Abstract
Although slow wave activity in the EEG has been linked to homeostatic sleep regulation, the neurobiological substrate of sleep homeostasis is not well understood. Whereas cortical neurons typically exhibit reduced discharge rates during slow wave sleep (SWS), a subpopulation of GABAergic interneurons, which express the enzyme neuronal nitric oxide synthase (nNOS), has recently been found to be activated during SWS. The extent of activation of these nNOS neurons is proportional to homeostatic sleep “drive”. These cells are an exception among cortical interneurons in that they are projection neurons. In this Opinion, we propose that cortical nNOS neurons are positioned to influence neuronal activity across widespread brain areas. Thus, they may provide a long-sought anatomical link to understand homeostatic sleep regulation.
Introduction
Numerous studies of sleep deprivation (SD) in humans and animals have provided evidence that sleep is homeostatically regulated. Sleep loss produces proportional increases in the "drive" to sleep, in the subsequent occurrence of sleep, and in slow wave activity (SWA) recorded in the electroencephalogram (EEG) during Non-Rapid Eye Movement (NREM) sleep (Glossary). This property of homeostatic regulation, along with a circadian input, was incorporated into the "two-process model" of sleep regulation (Figure 1) in which the homeostatic sleep-related "Process S" was proposed to interact with input from the circadian system ("Process C") to gate the occurrence of sleep and wakefulness [1]. Process S is suggested to reflect a biochemical process(es) that begins to increase at the onset of wakefulness. Once a threshold value is reached, sleep occurs only if Process C is in the appropriate circadian phase (Figure 1B). Although seemingly simplistic, this model accounts remarkably well for the timing of sleep in humans and other species.
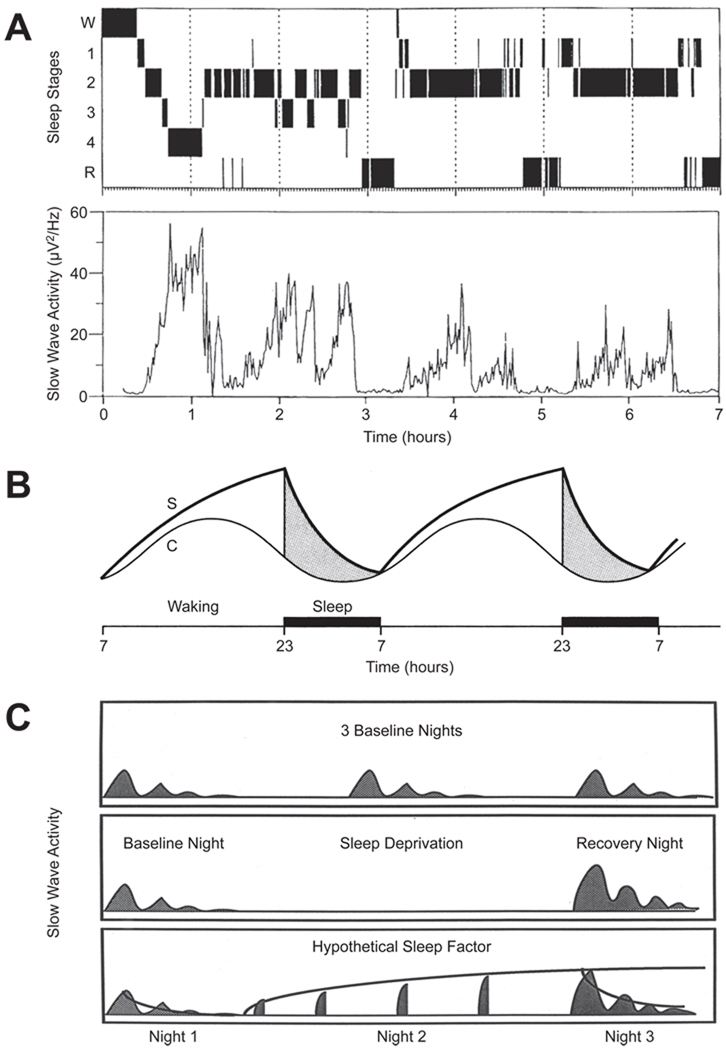
Figure 1. Homeostatic sleep regulation illustrated on three time scales.
A. Upper: Hypnogram illustrating the temporal progression through various stages of sleep across a 7 h night in a young adult. Abbreviations: W, wakefulness; 1–4, NREM Sleep Stages 1–4; R, REM Sleep. Lower: Slow Wave Activity (SWA; mean power density in the 0.75- to 4.0-Hz band of the EEG) determined in 1-min epochs. Note that the amplitude of the recurring peaks of SWA decline across the night. Adapted, with permission, from Ref. [100]. B. The "two-process model" of homeostatic sleep regulation proposed by Borbely [1] in which the homeostatic sleep-related "Process S" is proposed to interact with input from the circadian system ("Process C") to gate the occurrence of sleep and wakefulness. Process S is proposed to be a biochemical process(es) which begins to build up at the onset of wakefulness; SWA during NREM sleep (as illustrated in the lower panel of Figure 1A) is thought to be an index of the declining phase of Process S. C. Schematic illustrating the occurrence of SWA across three nights in basal conditions (top panel) and in response to a night of sleep deprivation (middle panel). Note that the amplitude of the SWA is greater on the recovery night after sleep deprivation than on the baseline night, indicating a homeostatic response. In the bottom panel, a curve has been fitted to the declining phase of Process S on Night 1, the rising phase of Process S on (Day and) Night 2 (during which sleep deprivation occurs), and the declining phase again on Night 3. The dynamics of the sleep/wake-dependent changes in SWA have been quantified with the use of computer simulations and SWA can now be predicted in great detail in several species
Cortical EEG is widely accepted to reflect thalamocortical activity [2–4]. A number of studies have suggested that SWA is related to the underlying Process S. SWA increases in proportion to prior wake duration and decreases over the course of nighttime sleep period (Figure 1A), reflecting a diminution of Process S during sleep. Thus, SWA has been interpreted to represent the cortical manifestation of recovery processes from prior waking activities that occur during sleep.
Despite elegant cellular electrophysiological studies indicating a role for a corticothalamocortical loop in the generation of cortical slow waves [2–4], identification of the neurobiological basis of homeostatic sleep regulation has been elusive. Sleep homeostasis likely involves a distributed network of sleep-active brain areas. Lesion, electrical stimulation and Fos immunohistochemistry experiments have identified sleep-active areas in the preoptic anterior hypothalamus (POAH) and the medulla. Curiously, despite the relationship between SWA and sleep homeostasis, sleep-active neurons have only recently been described in the cerebral cortex (discussed below).
“Sleep-active” Neurons in the Brain
Evidence from electrophysiological studies
The concept of a “sleep-active” neuron is straightforward: a cell whose firing rate is greater during either Rapid Eye Movement (REM) or non-REM (NREM) sleep than during wakefulness. Since neurons discharge in a variety of modes (tonic, phasic, burst firing, etc.), such studies typically calculate an average discharge rate over a defined period of time that is used to establish a ratio threshold between arousal states to label a cell as wake-active vs. NREM-active or REM-active. For example, monoaminergic neurons in the locus coeruleus (LC), dorsal raphé nuclei (DRN) and tuberomammillary nuclei (TMN) have their highest discharge rates during active wakefulness and are generally quiescent during REM sleep [5–8]; such cells are thus alternatively classified as either “wake-active” or “REM off” cells. More complex firing patterns also occur. For example, cholinergic neurons in the basal forebrain (BF) [9, 10] and pedunculopontine tegmental nuclei (PPT) [11, 12] have their greatest discharge when cortical EEG is desynchronized during both wakefulness and REM sleep; such neurons are called “Wake/REM active”. Lastly, the average firing rates of some neurons, such as dopaminergic cells in the ventral tegmental area (VTA), appear to be unchanged across states, although burst firing (typically associated with dopamine release) has been reported in the VTA during REM sleep [13, 14]. These firing patterns are thought to reflect the balance between excitatory and inhibitory inputs to specific brain regions across arousal states.
Using such criteria, only a few brain regions are known to contain neurons that show a marked increase in discharge rate during sleep. Sleep-active neurons have been found in the POAH [15–17] and a poorly-defined region in the vicinity of the nucleus tractus solitarius [18, 19]. The sleep-active neurons in the ventrolateral and median preoptic areas are known to be GABAergic and are thought to facilitate sleep by inhibiting wake-promoting neuronal populations in the histaminergic TMN [20, 21] and those in the perifornical and lateral hypothalamus that express the neuropeptide hypocretin/orexin [20, 21].
The cerebral cortex was among the first brain regions targeted in cellular electrophysiological studies of sleep and wakefulness. Early studies found that pyramidal neurons that discharge rapidly during sleep fire even more rapidly during waking [22, 23]. Subsequent studies established that cortical neurons that increase their firing rates during sleep are rare. For example, only 4 out of 177 neurons in the monkey orbitofrontal cortex increased their firing rates from 0.1 spikes/s during wakefulness to 1.0 spikes/s during NREM sleep [24]. Units that increase activity during NREM sleep compared to wakefulness have also been demonstrated in the guinea pig auditory cortex [25] and the monkey precentral gyrus [22], but clustering of such neurons has been found only in the monkey subgenual cingulate cortex [24].
Inhibitory neurons in the cerebral cortex are present in much smaller numbers than excitatory neurons [26], suggesting the possibility that sleep-active cortical neurons are rare because they are inhibitory neurons. Fast-spiking (FS) neuronal discharge is usually observed in inhibitory neurons [26] and FS neurons have been implicated in the generation of fast (20–40 Hz) rhythms that characterize spontaneous activity of the cortex during alert wakefulness [27–29]. Thus, FS neurons are unlikely to be sleep-active, although fast rhythms also occur episodically during slow-wave and REM sleep [30]. Other types of cortical neurons, categorized by their membrane response to depolarizing current pulses as regular-spiking, intrinsically bursting, or fast-rhythmic-bursting neurons, were not found to fire preferentially during NREM sleep [30]. However, a more recent study [31] found that nearly half of the regular-spiking neurons in the cat parietal cortex were “silent” during wakefulness, indicating that some cortical neurons only discharge during sleep.
Evidence from Fos expression studies
Since its introduction 20 years ago, Fos immunohistochemistry has been widely used as a marker of functional neuronal activity. Increased neuronal firing is typically accompanied by calcium influx and/or an increase in intracellular cyclic AMP, leading to the phosphorylation of the cyclic AMP response element binding protein (CREB), CRE-mediated c-fos transcription and, ultimately, translation of Fos protein. Although this cascade provides the basis for using Fos as a functional marker, Fos can also be induced under conditions that are unrelated to changes in neuronal firing such as during development, after seizures, hypoxemia or toxin treatment, and following lesions [32]. Nonetheless, Fos expression has been found to correspond well with the patterns of neuronal firing during sleep and wakefulness in the hypothalamus [16, 17, 33] and brainstem [34–36].
The cerebral cortex exhibits remarkable changes in Fos expression depending upon arousal state. Fos expression is high in all cortical regions after periods of spontaneous wakefulness [37] and very low during sleep [38, 39]. Moreover, there is a strong negative relationship between the number of Fos-immunoreactive cortical neurons and both the amount of NREM sleep and the number of sleep episodes in the 2 h preceding sacrifice [37].
Some studies have attempted to identify the cortical neuron types that express Fos during wakefulness. For example, the morphological and neurochemical identity of barrel cortex neurons that were Fos-immunoreactive after rats explored an enriched environment has been determined [40]. Although some GABAergic interneurons --identified by immunostaining for glutamic acid decarboxylase (GAD) and subclassified based on the presence of phenotypic markers such as parvalbumin, calbindin, calretinin and vasoactive intestinal polypeptide [41] -- were found to be immunopositive for Fos, the large majority of Fos+ neurons were pyramidal cells [40]. These and other observations indicate that most cortical neurons expressing Fos during wakefulness are excitatory neurons.
Identification of sleep-active neurons in the cerebral cortex
Since Fos immunhistochemistry has been used to identify sleep-active neurons in the POAH [15, 17], we used a similar approach to identify other sleep-active brain areas that might be related to homeostatic sleep regulation [42]. Using Fos expression in conjunction with immunolabelling for phenotypic markers to identify cortical interneurons [26], we found that a subset of GABAergic interneurons, which express neuronal nitric oxide synthase (nNOS), showed greatly elevated Fos expression during recovery sleep (RS) after SD in both mice and rats [42]. nNOS is a member of the family of NOS enzymes that produce nitric oxide (NO) from L-arginine and oxygen. This family includes three NOS isoforms: nNOS, endothelial (eNOS), and inducible (iNOS), by reference to the tissue from which they were originally purified [43]. nNOS neurons are distributed widely in the brain including the cerebral cortex and subcortical regions implicated in the control of sleep and wakefulness, such as the PPT, laterodorsal tegmental and lateral parabrachial nuclei [44]. The eNOS isoform is predominantly located in the endothelial cells but may also be present in other brain cells [45]. iNOS is normally present in brain only in trace amounts but can be activated in glial cells by immunological or stressful challenge [43] and in neurons during inflammation and neurodegeneration [46].
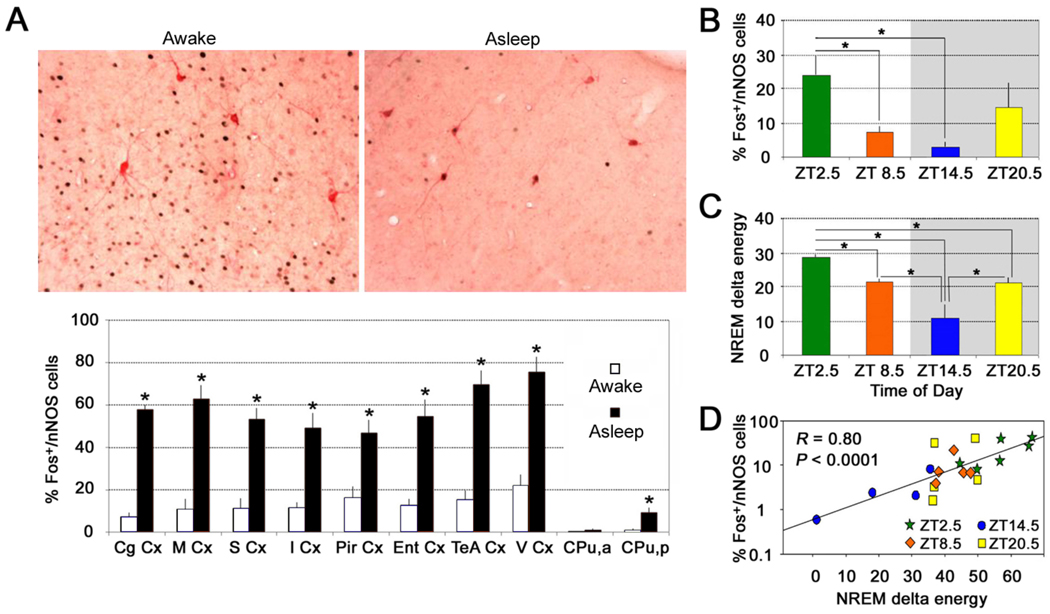
The number of Fos+/nNOS-immunoreactive cortical neurons was greatly increased during RS (Figure 2) [42], a period characterized by reduced wakefulness, increased total sleep time and increased SWA. This increase was observed in all cortical areas examined, with very few double-labeled cells being observed during SD. In contrast, the percentage of Fos/nNOS double-labelled cells did not differ between RS and SD in the perifornical area of the hypothalamus, a region in which the wake-active hypocretin/orexin cells are located. These results indicate that cortical nNOS-immunoreactive neurons are activated during RS when SWA is high and restoration from prior waking activities is presumed to be occurring, but that wake-active cells in areas such as the hypothalamus are not activated under these conditions.
Figure 2. Cortical nNOS neurons are activated during sleep.
A. Top: Immunostained sections of the cerebral cortex of mice sacrificed after 6-h of sleep deprivation (SD; left) or after 6-h of SD followed by 2.5-h recovery sleep (RS) period (right). Black=Fos-immunoreactive nuclei; pink=nNOS-immunoreactive cell bodies. Note that, although there are many Fos+ nuclei in the awake animal, none of them are located within the nNOS neurons whereas, in the animal that had a chance to sleep, a high proportion of the nNOS neurons contain Fos+ nuclei. Bottom: The proportion of Fos+/nNOS cells was significantly greater in the RS group (“asleep”) than in the SD group (“awake”) in every cortical region and in the posterior portion of the caudate-putamen. Data are mean ± s.e.m; *P<0.05 compared to corresponding SD group. Abbreviations: Cg Cx, Cingulate Cortex; M Cx, Motor Cortex; S Cx, Somatosensory Cortex; I Cx, Insular Cortex; Pir Cx, Piriform Cortex; Ent Cx, Entorhinal Cortex; TeA Cx, Temporal Association Cortex; V Cx, Visual Cortex; CPu, a, Caudate Putamen (+1.0 mm from bregma); CPu, p, Caudate Putamen (−1.5 mm from bregma). B and C. The proportion of Fos+/nNOS-immunoreactive cells (B) in the mouse cortex varied across the 24-h period in relation to prior sleep history, and was related to NREM delta energy (C), which is an index of homeostatic sleep drive. ZT= Zeitgeber Time, the time since light onset. Shaded area indicates the 12-h dark period. D. Using the data from B and C, the percentage of Fos+/nNOS cells in the mouse cortex was highly correlated with NREM delta energy across the 24-h period. Reproduced, with permission, from Ref. [42].
In order to determine whether nNOS neurons were also active during “normal” sleep, Fos expression in nNOS cells was also measured in mice undergoing spontaneous bouts of sleep and wakefulness [42]. The proportion of Fos+/nNOS cells was significantly higher in mice sacrificed at a time when SWA was high compared to a timepoint when SWA was low (Figure 2B). Furthermore, the number of Fos+/nNOS double-labelled cells was more strongly correlated with NREM delta energy during the 2.5 h prior to the animal’s sacrifice than with either NREM sleep time or SWA alone (Figure 2C, D). Similar findings were also observed in the brain of a third rodent species, the golden hamster [42]. Since NREM delta energy is an index of the homeostatic sleep response, identification of a neuronal population whose activation is proportional to this parameter suggests that these cells may be involved in the physiological response to prolonged wakefulness. The biochemical and cellular processes that underlie functional recovery from activation during wakefulness (Process S) presumably occurs during sleep. Thus, the existence of sleep-active cells in the cerebral cortex is intriguing, particularly since the cortex is the locus of cognition.
nNOS neurons are the smallest currently known subdivision of GABAergic interneurons in the mouse cortex and are a subpopulation of interneurons that express Neuropeptide Y (NPY) [41, 47]. Triple-labelling experiments of Fos, nNOS and NPY in mouse cortex during RS revealed that Fos was expressed only in neurons that expressed both nNOS and NPY (Figure 3A–C). Thus, nNOS appears to be the most specific phenotypic marker to identify the cortical interneurons that are activated during sleep.
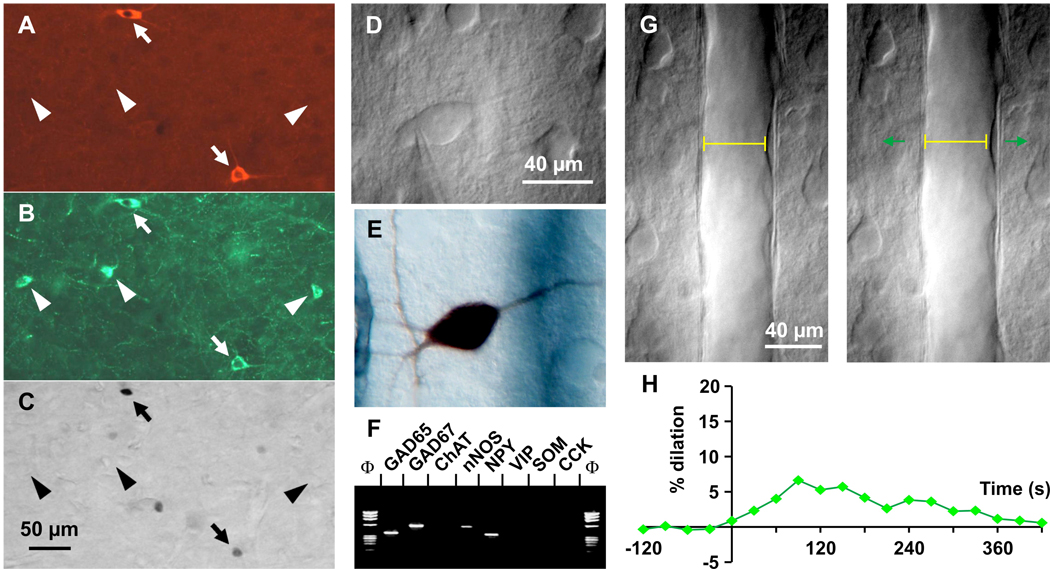
Figure 3. Physiology of cortical nNOS interneurons.
Triple staining for (A) nNOS, (B) NPY and (C) Fos immunoreactivity in mouse cortex during recovery sleep reveals that only neurons that express both nNOS and NPY contain Fos (arrows), whereas neurons that express only NPY (triangles) do not contain Fos (adapted, with permission, from Ref. [42]). D. Infrared videomicroscopy image of a cortical interneuron filled intracellularly with a marker (biocytin) in (E) showing a right horizontal dendritic arborization coursing toward the responsive blood vessel (blue gray color). F. Stimulation of a single nNOS interneuron induces reversible dilatation in vitro. Images of a blood vessel before (left) and after (right) onset of evoked firing (start time 0 sec, duration 30 sec); pial surface is upward. Vessel diameter is shown as a yellow line in both panels. G. Time course of the vasodilation illustrated in G, which was reversible (adapted, with permission, from Ref. [58]).
As mentioned above, nNOS neurons are also found in other brain areas besides the cortex, including in subcortical regions implicated in the control of sleep/wake states. Accordingly, we assessed whether subcortical nNOS neurons were also activated during RS. A significantly greater proportion of Fos+/nNOS neurons was observed during RS only in the cortex and in none of nine subcortical regions examined [48]. These results suggest that it is the localization of the nNOS neurons within a specific network -- rather than the presence of nNOS per se within cells -- that results in activation of these neurons during sleep and that, apparently, only cortical nNOS neurons are appropriately innervated by a network that results in activation during sleep.
Anatomy and physiology of cortical nNOS neurons
nNOS neurons have been identified in the cerebral cortex using two methods: nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) histochemistry and immunohistochemistry for NOS proteins [49]. NADPH-d-positive neurons in the cortex have been classified as Type I or Type II [50]. Type I neurons have a large somata, intense NADPH-d activity as well as intense nNOS immunoreactivity, and are located in the deeper layers of the cortex and even in the white matter. In contrast, Type II neurons have small somata, weak NADPH-d activity and nNOS immunoreactivity. The distribution of intensely-stained nNOS-immunoreactive neurons and NADPH-d-positive neurons in the cortex largely overlaps [51].
GABAergic interneurons are about 20% of the total number of cortical neurons and Type I nNOS neurons comprise about 0.5–2% of the interneuron population in the rat primary sensorimotor and occipital cortices [41, 52]. Although Type II nNOS neurons are less well-studied due to the lack of reliable reagents [53], they are roughly 10-fold more numerous than Type I cells. Whereas other types of interneurons, classified on the basis of morphological criteria, selectively innervate the soma, dendrite, or axon hillock of pyramidal neurons, nNOS-positive neurons exclusively form axo-dendritic connections and widely arborize in the layers in which their cell bodies are located [54]. The distribution of nNOS-positive neurons in the primate cortex is layer-specific, with cells being frequent in layers II/III but also in layer VI, especially at the layer VI–white matter interface [55]. In rats, nNOS-positive cells are found in all cortical layers [51], whereas these cells are most densely concentrated in layers V and VI of the mouse cortex [56]. nNOS cells are also found in the subcortical white matter in rodents [51, 56].
Cortical nNOS-containing cell bodies and their proximal dendrites receive massive input from the BF [57]. This input is likely cholinergic because substantia innominata lesions markedly reduce the number of choline acetyltransferase-immunoreactive fibers innervating nNOS neurons [57]. nNOS neurons and their proximal dendrites also receive serotonergic input [58] but, because of the absence of GAD terminals on nNOS somata and proximal dendrites [59], are unlikely to receive GABAergic innervation.
Cortical nNOS neurons exhibit several other molecular markers. Somatostatin (SST) and Neuropeptide Y (NPY) are expressed in all Type I nNOS neurons [41, 60]. In adult rat visual cortex, nNOS and the NR1 subunit of the NMDA glutamate receptor coexist within dendritic shafts, spines, and terminals [61]. In another study, 93% of nNOS-containing neurons were immunopositive for neurokinin 1 (NK1, substance P receptor) and, conversely, 95% of intensely-staining NK1 cortical neurons were nNOS-positive [62].
In addition to anatomical and phenotypic criteria mentioned above, cortical interneurons are classified according to physiological criteria such as their membrane response to intracellular current injection [26]. However, since cortical Type I nNOS neurons are so rare [60], few studies have reported electrophysiological properties of these cells. In one such study [58], electrophysiological recordings from cortical GABAergic interneurons were followed by single-cell PCR to determine the expression of specific phenotypic markers and the recorded neurons were then injected with a dye to enable morphological analyses (Figure 3D, E). Based on electrophysiological criteria, four of the five neurons that expressed nNOS adapted rapidly to depolarizing current injection with a decreasing spike discharge as injection proceeded, whereas the remaining cell that coexpressed NPY exhibited a delayed adapting response. The interneurons that expressed nNOS were multipolar or bitufted in morphology, and one NPY/NOS-immunopositive cell had neurogliaform morphology. These nNOS/NPY neurons are proposed to be involved in neurovascular coupling (see Box 1). A subsequent study [53] characterized NPY interneurons and found that 80% of the 15 nNOS neurons expressed NPY, adapted to current injection, and had a neurogliaform morphology similar to the neurogliaform “ivy” cells described in the hippocampus [63]. In contrast, neurons expressing all three markers (nNOS, NPY and SST) were rare and had larger somata suggestive of Type I neurons. We propose that sleep-active nNOS neurons correspond to Type I nNOS cells.[48].
Box 1. nNOS neurons and neurovascular coupling.
Cortical nNOS interneurons produce two potent vasoactive compounds: NO, a fast diffusible vasodilator, and NPY, a less diffusible vasoconstrictor. In addition to their neuronal targets, nNOS/NPY neurons can innervate arterioles both locally and remotely [58], but the precise role of these interneurons in neurovascular coupling remains elusive. Understanding the specific role of nNOS/NPY interneurons in this process is further complicated by the fact that numerous vasoactive messengers of various cellular origins are differentially recruited during neuronal activity [87]. Whereas brief (i.e., 1 sec) stimulations clearly demonstrate a vasodilatory role for nNOS neurons [86], in vivo studies using longer duration (i.e., 60 sec) sensory stimulation indicate only a permissive role for NO [96]. These observations suggest that NO is an early and transient mediator of functional hyperaemia and, when neuronal activity is sustained, NO modulates the action of other messengers [96]. This concept could explain the reversible dilation evoked by single nNOS neuron stimulation in vitro [58] (Figure 3F, G) and the in vivo spatiotemporal pattern of vascular dilation/constriction evoked by brief (1 sec) stimulations [97]. NO and NPY have also been proposed to exert their opposite vascular effects in a temporally- and/or spatially-dependent manner [58].
GABAergic cortical projection neurons and nNOS
Communication between cortical areas depends largely on glutamatergic neurons because these neurons constitute the majority of long-distance connections with either other pyramidal neurons or inhibitory interneurons. Until recently, the general view was that glutamatergic activation of local circuit GABAergic interneurons was required to produce inhibitory effects between distant cortical areas. However, several studies have now demonstrated that inhibitory effects can also be produced monosynaptically by a subgroup of GABAergic neurons that project long distances in the cortex [60]. The presence of nNOS in the majority (71–94%) of these GABAergic long-range projecting cortical neurons has been demonstrated in mice [47, 64], cats [50], and monkeys [65]. The vast majority of these GABAergic projection neurons also exhibit SST (>90%) and NPY (>80%) immunoreactivity [47, 50, 65]. Since SST and NPY are also expressed in a large number of local circuit GABAergic neurons, nNOS appears to be the most specific marker for the GABAergic projection neurons [47, 50, 65]. These projection neurons have large somata and thus appear to correspond to Type I nNOS cells. Therefore, Type I nNOS neurons may provide the morphological basis for direct intra-[66, 67] and inter-hemispheric inhibition [68–70] and are well-situated to produce widespread effects in the brain.
nNOS in the cortex: more than a phenotypic marker of sleep-active neurons?
Since Fos expression is increased only in the cortical and none of the subcortical nNOS neuronal populations during sleep [48], we are proposing that it is more likely the placement of the cortical nNOS neurons in a network (rather than the presence of nNOS per se) that accounts for activation of these cells during sleep. Nonetheless, strong evidence indicates that NO is an endogenous sleep-promoting substance [71]. Intracerebroventricular (icv) injection of the NO precursor, L-arginine, increases NREM sleep and does not affect REM sleep when administered to rats during the dark phase of the LD cycle [72]. Similar effects are also observed following treatment with nitric oxide donors, 3-morpholinosydnonimine (molsidomine; SIN-1) or S-nitroso-N-acetyl-DL-penicillamine (SNAP), in rats [72, 73] and cats [74]. In contrast to NO precursors or donors, systemic, icv [75], or local administration of NOS inhibitors into the PPT [76] decreases spontaneous sleep and reduces SWA during NREM sleep. Various doses of the NOS inhibitor, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), consistently decrease SWA at the onset of both light and dark [77] and after SD [78]. Thus, NOS inhibitors desynchronize the EEG and suppress SWA, whereas NO donors increase SWA [71].
NO is involved in sleep regulation by the BF but, in this region, iNOS seems to be more important than nNOS. In rats, SD causes induction of iNOS in the BF and inhibition of iNOS completely abolishes recovery of NREM sleep [79]. Without previous SD, iNOS inhibition does not affect NREM sleep, indicating that induction of iNOS occurs after SD but not during the spontaneous sleep-wake cycle [79]. In these experiments, accumulation of adenosine in the BF area was necessary for RS to occur [79, 80].
Higher levels of NO production have been consistently found in the cortex during the dark (i.e., active) period in nocturnal animals [81, 82] and the diurnal variations in NO level, nNOS expression, and activity are tightly correlated within the frontal cortex in rats [83]. Although the basis for this variation is unknown, the prevailing view is that NO production and nNOS activity are coupled to wakefulness. Indeed, NO plays an important role in mediating cortical activation produced by stimulation of BF cholinergic neurons [84] and shifts thalamic neurons from burst-firing associated with sleep to the tonic discharge firing mode [85]. However, our Fos/nNOS expression studies indicate that activation of Type I nNOS neurons occurs during sleep [42], particularly sleep following extended periods of wakefulness. Such coupling may not have been previously found because NO release, nNOS expression, and nNOS activity during wakefulness has not been compared with levels during sleep associated with increased homeostatic drive. The relative contributions of Type I vs. Type II nNOS neurons to NO release are also yet to be determined. Since Type II neurons are much more numerous than Type I, it is possible that much of the literature on NO release reflects activation of the non-sleep active nNOS population.
Role of nitric oxide in the CNS
NO is well-known to play a role in the regulation of regional cerebral blood flow [86, 87] (Figure 3; Box 1) and, indeed, a reduction in global cerebral blood flow occurs during NREM sleep [88, 89]. However, NO is also an important signaling molecule that can modulate neural activity more directly [43]. NO may play a key role in short-term dynamic variations of the strength of synapses on cortical pyramidal neurons [90], particularly with regard to the regulation of excitatory and inhibitory inputs [91]. NO is also implicated in long-term synaptic plasticity as a retrograde messenger in several regions of the brain, including the cortex [43]. Furthermore, NO may affect neuronal activity by modulating gap junction permeability [92, 93].
Rather than being released by exocytosis from synaptic vesicles and acting on membrane-bound receptor proteins, NO rapidly diffuses through membranes in target neurons where it can be stabilized through reaction with protein carriers, particularly soluble guanylyl cyclase, a well-established NO receptor [43]. Because NO readily crosses membranes, diffusing to act nearly simultaneously on a large number of cells throughout a volume of tissue, it is especially well-suited for large-scale modulation of brain activity [94, 95]. Indeed, NO has been found to modulate the rhythmic activity of neuronal ensembles [43, 85].
Behavioral state-dependent activation of nNOS neurons
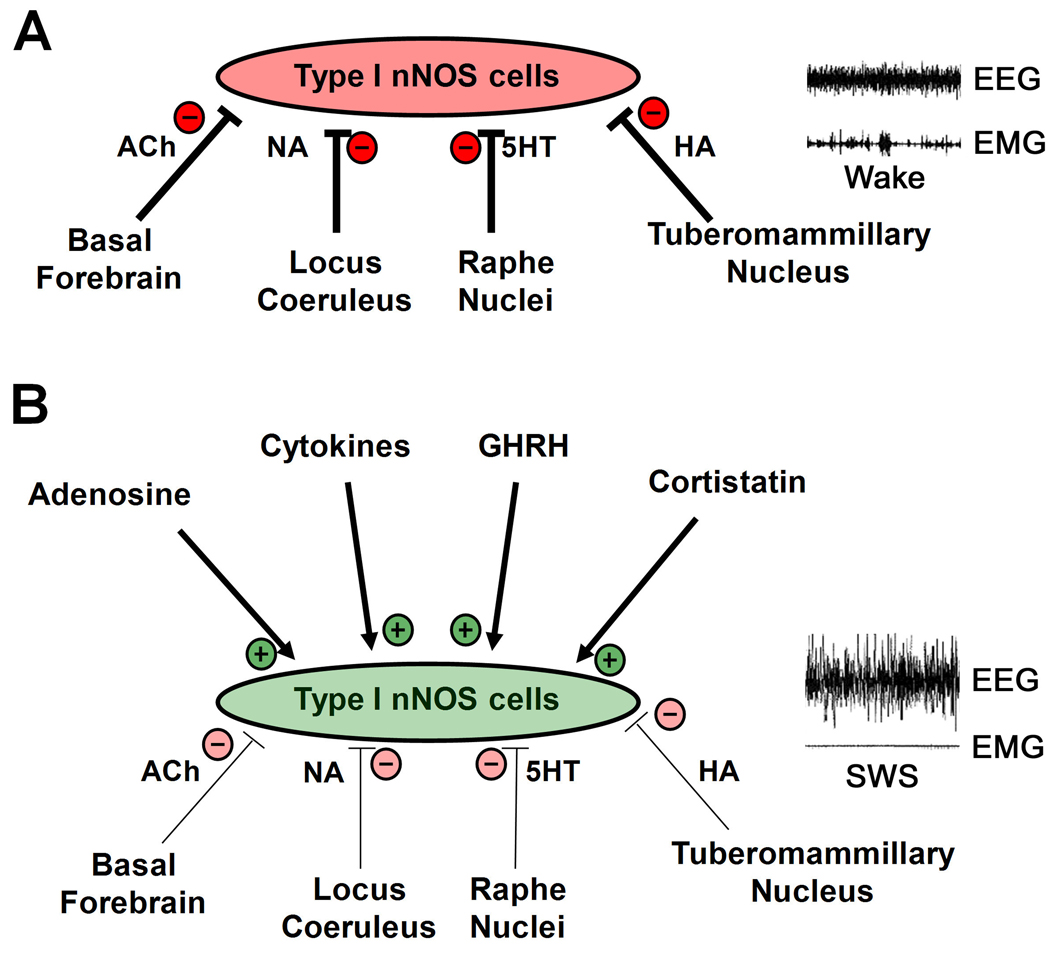
As described above, the activation of nNOS neurons during sleep appears to be an exception among cortical neurons and is likely a consequence of inputs from its afferent network. Unlike cortical nNOS neurons, the wake-active monoaminergic neurons in the LC, DRN and TMN decrease their discharge during sleep. Serotonergic and cholinergic inputs to the nNOS cells have been established [57, 58], although it unknown whether these inputs contact the sleep-active population of nNOS cells specifically. Nonetheless, as a working hypothesis, we propose that these inputs, along with afferents from other wake-active nuclei such as noradrenergic input from LC and histaminergic input from TMN, inhibit Fos expression in nNOS neurons during wakefulness (Figure 4A). As the firing rate of the wake-active regions declines during sleep onset, nNOS cells become disinhibited. Sleep-promoting substances, such as adenosine, cytokines and neuropeptides such as cortistatin and growth hormone-releasing hormone (GHRH), may also contribute to activate Fos expression in nNOS neurons (Figure 4B). Once activated, the widespread projections of nNOS neurons may play a role in synchronizing EEG activity across cortical regions through the release of NO and other colocalized transmitters such as GABA and NPY. In addition to NO effects on EEG activity, the release of NO and NPY may regulate cerebral blood flow in response to local neural events occurring during sleep, since cerebral blood flow is known to be markedly heterogeneous within the cortex during NREM sleep, despite a global reduction in cerebral blood flow during this state [88].
Figure 4. Proposed model for the activation of cortical nNOS neurons during sleep.
A. “Sleep-active” cortical neurons are nNOS cells that express nNOS, NPY and SST, and are likely to be of the Type I class. During wakefulness (illustrated by low amplitude EEG and high amplitude EMG recordings on the right), these cells are inhibited by afferent input from “wake-active” regions such as the histaminergic (HA) tuberomammillary nuclei, noradrenergic (NA) locus coeruleus, serotonergic (5HT) raphé nuclei, and/or basal forebrain cholinergic (ACh) neurons. These inhibitory inputs result in low activity of nNOS neurons during wakefulness. B. During slow wave sleep (SWS; illustrated by high amplitude EEG and low amplitude EMG relative to panel A), inhibitory input from wake-active regions is reduced (indicated by the thinner lines relative to A) and this disinhibition, along with putative excitatory input from sleep promoting substances such as adenosine, cytokines, and the neuropeptides cortistatin and growth hormone-releasing hormone (GHRH), results in activation of nNOS cells. In this model, “+” and “−“ refer to functional activation or inhibition of nNOS cells in vivo rather than direct excitation or inhibition at a cellular level. Functional activation (increased Fos expression) or inhibition could occur either directly or indirectly through disinhibition or disfacilitation, respectively.
Concluding remarks
More than 25 years after the two-process model of sleep regulation was proposed [1], our understanding of the neurobiological substrates underlying sleep homeostasis remains incomplete. Recent studies have demonstrated activation of a rare population of GABAergic cortical interneurons during sleep. Furthermore, the number of nNOS interneurons activated was found to be directly proportional to NREM delta energy, an index of homeostatic sleep drive. At this point, it is unclear whether Fos labelling of nNOS neurons during sleep indicates activation of a particular cortical neuronal cell type or NO production (Box 2), although strong evidence suggests that NO has a role as an endogenous sleep substance as well as in regulation of cerebral blood flow. Neuroanatomical studies have identified a unique type of cortical interneuron that sends long-range projections within the cerebral cortex; intriguingly, nNOS is the best phenotypic marker for these cells. Thus, nNOS neurons may be uniquely positioned to exert widespread effects on cortical activity, such as synchronizing the EEG across the brain.
Box 2. Outstanding questions.
1. What are the consequences of selective activation or loss of sleep-active cortical nNOS neurons?
At present, most of the evidence supporting the importance of cortical nNOS cells in sleep regulation is correlative. Testing the role of nNOS neurons more directly will require procedures that allow selective activation, inhibition or ablation of these cells. Since cortical nNOS neurons are sleep-active, we predict that such procedures will result in specific changes in sleep/wake activity. However, it is also possible that these procedures will reveal the role of cortical nNOS cells in other brain functions (e.g., blood flow, cognition, etc.).
2. What is the physiological significance of NO produced by sleep-active cortical nNOS neurons?
In nNOS knockout (KO) mice, hourly amounts of SWA are consistently higher across the 24 h period compared to control mice, suggesting that nNOS KO mice live under high sleep “pressure” [98]. Systemic administration of tumor necrosis factor-α (TNFα) increases NREM sleep and SWA in control and iNOS KO mice, but does not affect these parameters in nNOS KO mice [99]. These results suggest that NO production by nNOS plays a significant role in the regulation of sleep. However, two types of nNOS neurons (Type I and Type II) exist in the cortex, with sleep-active cells most likely being restricted to the Type I class [48]. Thus, the specific role of nNOS located within each of these distinct types of cortical nNOS neurons remains to be determined. One experimental approach would be to create cell type-specific nNOS knockout mice. Basal sleep/wake parameters and the response to sleep deprivation in these mice could then be assessed in these mice.
3. What is the neuronal circuitry that leads to cortical nNOS activation and inhibition during sleep and wakefulness, respectively?
According to our hypothesis, nNOS neurons receive input from neuronal groups involved in the regulation of sleep and wakefulness (Figure 4). However, the limited information on afferents to cortical nNOS neurons does not distinguish between Type I and Type II nNOS cells. Although nNOS neurons innervate neurons within the cerebral cortex, including those in distant cortical areas, the identity of the target cells is unknown. Furthermore, it has not been directly demonstrated that sleep-active nNOS neurons are projection neurons nor is it known whether cortical nNOS neurons project to subcortical brain regions. The roles of colocalized transmitters and modulators such as GABA, NPY and SST also remain to be clarified. Identifying the neural network in which the sleep-active nNOS neurons are located should enhance our understanding of the control of neural activity in the cortex during both sleep and wakefulness.
4. How do cortical nNOS neurons respond to known neurotransmitters, neuromodulators and exogenous substances such as anesthetics and hypnotics?
Figure 4 suggests some substances that may activate or inhibit cortical nNOS neurons. In vitro electrophysiology can be a powerful tool to assess the effects of various substances on specific cell types; such studies are facilitated by mouse strains that express enhanced green fluorescent protein (EGFP) under the control of a specific promoter. The development of nNOS-EGFP mouse strains that uniquely recognize Type I versus Type II nNOS cells will be invaluable to enable such assessments.
Acknowledgements
Research supported by NIH R01 HL059658, Human Frontiers Science Program RGY0070/2007 and the CNRS "Nitrex" project. We are grateful to Drs. Lars Dittrich for very helpful comments.
GLOSSARY
- Dorsal raphe nuclei (DRN)
midbrain nuclei that synthesize serotonin and have both ascending projections and descending projections. DRN neurons have their highest discharge rate during active wakefulness and are quiescent during REM sleep.
- Electroencephalogram (EEG)
integrated measure of brain electrical activity, usually recorded from the scalp or brain surface.
- Fos immunohistochemisty
Immunological detection of c-Fos, an immediate early gene transcription factor, is commonly used as an indicator of neuronal activity under a wide range of experimental conditions.
- Locus coeruelus (LC)
pontine nucleus that expresses tyrosine hydroxylase, the synthetic enzyme for noradrenaline. LC cells are wake-active and have both ascending and descending projections. LC innervation of the cortex is thought to have a role in selective attention and in the control of cerebral blood flow.
- NREM
sleep phase characterized by elevated arousal threshold, synchronized EEG, and moderate muscle tone. Large amplitude slow waves occur during the deepest stages of NREM sleep.
- NREM delta energy
mathematical product of NREM sleep duration multiplied by SWA during NREM sleep epochs. Due to the polyphasic nature of rodent sleep/wake patterns, NREM delta energy provides an integrated index of sleep homeostasis that is superior to SWA in many conditions.
- Preoptic-anterior hypothalamus (POAH)
rostral-most region of the brainstem, containing nuclei involved in autonomic functions and behaviors. Two nuclei within this region, the ventrolateral preoptic (VLPO) and median preoptic (MnPO) nuclei, contain GABAergic neurons that are thought to be sleep-active and which inhibit more caudal wake-active nuclei.
- Pedunculopontine tegmental nuclei (PPT)
dorsolateral midbrain nuclei that express the synthetic enzymes choline acetyltransferase and nitric oxide synthase and are thus both cholinergic and nitrergic. PPT cells are active in conjunction with EEG desynchronization during wakefulness and REM sleep.
- Process C
theoretical construct representing the output of the circadian system; usually modeled as a sine wave.
- Process S
theoretical construct representing a biochemical process(es) that begins to increase upon awakening and decays during sleep.
- REM
sleep phase characterized by desynchronized EEG, low muscle tone and rapid eye movements (REMs). Humans report dreaming after 80%, of REM awakenings.
- Recovery sleep (RS)
sleep that occurs after a period of sleep deprivation; characterized by decreased sleep latency, increased SWA and, sometimes, increased sleep duration.
- Sleep deprivation (SD)
experimental manipulation that imposes a homeostatic challenge to the sleep/wake regulatory system by extending wakefulness duration.
- Sleep homeostasis
regulated physiological response that results in the subjective experience of sleepiness, decreased sleep latency, increased sleep intensity as measured by SWA and, sometimes. increased sleep duration. Factors such as circadian and environmental influences affect sleep duration, limiting its value as a measure of sleep homeostasis.
- Slow wave activity (SWA)
spectral power of the EEG in the 0.5–4.0 Hz (delta) range during NREM sleep. SWA increases in proportion to prior wake duration and decreases during NREM sleep.
- Tuberomammillary nuclei (TMN)
posterolateral hypothalamic nuclei containing wake-active histaminergic neurons that innervate the cortex and likely promote wakefulness.
- Ventral tegmental area (VTA)
ventral midbrain region containing dopaminergic neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Destexhe A, et al. Are corticothalamic 'up' states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 5.John J, et al. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MF, et al. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MF, et al. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554:202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MG, et al. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassani OK, et al. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steriade M, et al. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 13.Miller JD, et al. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273:133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- 14.Dahan L, et al. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 15.Gong H, et al. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 16.Gvilia I, et al. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherin JE, et al. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi K, Satoh T. Characterization of the neurons in the region of solitary tract nucleus during sleep. Physiol Behav. 1980;24:99–102. doi: 10.1016/0031-9384(80)90020-7. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi K, Satoh T. Convergence of sleep-wakefulness subsystems onto single neurons in the region of cat's solitary tract nucleus. Arch Ital Biol. 1980;118:331–345. [PubMed] [Google Scholar]
- 20.Sherin JE, et al. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong H, et al. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evarts EV. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- 23.Evarts EV, et al. Spontaneous discharge of single neurons during sleep and waking. Science. 1962;135:726–728. doi: 10.1126/science.135.3505.726. [DOI] [PubMed] [Google Scholar]
- 24.Rolls ET, et al. Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol. 2003;90:134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- 25.Pena JL, et al. Sleep and wakefulness modulation of the neuronal firing in the auditory cortex of the guinea pig. Brain Res. 1999;816:463–470. doi: 10.1016/s0006-8993(98)01194-9. [DOI] [PubMed] [Google Scholar]
- 26.Ascoli GA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Sohal VS, et al. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steriade M, et al. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph M, et al. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 33.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maloney KJ, et al. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:774–778. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- 35.Leger L, et al. Noradrenergic neurons expressing Fos during waking and paradoxical sleep deprivation in the rat. J Chem Neuroanat. 2009;37:149–157. doi: 10.1016/j.jchemneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Sapin E, et al. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi-Zucconi G, et al. c-fos spontaneous expression during wakefulness is reversed during sleep in neuronal subsets of the rat cortex. J Physiol Paris. 1994;88:91–93. doi: 10.1016/0928-4257(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 38.Cirelli C, et al. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 39.Basheer R, et al. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–9750. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staiger JF, et al. Excitatory and inhibitory neurons express c-Fos in barrel-related columns after exploration of a novel environment. Neuroscience. 2002;109:687–699. doi: 10.1016/s0306-4522(01)00501-2. [DOI] [PubMed] [Google Scholar]
- 41.Kubota Y, et al. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- 42.Gerashchenko D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci U S A. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotti S, et al. Distribution of nitric oxide synthase immunoreactivity in the mouse brain. Microsc Res Tech. 2005;68:13–35. doi: 10.1002/jemt.20219. [DOI] [PubMed] [Google Scholar]
- 45.Siles E, et al. Age-related changes of the nitric oxide system in the rat brain. Brain Res. 2002;956:385–392. doi: 10.1016/s0006-8993(02)03575-8. [DOI] [PubMed] [Google Scholar]
- 46.Heneka MT, Feinstein DL. Expression and function of inducible nitric oxide synthase in neurons. J Neuroimmunol. 2001;114:8–18. doi: 10.1016/s0165-5728(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 47.Tomioka R, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- 48.Pasumarthi RK, et al. Further characterization of sleep-active neuronal nitric oxide synthase neurons in the mouse brain. Neuroscience. 2010;169:149–157. doi: 10.1016/j.neuroscience.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent SR. Nitric oxide neurons and neurotransmission. Prog Neurobiol. 2010;90:246–255. doi: 10.1016/j.pneurobio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Higo S, et al. Long-range GABAergic projection neurons in the cat neocortex. J Comp Neurol. 2007;503:421–431. doi: 10.1002/cne.21395. [DOI] [PubMed] [Google Scholar]
- 51.Oermann E, et al. Differential maturational patterns of nitric oxide synthase-I and NADPH diaphorase in functionally distinct cortical areas of the mouse cerebral cortex. Anat Embryol (Berl) 1999;200:27–41. doi: 10.1007/s004290050256. [DOI] [PubMed] [Google Scholar]
- 52.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- 53.Karagiannis A, et al. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seress L, et al. NOS-positive local circuit neurons are exclusively axo-dendritic cells both in the neo- and archi-cortex of the rat brain. Brain Res. 2005;1056:183–190. doi: 10.1016/j.brainres.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Barone P, Kennedy H. Non-uniformity of neocortex: areal heterogeneity of NADPH-diaphorase reactive neurons in adult macaque monkeys. Cereb Cortex. 2000;10:160–174. doi: 10.1093/cercor/10.2.160. [DOI] [PubMed] [Google Scholar]
- 56.Lee JE, Jeon CJ. Immunocytochemical localization of nitric oxide synthase-containing neurons in mouse and rabbit visual cortex and co-localization with calcium-binding proteins. Mol Cells. 2005;19:408–417. [PubMed] [Google Scholar]
- 57.Vaucher E, et al. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79:827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 58.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaucher E, et al. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–171. [PubMed] [Google Scholar]
- 60.Clancy B, et al. Cortical GABAergic neurons: stretching it Remarks, Main Conclusions and Discussion. Front Neuroanat. 2010;4:7. doi: 10.3389/neuro.05.007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoki C, et al. NMDA-R1 subunit of the cerebral cortex co-localizes with neuronal nitric oxide synthase at pre- and postsynaptic sites and in spines. Brain Res. 1997;750:25–40. doi: 10.1016/s0006-8993(96)01147-x. [DOI] [PubMed] [Google Scholar]
- 62.Vruwink M, et al. Substance P and nitric oxide signaling in cerebral cortex: anatomical evidence for reciprocal signaling between two classes of interneurons. J Comp Neurol. 2001;441:288–301. doi: 10.1002/cne.1413. [DOI] [PubMed] [Google Scholar]
- 63.Fuentealba P, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Higo S, et al. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- 66.Aroniadou-Anderjaska V, Keller A. Intrinsic inhibitory pathways in mouse barrel cortex. Neuroreport. 1996;7:2363–2368. doi: 10.1097/00001756-199610020-00017. [DOI] [PubMed] [Google Scholar]
- 67.Fabri M, Manzoni T. Glutamate decarboxylase immunoreactivity in corticocortical projecting neurons of rat somatic sensory cortex. Neuroscience. 1996;72:435–448. doi: 10.1016/0306-4522(95)00568-4. [DOI] [PubMed] [Google Scholar]
- 68.Kimura F, Baughman RW. GABAergic transcallosal neurons in developing rat neocortex. Eur J Neurosci. 1997;9:1137–1143. doi: 10.1111/j.1460-9568.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 69.Gonchar YA, et al. GABA-immunopositive neurons in rat neocortex with contralateral projections to S–I. Brain Res. 1995;697:27–34. doi: 10.1016/0006-8993(95)00746-d. [DOI] [PubMed] [Google Scholar]
- 70.Peters A, et al. Transcallosal non-pyramidal cell projections from visual cortex in the cat. J Comp Neurol. 1990;302:124–142. doi: 10.1002/cne.903020110. [DOI] [PubMed] [Google Scholar]
- 71.Gautier-Sauvigne S, et al. Nitric oxide and sleep. Sleep Med Rev. 2005;9:101–113. doi: 10.1016/j.smrv.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Monti JM, Jantos H. Effects of L-arginine and SIN-1 on sleep and waking in the rat during both phases of the light-dark cycle. Life Sci. 2004;75:2027–2034. doi: 10.1016/j.lfs.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 73.Kapas L, Krueger JM. Nitric oxide donors SIN-1 and SNAP promote nonrapid-eye-movement sleep in rats. Brain Res Bull. 1996;41:293–298. doi: 10.1016/s0361-9230(96)00227-4. [DOI] [PubMed] [Google Scholar]
- 74.Datta S, et al. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse. 1997;27:69–78. doi: 10.1002/(SICI)1098-2396(199709)27:1<69::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 75.Kapas L, et al. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- 76.Hars B. Endogenous nitric oxide in the rat pons promotes sleep. Brain Res. 1999;816:209–219. doi: 10.1016/s0006-8993(98)01183-4. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro AC, Kapas L. Day- and nighttime injection of a nitric oxide synthase inhibitor elicits opposite sleep responses in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R521–R531. doi: 10.1152/ajpregu.00605.2004. [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro AC, et al. Systemic injection of a nitric oxide synthase inhibitor suppresses sleep responses to sleep deprivation in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1048–R1056. doi: 10.1152/ajpregu.2000.278.4.R1048. [DOI] [PubMed] [Google Scholar]
- 79.Kalinchuk AV, et al. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 2006;24:1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 80.Kalinchuk AV, et al. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 81.Ayers NA, et al. Circadian variation of nitric oxide synthase activity and cytosolic protein levels in rat brain. Brain Res. 1996;707:127–130. doi: 10.1016/0006-8993(95)01362-8. [DOI] [PubMed] [Google Scholar]
- 82.Clément P, et al. Changes in the sleep-wake cycle architecture and cortical nitric oxide release during ageing in the rat. Neuroscience. 2003;116:863–870. doi: 10.1016/s0306-4522(02)00761-3. [DOI] [PubMed] [Google Scholar]
- 83.Clément P, et al. Changes occurring in cortical NO release and brain NO-synthases during a paradoxical sleep deprivation and subsequent recovery in the rat. J Neurochem. 2004;90:848–856. doi: 10.1111/j.1471-4159.2004.02529.x. [DOI] [PubMed] [Google Scholar]
- 84.Marino J, Cudeiro J. Nitric oxide-mediated cortical activation: a diffuse wake-up system. J Neurosci. 2003;23:4299–4307. doi: 10.1523/JNEUROSCI.23-10-04299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang S, Cox CL. Excitatory and anti-oscillatory actions of nitric oxide in thalamus. J Physiol. 2008;586:3617–3628. doi: 10.1113/jphysiol.2008.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitaura H, et al. Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci Res. 2007;59:160–171. doi: 10.1016/j.neures.2007.06.1469. [DOI] [PubMed] [Google Scholar]
- 87.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 88.Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 89.Corfield DR, Meadows GE. Control of cerebral blood flow during sleep and the effects of hypoxia. Adv Exp Med Biol. 2006;588:65–73. doi: 10.1007/978-0-387-34817-9_7. [DOI] [PubMed] [Google Scholar]
- 90.Kara P, Friedlander MJ. Dynamic modulation of cerebral cortex synaptic function by nitric oxide. Prog Brain Res. 1998;118:183–198. doi: 10.1016/s0079-6123(08)63208-2. [DOI] [PubMed] [Google Scholar]
- 91.Le Roux N, et al. Roles of nitric oxide in the homeostatic control of the excitation-inhibition balance in rat visual cortical networks. Neuroscience. 2009;163:942–951. doi: 10.1016/j.neuroscience.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 92.Strata F, et al. Nitric oxide sensitive depolarization-induced hyperpolarization: a possible role for gap junctions during development. Eur J Neurosci. 1998;10:397–403. doi: 10.1046/j.1460-9568.1998.00047.x. [DOI] [PubMed] [Google Scholar]
- 93.O'Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 94.Jansson A, et al. Effects of nitric oxide inhibition on the spread of biotinylated dextran and on extracellular space parameters in the neostriatum of the male rat. Neuroscience. 1999;91:69–80. doi: 10.1016/s0306-4522(98)00575-2. [DOI] [PubMed] [Google Scholar]
- 95.Gally JA, et al. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990;87:3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindauer U, et al. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- 97.Devor A, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen L, et al. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- 99.Chen L, et al. The role of nitric oxide synthases in the sleep responses to tumor necrosis factor-alpha. Brain Behav Immun. 2004;18:390–398. doi: 10.1016/j.bbi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, et al., editors. Principles and Practice of Sleep Medicine. 3rd edn. W. B. Saunders Company; 2000. pp. 377–390. [Google Scholar]