Abstract
Five new benzenoids, benzocamphorins A–E (1–5), and ten recently isolated triterpenoids, camphoratins A–J (16–25), together with 23 known compounds including seven benzenoids (6–12), three lignans (13–15), and 13 triterpenoids (26–38) were isolated from the fruiting body of Taiwanofungus camphoratus. Their structures were established by spectroscopic analysis. Selected compounds were examined for cytotoxic and anti-inflammatory activities. Compounds 9 and 21 showed moderate cytotoxicity against MCF-7 and Hep2 cell lines with ED50 values of 3.4 and 3.0 µg/mL, respectively. Compounds 21, 25, 26, 29–31, 33, and 36 demonstrated potent anti-inflammatory activity by inhibiting lipopolysaccharide (LPS)-induced nitric oxide (NO) production with IC50 values of 2.5, 1.6, 3.6, 0.6, 4.1, 4.2, 2.5, and 1.5 µM, respectively, which were better than those of the nonspecific nitric oxide synthase (NOS) inhibitor N-nitro-L-arginine methyl ester (L-NAME) (IC50: 25.8 µM). These results may substantiate the use of T. camphoratus in traditional Chinese medicine (TCM) for the treatment of inflammation and cancer-related diseases. The newly discovered compounds deserve further development as anti-inflammatory candidates.
Keywords: Taiwanofungus camphoratus, Fruiting bodies, Anti-inflammatory, NOX, ROS, Cytotoxicity
1. Introduction
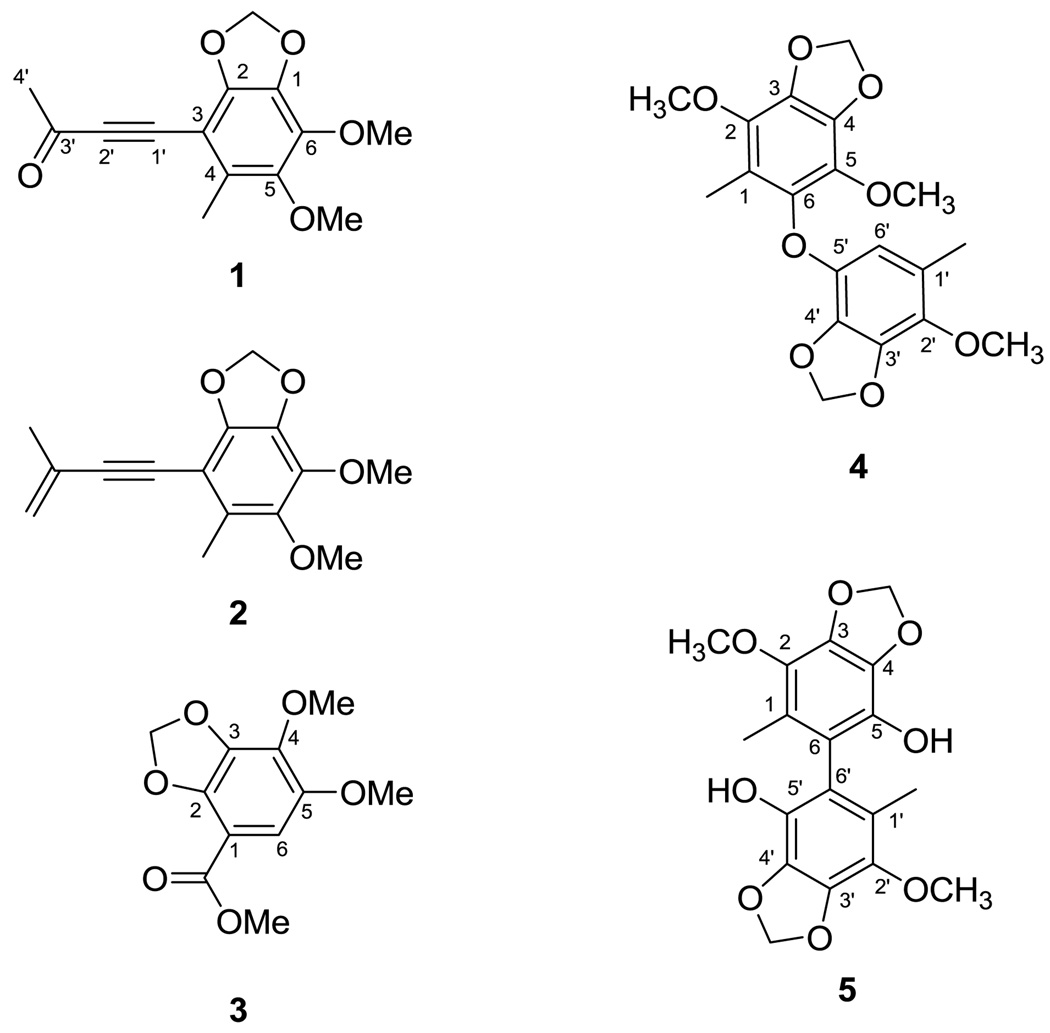
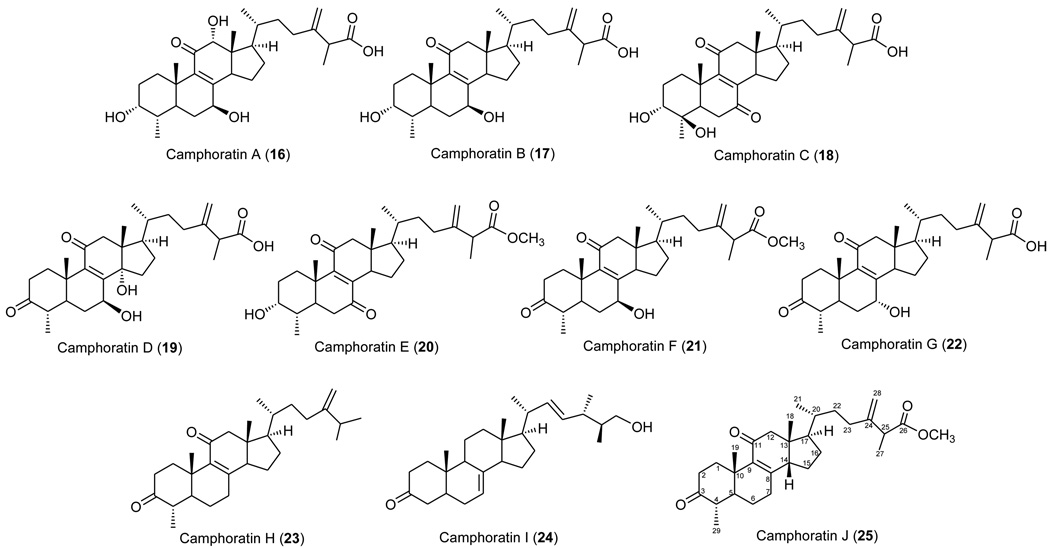
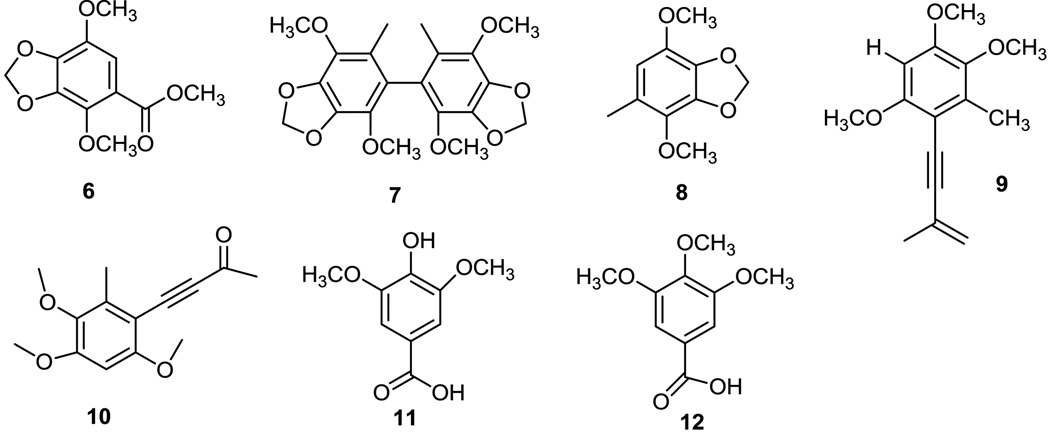
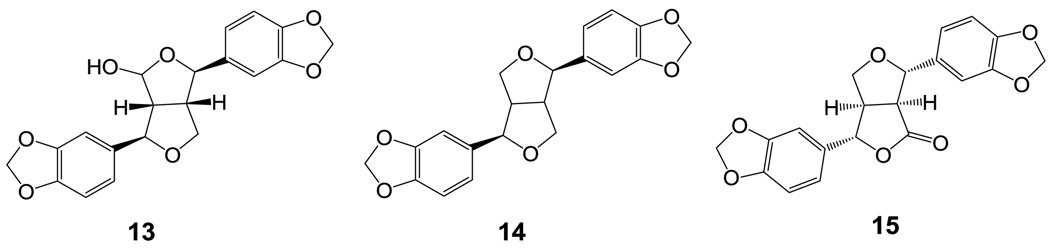
Niu-chang-chih also named Taiwanofungus camphoratus (synonym: Ganoderma camphoratum, Antrodia cinnamomea, Antrodia camphorata) (Polyporaceae, Aphyllophorales) is a rare and precious medical fungus in Taiwan.1 The fruiting bodies of Niu-chang-chih have been used as a Chinese folk medicine for the treatment of liver diseases, food and drug intoxication, diarrhea, abdominal pain, hypertension, itchy skin and tumorigenic diseases in Taiwan.2,3 Niu-chang-chih has thus received huge attention by the public. Previous studies have revealed that Niu-chang-chih exerts several biological activities, such as hepatoprotective effects, anti-hepatitis B virus effects, anticancer activity, antioxidant properties, and anti-inflammatory activities.4,5 Our ongoing study on the chemical constituents of an ethanol extract of the fruiting body of T. camphoratus has now led to the isolation of five new benzenoids, benzocamphorins A–E (1–5) (Figure 1), ten recently isolated triterpenoids, camphoratins A–J (16–25) (Figure 2), together with 23 known compounds including seven benzenoids (6–12) (Figure 3), three lignans (13–15) (Figure 4), and 13 triterpenoids (26–38) (Figure 5).
Figure 1.
Structures of compounds 1–5.
Figure 2.
Structures of compounds 16–25.
Figure 3.
Structures of compounds 6–12.
Figure 4.
Structures of compounds 13–15.
Figure 5.
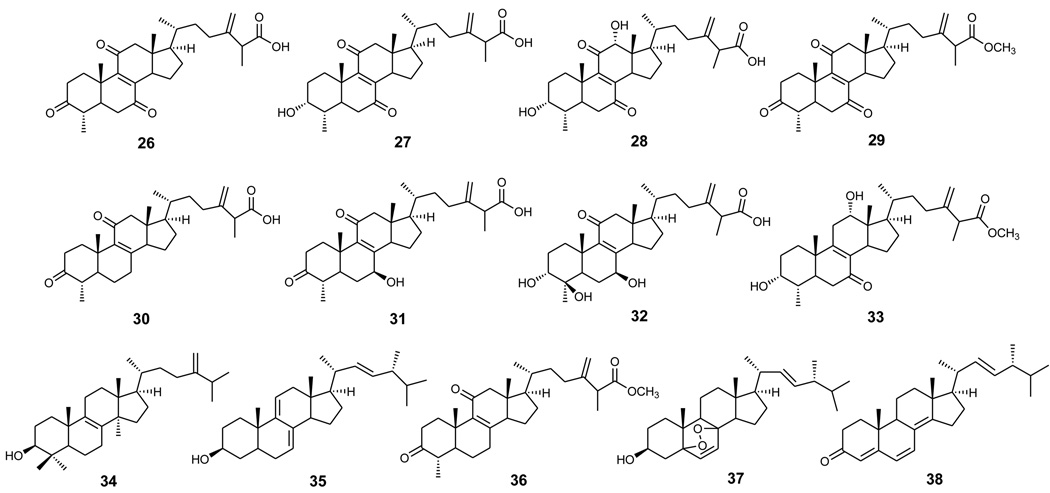
Structures of compounds 26–38.
Inflammation, which is related to morbidity and mortality of many diseases, is part of the complex biological response of vascular tissues to harmful stimuli, and is the host response to infection or injury, which involves the recruitment of leukocytes and the release of inflammatory mediators, including nitric oxide (NO). NO is the metabolic by-product of the conversion of L-arginine to L-citrulline by a class of enzymes termed NO synthases (NOS). Numerous cytokines can induce the transcription of inducible NO synthase (iNOS) in leukocytes, fibroblasts, and other cell types, accounting for enhanced levels of NO. Although NO is a microbicide and may have important roles in tissue adapting to inflammatory states, overproduction of NO may exacerbate tissue injury in both acute and chronic inflammatory conditions. In experimental models of acute inflammation, inhibition of iNOS can have a dose-dependent protective effect, suggesting that NO promotes edema and vascular permeability. NO also has a detrimental effect in chronic models of arthritis, whereas protection is seen with iNOS inhibitors. Glucocorticoids, which are often used in the treatment of inflammation, are able to inhibit the expression of iNOS. Therefore, the compounds isolated from T. camphoratus were tested for anti-inflammatory activity based on inhibition of NO production. In this paper, we report the structural determination of the new compounds from T. camphoratus, as well as evaluation of the cytotoxicity and anti-inflammatory activity of the isolates.
2. Chemistry: extraction and isolation
The wild fruiting bodies of T. camphoratus, growing in Ping-Tung Hsien, Taiwan, were purchased from the Kaohsiung Society for Wildlife and Nature in June 2003. The fungus was identified by Dr. Tun-Tschu Chang. A voucher specimen (TSWu 2003005) was deposited in the Department of Chemistry, National Cheng Kung University, Tainan, Taiwan.
The fresh fruiting body of T. camphoratus (1.0 kg) was extracted with EtOH (4 × 10 L) under reflux. The EtOH extract was concentrated to afford a brown syrup (161 g) and then partitioned between MeOH/H2O (1:1) and n-hexane. The water layer was filtered to obtain a filtrate and a water-insoluble portion. The filtrate (55.5 g) was subjected to column chromatography on Diaion HP-20 (10 × 60 cm) using increasing concentrations of MeOH in H2O as the eluent to obtain ten fractions (ACEW 1–10). Compounds 11 (2.6 mg, 0.0016%) and 12 (2.2 mg, 0.0014%) were obtained from fraction ACEW 1 by silica gel column chromatography using benzene-CHCl3 (9:1) as the eluent. Fraction ACEW 8 was rechromatographed on a silica gel column using CHCl3–Me2CO (25:1) as the eluent and purified further by preparative TLC (silica gel, i-Pr2O–Me2CO, 15:1) to obtain compounds 7 (40.0 mg, 0.025%), 4 (2.7 mg, 0.0017%), 5 (2.0 mg, 0.012%), and 6 (2.5 mg, 0.0016%). ACWE 10 was separated on a silica gel column using i-Pr2O–MeOH (6:1) as the eluent to afford four subfractions (ACEW10-1–10-4). Compounds 2 (10.0 mg, 0.0062%), 1 (2.0 mg, 0.0012%), 10 (3.2 mg, 0.002%), 9 (10.2 mg, 0.0063%), and 8 (30.0 mg, 0.0186%) were obtained from subfraction ACEW10-1 using preparative TLC (silica gel, n-hexane–Me2CO, 15:1). Compounds 13 (7.0 mg, 0.0043%), 14 (6.1 mg, 0.0038%), and 15 (3.5 mg, 0.0022%) were isolated from subfraction ACEW10-3 by column chromatography over silica gel using n-hexane–EtOAc (1:1) as the eluent. Subfraction ACEW10-4 was chromatographed on a silica gel column using n-hexane–EtOAc (1:1.5) as the eluent to yield compound 3 (3.0 mg, 0.0019%).
The n-hexane layer (9.3 g) was chromatographed on silica gel and eluted with EtOAc in n-hexane (gradient of 0–100% EtOAc) to obtain ten fractions. Fraction 4 was chromatographed repeatedly on a silica gel column using n-hexane–Me2CO (19:1) as the eluent to yield 23 (3.0 mg), 24 (6.0 mg, 0.0037%), 25 (4.5 mg, 0.0028%), 38 (3.0 mg, 0.0019%), 34 (22.0 mg, 0.0137%), 35 (90.2 mg, 0.056%), 36 (22.1 mg, 0.0137%), and 37 (16.5 mg, 0.0102%). Compound 37 (41.1 mg, 0.0255%) was also obtained in the same way from fraction 8. The water-insoluble portion (89.5 g) was chromatographed on a silica gel column using CHCl3–MeOH mixtures of increasing polarity for elution to obtain ten fractions (WI-1–WI-10). Compounds 16 (2.2 mg, 0.0014%), 20 (2.0 mg, 0.0012%), 21 (14.2 mg, 0.0088%), 24 (1.0 mg, 0.0006%), 29 (1.29 g, 0.801%), 30 (53.8 mg, 0.0334%), and 36 (62.2 mg, 0.0386%) were obtained from a combined fraction (fractions WI-1 and WI-2) by silica gel column chromatography with gradient elution (CHCl3–Me2CO, 39:1 to 14:1). Fraction WI-3 was separated on a silica gel column using i-Pr2O–MeOH (19:1) as the eluent to yield 26 (4.1 g, 2.55%), 33 (11.0 mg, 0.0068%), 31 (122.9 mg, 0.0763%), and 27 (53.0 mg, 0.0329%). Fraction WI-4 was chromatographed on a silica gel column with i-Pr2O–MeOH (12:1) to give 22 (11.3 mg, 0.007%), 33 (38.0 mg, 0.0236%), 31 (708.0 mg, 0.44%), and 27 (66.5 mg, 0.041%). Fractions WI-5–WI-7 were combined and rechromatographed on a silica gel column with CHCl3–MeOH (6:1) as the mobile phase to afford 17 (5.0 mg, 0.0031%), 19 (2.2 mg, 0.0014%), 18 (3.8 mg, 0.0024%), 22 (3.4 mg, 0.00211%), and 28 (2.10 g, 1.3%). Compound 32 (1.16 g, 0.72%) was isolated from a combined fraction (fractions WI-8 and WI-9) by silica gel column chromatography using i-Pr2O–MeOH (4:1) as the eluent.
3. Results and discussion
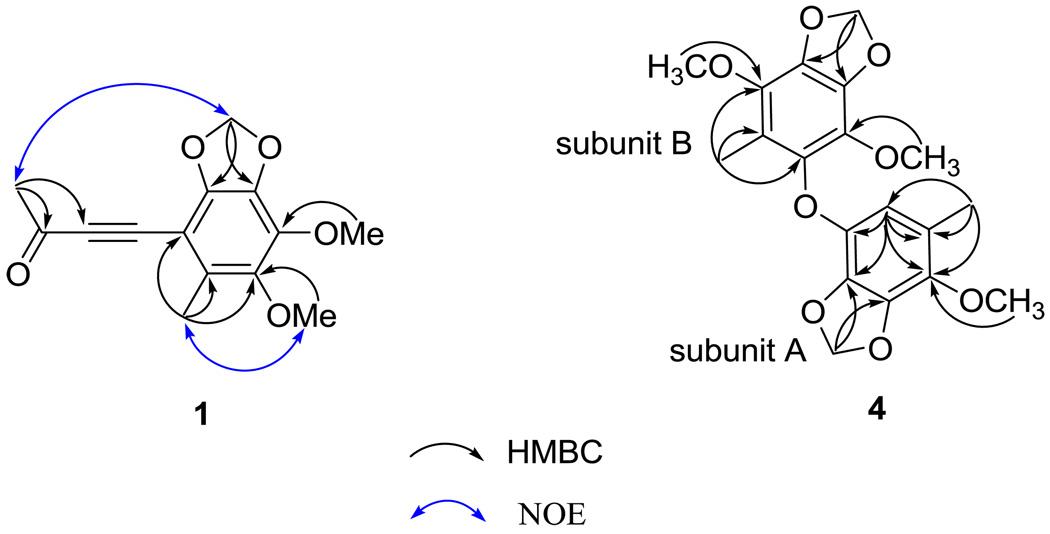
The structural elucidation of the five newly isolated benzenoids, benzocamphorins A–E, (1–5) is described below. Compound 1 was isolated as a pale yellow oil. HRESIMS showed an [M + Na]+ ion peak at m/z 285.0740, consistent with the molecular formula C14H14O5Na. The IR spectrum suggested that 1 was a benzenoid (νmax 1610, 1475, and 1446 cm−1) bearing a conjugated carbonyl (νmax 1663 cm−1). The latter was identified as a methyl ketone based on the carbon resonances at δ 33.2 (CH3) and 184.8 (qC) as well as the proton resonance at δ 2.45 (3H, s).6 The 1H NMR spectra of 1 showed signals for one methylenedioxy [δH 5.98 (2H, s)], one aromatic methyl [δH 2.31 (3H, s)], and two methoxy [δH 3.88 (3H, s) and 4.02 (3H, s)] groups. In addition, a 1,2-disubstituted alkyne [δC 87.6 (qC) and 96.0 (qC)] was also observed in the 13C NMR spectrum of 1. The above characteristic NMR signals were similar to those of the known benzenoid antrocamphin B (10), isolated from the same organism.6 Detailed inspection of the HMBC spectrum of 1 led to the assignment of a 3-oxobut-1-ynyl group (Figure 2). The NOE correlations between Me-4 and OMe-5 and between Me-3′ and the methylenedioxy protons helped to establish the structure of 1 (Figure 6).
Figure 6.
Selected HMBC correlations of 1 and 4 and NOE correlations of 1.
The molecular formula of 2, C15H16O4, was established from HRESIMS and 13C NMR spectroscopic data. The IR spectrum showed absorption bands attributable to a benzonoid at νmax 1611, 1473, and 1449 cm−1. The 1H NMR spectrum of 2 was similar to that of 1, except that the 3-oxobut-1-ynyl group in 1 was replaced by a 3-methylbut-3-en-1-ynyl group in 2. This assignment was enforced by the presence of the proton resonances for a 1,1-disubstituted double bond at δ 5.36 (1H, s) and 5.25 (1H, s), which correlated to C-2′, C-3′, and C-4′ in the HMBC spectrum of 2. In the NOESY spectrum of 2, a NOE correlation between the methylenedioxy proton and the Me-3′ protons suggested that the methylenedioxy group was located at C-1 and C-2. In addition, the Me-4 protons showed NOE correlations with one proton of the sp2 methylene group at δ 5.36 and with the OMe-5 protons at δ 3.85, which suggested that Me-4 was adjacent to C-3 and C-5. The locations of all functionalities borne by the benzene ring were thus determined.
The molecular formula of 3 was deduced as C11H12O6 based on the pseudomolecular ion peak at m/z 263.0534 [M + Na]+ obtained from HRESIMS. The 13C NMR spectrum of 3 displayed 11 signals including a methyl ester [δC 164.9 (C=O) and 52.0 (OMe)], two methoxy [δC 60.2 (OMe-4) and 56.7 (OMe-5)], a methylenedioxy group [δC 102.1], an aromatic methine [δC 104.3 (C-6)], a quaternary carbon [δC 104.8 (C-1)], and four oxygenated aromatic [δC137.5 (C-3), 137.7 (C-4), 144.8 (C-2), and 146.4 (C-5)] carbons. The 1H NMR spectrum showed a single aromatic proton resonance [δH 6.90 (s, H-6)], which indicated a pentasubstituted benzene ring in 3. The above characteristic NMR signals were similar to those of the known compound, methyl 2,5-dimethoxy-3,4-methylenedioxybenzoate (6),7 suggesting an isomeric relationship between both compounds. The aromatic methine proton showed an HMBC correlation with the carbonyl carbon of the methyl ester and an NOE correlation with the methoxy protons at C-5, suggesting that this proton (H-6) was located between C-1 and C-5. Considering the above evidence coupled with a comparison of the NMR spectroscopic data of 3 with those of 6, the structure of 3 was thus determined as methyl 4,5-dimethoxy-2,3-methylenedioxybenzoate.
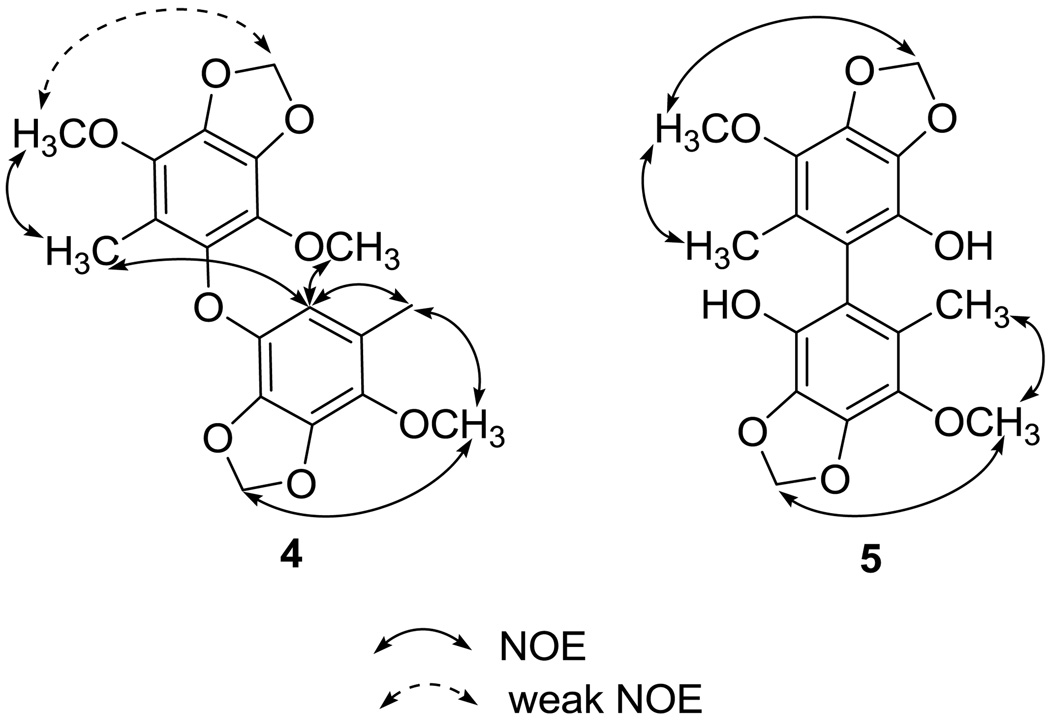
Compound 4 was isolated as a white powder and exhibited a [M + Na]+ peak at m/z 399.1051, corresponding to the molecular formula of C19H20O8Na and ten degrees of unsaturation. Its IR spectrum showed absorption bands at 1619, 1497, 1489, 1448, and 1427 cm−1, disclosing that 4 is a benzenoid. Its 1H NMR spectrum, coupled with a HMQC experiment, showed signals for two methylenedioxy groups at δ 5.98 and 5.94, three methoxy groups at δ 3.93, 3.88, and 3.82, and two aromatic methyl groups at δ 2.06 and 2.03. One remaining aromatic methine and eleven aromatic quaternary carbons were also observed in the 13C NMR spectrum of 4. Thus, 4 might be a benzoic dimer. In subunit A, the HMBC correlations from the methyl proton at δH 2.06 to the carbons at C-1′, C-2′, and C-6′, from the methylenedioxy protons at δH 5.98 to the carbons at C-3′ and C-4′, and from the phenyl proton at δH 5.92 (H-6′) to the carbons at C-1′, C-2′, C-4′ and C-5′ (Figure 2) in combination with selective 1D NOESY experiments (Me-1′ / OMe-2′, Me-1′ / H-6′, and OMe-2′ / methylenedioxy protons at δH 5.85) (Figure 7) enforced the locations of all functional groups on the benzene ring. The carbons in subunit B were deduced as a benzenoid containing a methyl, one methylenedioxy, and two methoxy substituents. The HMBC correlations from Me-1 protons to C-2 and C-6 carbons as well as the selective 1D NOESY enhancements of Me-1 /OMe-2, H-6′ / Me-1 and H-6′ / OMe-5 established the structure of subunit B. Based on the carbon resonances of C-6 and C-5′ at 139.5 and 137.25, respectively, the two subunits were connected by an oxygen atom, which also was consistent with the molecular formula of 4.
Figure 7.
NOE correlations of 4 and 5.
The HRESIMS spectrum of 5 disclosed that it possesses a molecular ion peak at m/z 385.0897, corresponding to the molecular formula C18H18O8Na. The IR absorption bands at νmax 3526, 1512 and 1460 cm−1 suggested that 5 was a phenolic derivative. The appearance of only nine signals in the 13C NMR spectrum revealed that the compound is symmetric and homodimeric. Analysis of the 1H and 13C NMR spectra of 5 suggested that half of the molecular possesses methoxy [δH 3.93 (s); δC 60.1(CH3)], methylenedioxy [δH 6.02 (s); δC 101.8 (CH2)], and aromatic methyl [δH 1.85 (s); δC 12.6 (CH3)] groups, as well as four oxygenated aromatic carbons [δC 139.1 (qC), 136.4 (qC), 133.6 (qC), and 133.3 (qC)] and two quaternary carbons [δC 123.8 (qC) and δC 114.5 (qC)]. The aromatic methyl protons showed HMBC correlations to C-1, C-2, and C-6, suggesting that this methyl group was positioned between C-2 and C-6, to which the other molecular half was attached. In the NOESY spectrum of 5 (Figure 7), the methylenedioxy protons showed an NOE correlation with the OMe-2 carbon, suggesting that this substituent was positioned at C-3 and C-4. Thus, the one remaining hydroxy group should be positioned at C-5. The structure of 5 was unambiguously determined as shown in Figure 1.
The structural characterization of ten newly isolated triterpenoids, camphoratins A–J (16–25), was reported previously in Ref 13. In addition, 23 known compounds were identified by the comparison of their physical and spectroscopic data with those of corresponding authentic samples, including seven benzenoids [2,5-dimethoxy-3,4-methylenedioxybenzoate (6),7 2,2′,5,5′-tetramethoxy-3,4,3′,4′-bi-methylenedioxy-6,6’-dimethylbiphenyl (7),8 4,7-dimethoxy-5-methyl-1,3-benzodioxole (8),8 antrocamphins A and B (9 and 10),6 syringic acid (11),9 and 3,4,5-trimethoxybenzoic acid (12)],10 three lignans [4-hydroxysesamin (13),11 (+) sesamin (14),11 and aptosimon (15)],12 and 13 triterpenoids [zhankuic acids A–C (26–28),14, 15 zhankuic acid A methyl ester (29),14 antcin A (30),15 antcin C (31),15 antcin K (32),16 methyl antcinate H (33),17 eburicol (34),18 ergosterol D (35),19 methyl 4α-methylergost-8,24(28)-dien-3,11-dion-26-oate (36),20 ergosterol peroxide (37),21 and ergosta-2,4,8(14),22-tetraen-3-one (38)].22
Compounds 7–9, 13, 14, 20, 21, 25–33, and 36 were first assayed for cytotoxic activity against Doay (human medulloblastoma), Hep2 (human laryngeal carcinoma), MCF-7 (human breast adenocarcinoma), and Hela (human cervical epitheloid carcinoma) cell lines. Compounds 9 and 21 showed moderate cytotoxicity against MCF-7 and Hep2 cell lines with ED50 values of 3.4 and 3.0 µg/mL, respectively (Table 1). The other tested compounds showed marginal or little cytotoxicity (threshold for activity is considered 4 µg/mL) against the above cancer cell lines.
Table 1.
Cytotoxic activity of 7–9, 13 and 14, 20 and 21, 25–33, and 36
| cell lines ED50 (µg/mL ) | ||||
|---|---|---|---|---|
| compound | Daoy | Hep2 | MCF-7 | Hela |
| 7 | - | - | - | - |
| 8 | - | - | - | - |
| 9 | 5.9 | 10.5 | 3.4 | 6.9 |
| 13 | - | - | - | - |
| 14 | - | - | - | - |
| 20 | 5.2 | 7.0 | 6.6 | 9.0 |
| 21 | 4.4 | 3.0 | 7.9 | 8.9 |
| 25 | –a | – | 8.7 | 11.3 |
| 26 | – | 16.6 | – | – |
| 27 | - | - | - | - |
| 28 | - | - | - | - |
| 29 | - | - | - | - |
| 30 | 13.2 | – | 13.3 | – |
| 31 | - | - | - | - |
| 32 | - | - | - | - |
| 33 | - | - | - | - |
| 36 | - | - | - | - |
| mitomycin | 0.1 | 0.1 | 0.1 | 0.2 |
ED50 >20 µg/mL.
The anti-inflammatory effects of 2, 7–9, 17, 21, and 24–37 were then evaluated by examining their effects on lipopolysaccharide (LPS)-induced iNOS-dependent NO production and NADPH oxidase (NOX)-dependent reactive oxygen species (ROS) production in BV2 murine microglial cells and polymorphonuclear neutrophils (PMNs) (Table 2). Triterpenoids 21, 25, 26, 29–31, 33, and 36 inhibited NOS activity significantly with IC50 values of 2.5, 1.6, 3.6, 0.6, 4.1, 4.2, 2.5, and 1.5 µM, respectively. They were more potent than N-nitro-L-arginine methyl ester (L-NAME) (IC50: 25.8 µM), a nonspecific NOS inhibitor, at inhibiting LPS-induced NO production. Except for 8 and 35, the remaining tested compounds also effectively inhibited NOS activity with IC50 values ranging from 6.3 to 22.3 µM. NOX is the major ROS-producing enzyme in activated inflammatory cells.23 We previously reported that drugs with anti-inflammatory activity also show potent NOX-inhibitory action.24,25 Therefore, we evaluated the isolates for effects on NOX activity in lysates of microglial cells and PMNs. Our data suggest that none of the tested compounds were potent inhibitors of NOX, relative to the specific NOX inhibitor diphenyleneiodonium (DPI) (IC50 0.4 and 0.3 µM in lysates of microglial cells and PMNs, respectively) (Table 2).
Table 2.
Effects of 2, 7–9, 17, 21, and 24–37 on NOX activity a in BV2 murine microglial cells and PMNs and NOS activity b in murine microglial cells
| IC50 (µM) in NOX | |||
|---|---|---|---|
| compound | activity from BV2 cell lysate |
fMLP-induced NOX activation in PMN |
IC50 (µM) in NOS |
| 2 | ND | 14.4 ± 4.9* | 12.1± 0* |
| 7 | ND | 15.5 ± 3.3* | 16.2 ± 1.4* |
| 8 | ND | 19.9 ± 3.0* | 29.1 ± 4.4* |
| 9 | 50.1± 3.3* | 15.1 ± 4.1* | 7.2 ± 1.0* |
| 17 | ND | 32.1 ± 3.5* | 15.7 ± 0.9* |
| 21 | ND | 11.2 ± 2.3* | 2.5 ± 0.6* |
| 24 | ND | 17.5 ± 3.9* | 12.7 ± 2.2* |
| 25 | ND | 15.8 ± 4.0* | 1.6 ± 0.6* |
| 26 | ND | 22.1 ± 6.7* | 3.6 ± 0.8* |
| 27 | ND | ND | 9.6 ± 0.7* |
| 28 | 40.3 ± 3.5* | ND | 16.2 ± 0.9* |
| 29 | ND | 8.4 ± 2.1* | 0.6 ± 0.3* |
| 30 | 45.9 ± 7.9* | 29.2 ± 6.7* | 4.1 ± 0.5* |
| 31 | ND | 22.6 ± 3.3* | 4.2 ± 1.2* |
| 32 | ND | 47.2 ± 8.4* | ND |
| 33 | 16.0 ± 8.1* | 18.1 ± 5.9* | 2.5 ± 0.3* |
| 34 | ND | 21.9 ± 6.3* | 22.3 ± 2.9* |
| 35 | ND | 27.9 ± 5.6* | 30.6 ± 0.8* |
| 36 | ND | 16.2 ± 4.3* | 1.5 ± 0.7* |
| 37 | ND | 20.3 ± 6.4* | 6.3 ± 1.8* |
| DPI | 0.4 ± 0.2 | 0.3 ± 0.1 | – |
| L-NAME | – | – | 25.8 ± 2.5 |
NOX activity was measured as ROS production by triggering with NADPH (200 µM) or fMLP (2 µM) in the presence of test drugs (1–50 µM) in BV2 cell lysate or PMN. DPI (a NOX inhibitor) was included as a positive control for NOX inhibition.
NO production was measured in the presence of test drugs (1–50 µM). L-NAME (a non-selective NOS inhibitor) was included as a positive control. Data were calculated as 50% inhibitory concentration (IC50) and expressed as the mean ± S.E.M. from 3–6 experiments performed on different days using BV2 cell lysate or PMN from different passages or donors. ND: values not detectable. “–”: samples not tested * P < 0.05 as compared with relative positive control, respectively.
Based on an overview of the biological data, the following structure-activity relationship (SAR) conclusions were noted regarding the NOS inhibitory activity for compounds 17, 21, and 24–37 (Table 2). Among the tested compounds, the 3-ketone triterpenoids were more active than the corresponding 3-hydroxy analogues (e.q., 21 vs 17; 27 vs 16). Similarly, introduction of a hydroxy group to C-4 resulted in a dramatic loss of activity (e.q., 27 vs 32). It is apparent that a less polar A ring contributed to increased activity. In addition, comparison of triterpenoids with different substituents at C-7 revealed that the rank order of potency was 7-ketone (29) > 7-methylene (30) ≈ 7-hydroxy (31). On the other hand, compound 25, the rare example with a 14β-hydrogen configuration, was less potent than the normal triterpene (29, 14α-hydrogen). A comparison of the carboxylic acids and methyl carboxylates revealed that the latter were slightly more potent than the former (e.g., 21 vs 31; 29 vs 26). Consequently, the most active 29 could be reasonably explained by the fact that it possesses 7-ketone, less polar A ring, and methyl carboxylate functionalities.
In conclusion, the results from the anti-inflammatory assays revealed that the triterpenoids 21, 25, 26, 29–31, 33, and 36 have potent NO-reducing activity in microglial cells. Thus, triterpenoids rather than benzenoids might be the active components of the folk remedy using T. camphoratus for the treatment of some inflammatory related disorders. However, continued investigation of related triterpernoids coupled with structure modification studies could be helpful to develop and optimize lead chemotherapeutic agents.
4. Experimental
4.1. General experimental procedures
Melting points were determined on a Yanagimoto MP-S3 micro-melting point apparatus. IR spectra were recorded on a Shimadzu FTIR spectrometer Prestige-21. Optical rotations were measured using a Jasco DIP-370 polarimeter. UV spectra were obtained on a Hitachi UV-3210 spectrophotometer. ESI and HRESI mass spectra were recorded on a Bruker APEX II mass spectrometer. The NMR spectra, including 1H NMR, 13C NMR, COSY, NOESY, HMBC, HMQC experiments, were recorded on Bruker AVANCE-500 and AMX-400. Silica gel (E. Merck 70–230, 230–400 mesh) was used for column chromatography.
4.1.1. Benzocamphorin A (1)
Pale yellow oil; UV (MeOH) λmax (log ε) 214 (3.44), 275 (2.63), 315 (2.94) nm; IR (KBr) νmax 2925, 2854, 1663, 1610, 1475, 1446, 1381, 1277, 1212, 1054 cm−1; 1H NMR (CDCl3 400 MHz) δH 5.98 (2H, s, OCH2O), 4.02 (3H, s, OMe-6), 3.88 (3H, s, OMe-5), 2.45 (3H, s, 4′), 2.31 (3H, s, Me-4); 13C NMR(CDCl3, 100 MHz) δC 184.8 (C-3′), 142.5 (C-2), 142.0 (C-6), 137.5 (C-5), 136.2 (C-1), 131.3 (C-4), 106.6 (C-3), 102.2 (OCH2O), 96.0 (C-2′), 87.6 (C-1′), 60.7 (OMe-6), 60.5 (OMe-6), 33.2 (C-4′), 14.4 (Me-4); ESIMS m/z 285 [M + Na]+; HRESIMS m/z 285.0740 (calcd for C14H14O5Na, 285.0739)
4.1.2. Benzocamphorin B (2)
Pale yellow oil; UV (MeOH) λmax (log ε) 215 (4.38), 254 (3.78), 287 (4.04) nm; IR (KBr) νmax 2943, 2781, 1611, 1473, 1449, 1389, 1274, 1207, 1050 cm−1; 1H NMR (CDCl3, 400 MHz) δH 5.36 (1H, br s, H-5′b), 5.26 (1H, br s, H-5′a), 5.92 (2H, s, OCH2O), 3.97 (3H, s, OMe-6), 3.85 (3H, s, OMe-5), 2.26 (3H, s, Me-4), 2.00 (3H, s, Me-3′); 13C NMR(CDCl3, 100 MHz) δC 139.8 (C-6), 139.4 (C-1), 137.1 (C-5), 136.2 (C-2), 127.8 (C-4), 127.2 (C-3′), 120.9 (C-5′), 109.8 (C-3), 101.4 (OCH2O), 97.5 (C-2′), 83.5 (C-1′), 60.3 (OMe-6), 59.9 (OMe-5), 23.5 (Me-4), 13.8 (Me-3′); ESIMS m/z 283 [M + Na]+; HRESIMS m/z 283.0944 (calcd for C15H16O4Na, 283.0946 )
4.1.3. Benzocamphorin C (3)
Colorless oil; UV (MeOH) λmax (log ε) 220 (3.69), 263 (3.36), 320 (2.95) nm; IR (KBr) νmax 2920, 2851, 1699, 1629, 1503, 1437, 1201, 1097 cm−1; 1H NMR (CDCl3, 300 MHz) δH 6.90 (1H, s, H-6), 6.04 (2H, s, OCH2O), 4.10 (3H, s, OMe-4), 3.89 (3H, s, COOCH3), 3.85 (3H, s, OMe-5); 13C NMR(CDCl3, 75 MHz) δC 164.9 (COOCH3), 146.4 (C-5), 144.8 (C-2), 137.7 (C-4), 137.5 (C-3) 104.8 (C-1), 104.3 (C-6), 102.1 (OCH2O), 60.2 (OMe-4), 56.7 (OMe-5), 52.0 (COOCH3); ESIMS m/z 263 [M + Na]+; HRESIMS m/z 263.0534 (calcd for C11H12O6Na, 263.0532)
4.1.4. Benzocamphorin D (4)
White powder; mp 73–74 °C; UV (MeOH) λmax (logε) 207 (4.80), 279 (3.39) nm; IR (KBr) νmax 2939, 2892, 1619, 1497, 1448, 1427, 1254, 1232, 1119, 1085, 1057, 1024, 956 cm−1; 1H NMR (CDCl3 500 MHz) δH 2.03 (3H, s, CH3-1), 2.06 (3H, s, CH3-1′), 3.82 (3H, s, OCH3-5), 3.88 (3H, s, OCH3-2′), 3.93 (3H, s, OCH3-2), 5.92 (1H, s, H-6′), 5.94 (2H, s, OCH2O-3, 4), 5.98 (2H, s, OCH2O-3′, 4′); 13C NMR (CDCl3, 125 MHz) δC 9.3 (CH3-1), 15.8 (CH3-1′), 59.8 (OCH3-2′), 60.0 (OCH3-2), 60.6 (OCH3-5), 101.4 (OCH2O-3, 4), 101.6 (OCH2O-3′, 4′), 109.5 (C-6′), 117.6 (C-1), 123.6 (C-1′), 133.0 (C-5), 134.3 (C-3′), 135.6 (C-4), 136.7 (C-2′), 136.8 (C-2), 137.25 (C-5′), 137.29 (C-3), 138.7 (C-4′), 139.5 (C-6); ESIMS m/z 399 [M + Na]+; HRESIMS m/z 399.1052 (calcd for C19H20O8Na, 399.1056 )
4.1.5. Benzocamphorin E (5)
Colorless oil; UV (MeOH) λmax (logε) 208 (4.91), 283 (3.80) nm; IR (KBr) νmax 3526, 2928, 2859, 1713, 1492, 1460, 1261, 1035 cm−1; 1H NMR (CDCl3, 500 MHz) δH 1.85 (6H, s, CH3-1, 1′), 3.93 (6H, s, OCH3-2, 2′), 6.02 (4H, s, OCH2O-3, 4; 3′, 4′); 13C NMR(CDCl3, 125 MHz) δC 12.6 (CH3-1, 1′), 60.1 (OCH3-2, 2′), 101.8 (OCH2O-3, 4; 3′, 4′), 114.5 (C-6, 6′), 123.8 (C-1, 1′), 133.3 (C-5, 5′), 133.6 (C-4, 4′ or C-3, C-3′), 136.4 (C-2, 2′), 139.1 (C-4, 4′ or C-3, C-3′); ESIMS m/z 385 [M + Na]+; HRESIMS m/z 385.0897 (calcd for C18H18O8Na, 385.0899)
4.2. Cytotoxicity assay
Cytotoxicity was tested against Doay, Hep2, MCF-7, and Hela cell lines, using a MTT colorimetric assay method. The assay procedure was carried out as previously described,26 and mitomycin was used as positive control.
4.3. Microglial cell culture and measurements of NO
The BV2 murine microglial cell line was cultured and production of NO was measured by the methods as described in our prior report.27 L-NAME (a non-selective NOS inhibitor) was included as a positive control.
4.4. Measurement of NOX activity
NOX activity was measured as described previously.27 DPI (a NOX inhibitor) was included as a positive control.
Acknowledgments
The authors acknowledge the financial support from the National Science Council, Taiwan, Republic of China and Dr. Tun-Tschu Chang (Division of Forest Protection, Taiwan Forestry Research Institute, Taipei, Taiwan) for his identification of T. camphoratus. Thanks are also due to the partial support from NIH grant CA-17625 awarded to K. H. Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Wu SH, Yu ZH, Dai YC, Chen CT, Su CH, Chen LC, Hsu WC, Hwang GY. Fung. Sci. 2004;19:109. [Google Scholar]
- 2.Tsai ZT, Liaw SL. The Use and the Effect of Ganoderma. Taichung, Taiwan: Sang-Yun Press; 1982. pp. 116–117. [Google Scholar]
- 3.Chen CJ, Su CH, Lan MH. Fung. Sci. 2001;16:65. [Google Scholar]
- 4.Liu DZ, Liang HJ, Chen CH, Su CH, Lee ZH, Huang CT, Hou WC, Lin SY, Zhong WB, Lin PJ, Hung LF, Liang YC. J. Ethnopharmacol. 2007;113:45. doi: 10.1016/j.jep.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Rao YK, Fang SH, Tzeng YM. J. Ethnopharmacol. 2007;114:78. doi: 10.1016/j.jep.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Chen JJ, Lin WJ, Liao CH, Shieh PC. J. Nat. Prod. 2007;70:989. doi: 10.1021/np070045e. [DOI] [PubMed] [Google Scholar]
- 7.Huang KF, Huang WM, Chiang HC. Chin. Pharm. J. 2001;53:327. [Google Scholar]
- 8.Chiang HC, Wu DP, Cherng IW, Ueng CH. Phytochemistry. 1995;39:613. [Google Scholar]
- 9.Herz W, Kumar N. Phytochemistry. 1981;20:247. [Google Scholar]
- 10.Tezuka Y, Yoshida Y, Kikuchi T, Xu GJ. Chem. Pharm. Bull. 1993;41:1346. doi: 10.1248/cpb.41.1866. [DOI] [PubMed] [Google Scholar]
- 11.Wu DP, Chiang HC. J. Chin. Chem. Soc. 1995;42:797. [Google Scholar]
- 12.Brieskorn CH, Huber H. Tetrahedron Lett. 1976:2221. [Google Scholar]
- 13.Wu SJ, Leu YL, Chen CH, Chao CH, Shen DY, Chan HH, Lee EJ, Wu TS, Wang YH, Shen YC, Lee KH. J. Nat. Prod. in press. [Google Scholar]
- 14.Chen CH, Yang SW, Shen YC. J. Nat. Prod. 1995;58:1655. doi: 10.1021/np50125a002. [DOI] [PubMed] [Google Scholar]
- 15.Cherng IH, Chiang HC, Cheng MC, Wang Y. J. Nat. Prod. 1995;58:365. [Google Scholar]
- 16.Shen CC, Kuo YC, Huang RL, Lin LC, Don MJ, Chang TT, Chou CJ. J. Chin. Med. 2003;14:247. [Google Scholar]
- 17.Cherng IW, Wu DP, Chiang HC. Phytochemistry. 1996;41:263. [Google Scholar]
- 18.Shirane N, Takenaka H, Ueda K, Hashimoto Y, Katoh K, Ishii H. Phytochemistry. 1996;41:1301. [Google Scholar]
- 19.Abraham RJ, Monasterios JR. J. Chem. Soc. Perkin Trans. II. 1974:662. [Google Scholar]
- 20.Wu DP, Chiang HC. J. Chin. Chem. Soc. 1995;42:797. [Google Scholar]
- 21.Rösecke J, König WA. Phytochemistry. 2000;54:757. doi: 10.1016/s0031-9422(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Quang DN, Bach DD. Nat. Prod. Res. 2008;22:901. doi: 10.1080/14786410701642706. [DOI] [PubMed] [Google Scholar]
- 23.Van den Worm E, Beukelman CJ, Van den Berg AJ, Kroes BH, Labadie RP, Van Dijk H. Eur. J. Pharmacol. 2001;433:225. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 24.Lin LC, Wang YH, Hou YC, Chang S, Liou KT, Chou YC, Wang WY, Shen YC. J. Pharm. Pharmacol. 2006;58:129. doi: 10.1211/jpp.58.1.0016. [DOI] [PubMed] [Google Scholar]
- 25.Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Eur. J. Pharmacol. 2003;475:19. doi: 10.1016/s0014-2999(03)02121-6. [DOI] [PubMed] [Google Scholar]
- 26.Shen YC, Wang SS, Pan YL, Lo KL, Chakraborty R, Chien CT, Kuo YH, Lin YC. J. Nat. Prod. 2002;65:1848. doi: 10.1021/np0202273. [DOI] [PubMed] [Google Scholar]
- 27.Wang YH, Wang WY, Chang CC, Liou KT, Sung YJ, Liao JF, Chen CF, Chang S, Hou YC, Chou YC, Shen YC. J. Biomed. Sci. 2006;13:127. doi: 10.1007/s11373-005-9031-0. [DOI] [PubMed] [Google Scholar]