.001). The ADA/arachidonic acid ratio was not correlated with plasma IL-6 (r = −0.32, p P=0.18), TNFα (r = +0.02, P=0.92), or CRP (r = +0.02, P=0.94). Among risperidone-treated control and n-3 fatty acid deficient rats (n=20), erythrocyte arachidonic acid composition was not significantly correlated with IL-6 (r = −0.17, P=0.53), TNFα (r = −0.24, P=0.33), or CRP (r = −0.09, P=0.71), and erythrocyte EPA+DHA composition was not significantly correlated IL-6 (r = +0.18, P=0.46), TNFα (r = +0.07, P=0.77), or CRP (r = +0.06, P=0.82). The ADA/AA ratio was inversely correlated with TNFα (r = −0.49, P=0.03), and similar trends were found for IL-6 (r = −0.38, P=0.09) and CRP (r = −0.35, P=0.16).
4. Discussion
A principal finding of the present study is that chronic risperidone treatment normalized constitutive elevations in pro-inflammatory cytokine (IL-6, TNFα) and CRP production in n-3 fatty acid deficient rats. The differences in pro-inflammatory cytokine and CRP levels observed in risperidone-treated versus drug-free n-3 fatty acid deficient rats is similar to those previously observed between n-3 fatty acid deficient and n-3 fatty acid repleted rats (McNamara et al., 2010). In controls, chronic risperidone treatment did not significantly alter IL-6, TNFα, or CRP production, though trends for greater concentrations were observed. Consistent with our prior study (McNamara et al., 2009), chronic risperidone treatment increased erythrocyte membrane long-chain n-3 fatty acid composition in rats maintained on a diet containing the short-chain n-3 fatty acid precursor (ALA, 18:3n-3), but not in rats maintained on the ALA-free diet. Contrary to our hypothesis, however, risperidone decreased pro-inflammatory cytokine and CRP production in rats maintained on the ALA-free diet in the absence of corresponding increases in n-3 fatty acid composition, and did not significantly alter cytokine and CRP production in rats maintained on the ALA-fortified diet despite increased n-3 fatty acid composition. Nevertheless, these data are in agreement with prior preclinical reports that risperidone has immunosuppressive and anti-inflammatory properties (Kato et al., 2007; Sugino et al., 2009), and further indicate that this effect is not mediated by augmentation of n-3 fatty acid biosynthesis.
Consistent with prior studies finding that n-3 fatty acid deficiency is associated with reciprocal increases in n-6 fatty acid biosynthesis (Igarashi et al., 2007), we found that erythrocyte membrane arachidonic acid (20:4n-6) composition was significantly elevated in untreated n-3 fatty acid deficient rats. Prior studies indicate that elevated arachidonic acid composition in immune cell membranes is positively correlated with PGE2 production (Calder, 2008). In the present study, erythrocyte membrane arachidonic acid composition was positively correlated with pro-inflammatory cytokine and CRP levels in untreated control and n-3 deficient rats. However, positive correlations were not observed in risperidone treated rats which exhibited similar erythrocyte arachidonic acid composition to untreated rats. This finding suggests that the immunosuppressant and anti-inflammatory effects of risperidone are dissociable from its effects on arachidonic acid membrane composition, and implicate involvement of down-stream signaling events potentially including COX-2-mediated arachidonic acid→PGE2 production. It is relevant, therefore, that combined treatment with the COX-2 inhibitor celecoxib and risperidone was found to be superior to risperidone alone in reducing symptom severity in schizophrenic patients (Akhondzadeh et al., 2007).
Chronic risperidone treatment differentially altered erythrocyte composition of adrenic acid (ADA, 22:4n-6) and the ADA/AA ratio, an index of arachidonic acid→ADA elongation, in controls and n-3 fatty acid deficient rats. Specifically, risperidone-treated controls exhibited a lower ADA/AA ratio relative to untreated controls, consistent with risperidone shunting arachidonic acid away from elongation to ADA to alternate pathways including COX-2 mediated arachidonic acid→PGE2 production. In contrast, risperidone-treated n-3 fatty acid deficient rats exhibited a higher erythrocyte ADA/AA ratio relative to untreated n-3 fatty acid deficient rats, and the ADA/AA ratio was inversely correlated with plasma TNFα concentrations. The latter findings are consistent with risperidone shunting arachidonic acid to arachidonic acid→ADA fatty acid elongation and away from arachidonic acid→PGE2 production. This significant Drug by Diet interaction suggests that n-3 fatty acid status is an important determinant of the immunosuppressant and anti-inflammatory effects of risperidone, and may contribute in part to the variability in immune responses observed in schizophrenic patients following treatment with atypical antipsychotic medications including risperidone (Drzyzga et al., 2006).
In conclusion, the present study found that chronic risperidone treatment has immunosuppressant and anti-inflammatory properties in n-3 fatty acid deficient rats, but not in n-3 adequate rats. These findings add to a growing body of preclinical data demonstrating that n-3 fatty acid deficiency (Calder, 2008; Groeger et al., 2010; Khalfoun et al., 1997; Lu et al., 2010; McNamara et al., 2010; Mingam et al., 2008; Serhan, 2005) and atypical antipsychotic medications including risperidone (Bian et al., 2008; Kato et al., 2007; Sugino et al., 2009) have opposing effects on inflammatory immune signaling pathways. Moreover, the present data support our prior finding that chronic risperidone augments long-chain n-3 fatty acid biosynthesis (McNamara et al., 2009), and demonstrate that this mechanism does not contribute to its immunosuppressive effects. The present data therefore suggest that the anti-inflammatory and immunosuppressive actions of risperidone may be mediated by alternate signaling pathways, including down-regulation of arachidonic acid→PGE2 production. Future studies will be required to determine whether other antipsychotic medications also exhibit anti-inflammatory and immunosuppressive actions in this model, and whether the effects are correlated with receptor binding profiles. Lastly, in conjunction with previous findings, the present data support the view that elevations in inflammatory and immunological signaling may contribute to the pathophysiology of schizophrenia.
Acknowledgments
This work was supported in part by NIH grants MH073704 to R.K.M. and DK59630 to P.T. The authors thank the laboratory of Dr. M. Wills-Karp for performing the cytokine and CRP assays, and Ortho-McNeil Janssen Pharmaceuticals for providing the risperidone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, Kanba S. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:42–48. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20:532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Kato T, Monji A, Hashioka S, Kanba S. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res. 2007;92:108–115. doi: 10.1016/j.schres.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400:589–597. [PubMed] [Google Scholar]
- Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments the development of behavioral sensitization in adult mice: Prevention by chronic lithium treatment. J Psychiatric Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek R, Rider T, Tso P. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: Evidence for augmented biosynthesis. Schizophr Res. 2009;107:150–157. doi: 10.1016/j.schres.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK. Modulation of polyunsaturated fatty acid biosynthesis by antipsychotic medications: Implications for the pathophysiology and treatment of schizophrenia. Clinical Lipidology. 2009;4:809–820. [Google Scholar]
- McNamara RK, Rider T, Jandacek R, Tso P, Straus A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: Relationship with central serotonin turnover. Prostogland Leukotrienes Essential Fatty Acids. 2010 doi: 10.1016/j.plefa.2010.08.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingam R, Moranis A, Bluthé RM, De Smedt-Peyrusse V, Kelley KW, Guesnet P, Lavialle M, Dantzer R, Layé S. Uncoupling of interleukin-6 from its signaling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:303–307. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]