Abstract
Rationale
Minocycline, a tetracycline antibiotic, interacts with brain glutamate and dopamine neurotransmission. In preclinical studies, minocycline attenuated amphetamine-induced acute dopamine release and subsequent behavioral sensitization. The goal of this study was to determine minocycline’s effects on the acute physiological, behavioral, and subjective responses to dextroamphetamine (DAMP) in healthy volunteers.
Methods
Ten healthy volunteers participated in an outpatient double-blind, placebo-controlled, crossover study. Subjects had a 5-day treatment period with either minocycline (200 mg/day) or placebo and then were crossed over for 5-days of the other treatment. After two days of taking the study medication, on days 3 and 4, subjects were randomly assigned to double-blind acute challenge with either 20 mg/70 kg DAMP or placebo DAMP (randomly labeled as drug A or B) and then crossed-over to the other challenge. On Day 5 (Experimental Session 3), subjects had the opportunity to self-administer either placebo or DAMP capsules by working on a progressive ratio computer task.
Results
Minocycline attenuated DAMP-induced subjective-rewarding effects but did not change DAMP choice behavior. Minocycline treatment speeded reaction times on a Go No-Go task and reduced plasma cortisol levels.
Conclusions
These findings warrant further studies examining the potential use of minocycline for stimulant addiction.
Keywords: dextroamphetamine, dopamine, glutamate psychostimulant, minocycline
Introduction
A growing body of evidence suggests that glutamate, in conjunction with dopamine, is essential in mediating the rewarding and addictive effects of psychostimulants, amphetamines and cocaine (Kalivas 2007). Glutamatergic input to the ventral tegmental area (VTA) or nucleus accumbens enhances dopaminergic transmission in the mesocorticolimbic pathway (Tzschentke and Schmidt 2003). The glutamatergic input to the VTA are strengthened within hours following a single cocaine administration (Ungless et al. 2001). Blockage of NMDA or AMPA type glutamate receptors prevents behavioral sensitization or reinstatement of psychostimulant use in preclinical models of addiction (Cornish and Kalivas 2000; Di Ciano and Everitt 2001; Knackstedt and Kalivas 2009; Vezina and Queen 2000). Since glutamate is the primary neurotransmitter for learning and memory, its simultaneous activation with the dopaminergic system may underlie the changes in neuronal plasticity that lead to the initiation of addiction (Thomas et al. 2008).
The contribution of glutamate activation to psychostimulant responses has not been well characterized in humans, partly due to the limited number of glutamatergic medications available for human use. One of these medications is minocycline, an antibiotic derived from tetracycline and commonly used to treat acne. Minocycline readily crosses the blood brain barrier and has significant effects on the dopamine and glutamate transmission. In preclinical studies, minocycline attenuated the development of behavioral sensitization to amphetamines (Kofman et al. 1990; Zhang et al. 2006) and cocaine (Chen et al. 2009) as well as dopamine-releasing effects of amphetamines in the striatum (Zhang et al. 2006). The inhibitory effects of minocycline on amphetamine-induced dopaminergic activation are possibly mediated by its glutamatergic actions. Minocycline blocked the behavioral responses mediated by the NMDA-type glutamate receptor in mice (Fujita et al. 2008) and rats (Levkovitz et al. 2007; Munzar et al. 2002).
Furthermore, minocycline attenuated the reduction of dopamine transporters produced by repeated administration of methamphetamine in the monkey brain (Hashimoto et al. 2007). In other studies, minocycline reduced glutamate release in hippocampal neurons of rats (Gonzalez et al. 2007) and increased phosphorylation and surface expression of GluR1-type AMPA receptor subunits in mouse striatal neurons (Imbesi et al. 2008). Minocycline is being examined for the treatment of methamphetamine addiction, depression, schizophrenia and neurodegenerative disorders including Amyotrophic Lateral Sclerosis (ALS) and Alzheimer’s disease (Chaves et al. 2009; Hashimoto 2009; Levkovitz et al. 2009; Molina-Hernandez et al. 2008).
In spite of its potential use as a novel treatment medication for several psychiatric disorders, the behavioral pharmacology of minocycline has not been well-characterized in humans. In this study, we investigated minocycline’s effects on physiological, cognitive, subjective, and behavioral responses to amphetamines. Amphetamines activate the mesocorticolimbic dopamine system, an essential component of the brain reward circuit (Bardo 1998; Koob 1992; Tzschentke 2001). Based on the preclinical studies indicating inhibition of behavioral and neurochemical responses of amphetamine by minocycline, we hypothesized that minocycline would attenuate the subjective-rewarding effects of oral dextroamphetamine (DAMP) and improve cognitive performance. We also hypothesized that minocycline would reduce DAMP reinforcement, as assessed with an amphetamine choice paradigm.
Materials and Methods
Participants
A total of 15 healthy controls were recruited from the New Haven area by newspaper advertisements and flyers. Five of 15 dropped out before completing the study because of a positive urine toxicology (n=1), cold symptoms (n=2) and personal reasons (n=2). The 10 completers were eight male and two females (seven African-Americans, two Caucasian, and one Hispanic) with an average age (SD) of 33.7 years (7.7). All subjects had normal physical, laboratory, and psychiatric examinations, and none were dependent on alcohol or other drugs except nicotine. In order to minimize confounding effects from nicotine or caffeine withdrawal, those who smoked more than five cigarettes or drank more than three cups of caffeinated beverages per day were excluded. All subjects provided informed consent prior to study entry and were paid for participation. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System, West Haven campus. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
Procedures
In this double-blind, placebo-controlled, crossover study, subjects had two 5-day treatment periods, in which they were randomized to minocycline (200 mg/day) or placebo. Each treatment period was separated by a minimum 4-day “washout” period in order to minimize residual effects from study medications. During the first 2 days of each treatment period, subjects had daily morning clinic visits at about 8:00 A.M. for medication administration and monitoring of adverse events. On Days 3 and 4, subjects came to the laboratory for experimental sessions 1 and 2. In these sessions, subjects received a random order of placebo or 20 mg/70 kg DAMP (randomly labeled as drug A or B) in a double-blind fashion. At the start of the sessions 1 and 2, an indwelling intravenous catheter was placed in the antecubital vein both for blood drawing and as a safety precaution. Baseline measures were obtained, and then subjects were given that day’s dose of the study medication (minocycline or placebo) and DAMP followed by a light meal. Outcome measures were collected over four hours of the experimental session.
On Day 5 (Experimental Session 3), subjects had the opportunity to self-administer either Drug A or Drug B (placebo or DAMP) capsules working on a progressive ratio computer task, adapted from Stoops et al. (2004 and 2005). For this task, subjects first chose on the computer screen whether they wanted to work for Drug A or B. Subjects then had the option to earn one fourth of the medication dose (5 mg for DAMP) by pressing the A or B button of the keyboard 200 times. If subjects wanted to work on getting another capsule, they had to press the keyboard 400 for the second, 800 for the third, and 1,600 times for the fourth, which was the maximum number of capsules (20 mg for DAMP) allowed. All experimental sessions started at 8:00 A.M. and urine toxicology screenings were done before each session.
Drugs
DAMP (the dextrorotatory isomer of amphetamine) was obtained from Barr Laboratories (Pomona, New York). DAMP is used for the treatment of attention-deficit hyperactivity disorder (ADHD) and narcolepsy at a dose of 5 to 60 mg/day (PDR, 2009). In this study, we used a single 20 mg/70 kg oral dose, which has been shown to produce typical amphetamine effects in healthy volunteers (Brauer and de Wit 1996; Fillmore et al. 2003; Sofuoglu et al. 2008a; Sofuoglu et al. 2007; 2008b; Tidey et al. 2000). Plasma levels of DAMP peak within one to three hours following oral administration, and the elimination half-life is 10 to 13 hours (PDR, 2009).
Minocycline (Dynacin®) was administered at 200 mg/day, as a single dose, for 4 days. This dose is within the range of usual daily dose of minocycline used for the treatment of infections (Jonas and Cunha 1982). Further, two recent trials have utilized a 200 mg/day dose of minocycline in studies of stroke and schizophrenia (Lampl et al. 2007; Levkovitz et al. 2009; The NINDS NET-PD Investigators 2006). Following oral administration, peak plasma levels of minocycline are reached within 1–4 hours. The elimination half-life of minocycline ranges from 11 to 24 hours. Minocycline was administered in the clinic daily by the study nurse.
Measures
The outcome measures included physiological, endocrine, subjective, behavioral, and cognitive performance measures. The physiological measures consisted of systolic and diastolic blood pressure and heart rate, which were measured at baseline then at 30, 60, 90, 120, 150, and 180 minutes after DAMP administration. Plasma cortisol was obtained at baseline and then at one, two, and three hours after DAMP administration. Cortisol release is sensitive to DAMP through central noradrenergic pathways (Grady et al. 1996; Seiden et al. 1993).
Subjective measures were obtained at baseline, and then at 30, 60, 90, 150, and 180 minutes after the medication treatment and were comprised of the Drug Effects Questionnaire (DEQ), the Addiction Research Center Inventory-Short Form (ARCI), and the Profile of Mood States (POMS). The DEQ assessed the acute subjective effects of DAMP, and asked subjects to rate “stimulated”, “high”, “anxious”, “sedated”, “down”, “feeling the drug strength”, “feel good drug effects”, “feel bad drug effects”, “want more drug”, and “like the drug” on a 100 mm scale, from 0 (“not at all”) to 100 (“extremely”). The ARCI consists of 49 true-or-false questions with five subscales: drug-induced euphoria (Morphine-Benzedrine Group; MBG), stimulant-like effects (Amphetamine; A), intellectual efficiency and energy (Benzedrine Group; BG), dysphoria (Lysergic Acid; LSD), and sedation (Pentobarbital-Chlorpromazine; PCAG) (Martin et al., 1971). The POMS is a 65–item rating scale used to measure the effects of medication treatments on mood using six subscales: Tension, Depression, Anger, Vigor, Fatigue, and Confusion (McNair et al. 1971).
The behavioral outcome was the number of capsules subjects earned for self-administration on day 5 of each treatment period. Cognitive performance was assessed with the Sustained Attention to Response Test (SART), which was administered two hours after DAMP administration. The SART (Robertson et al. 1997) is a Go No-Go task. It assesses the ability to withhold responses to an infrequently occurring target (No-Go trials). Reaction times (RTs) and errors on Go trials are also assessed. 225 single digits (25 × 9 digits) are presented on a computer monitor for 250 ms each, immediately followed by a mask for 900 ms. Subjects must press a spacebar in response to every digit except the “3” They are instructed to give equal importance to speed and accuracy, (see (Sofuoglu et al. 2008b) for details).
Statistical Analysis
To assess treatment effects, we used a mixed-effect repeated-measures crossover analysis using SAS Proc Mixed (version 9.1.3.). The structure of the analysis included a fixed main effects for level of minocycline (placebo or minocycline), DAMP (DAMP or placebo), the time point of the measure after giving DAMP or placebo, and the interaction of the main effects of minocycline or placebo and DAMP or placebo. Also included were a random effect participant and a blocking factor for treatment sequence. On the SART, the primary outcome measure was the number of errors of commission (out of 25) pressing the spacebar when “3” was displayed. The number of errors of omission (not pressing the spacebar on digits other than 3), and the mean correct reaction times to those digits were also measured, with reaction times quicker than 100 ms discarded from analysis. A significance level of p<0.05 was used for all analyses.
Results
Physiological Responses
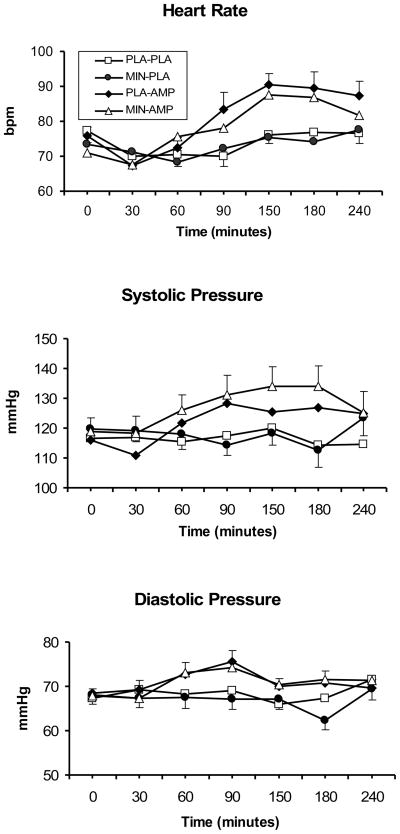
As shown in Figure 1, DAMP increased the heart rate (DAMP main effect; F (1, 26) = 15.2; p<0.001), systolic (DAMP main effect; F (1, 265) = 42.9; p<0.0001), and diastolic blood pressure (DAMP main effect; F (1, 265) = 23.5; p<0.0001), compared to placebo. Minocycline, compared to placebo, increased the systolic blood pressure (minocycline main effect; F (1, 265) = 5.1; p<0.05). Minocycline did not affect the daily baseline heart rate, systolic or diastolic blood during the treatment phase (ps<0.05).
Fig. 1.
The average (with standard error of the mean - SEM) systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) responses to 20 mg/70 kg DAMP (AMP) or placebo (PLA) under 200 mg/day minocycline (MIN) or placebo (PLA) conditions. Measurements were taken at baseline, and then at 30, 60, 90, 120, 150, 180, and 240 minutes after DAMP administration. Some of the error bars were removed for clarity. For systolic blood pressure, a significant main effect for minocycline was observed (p<0.05).
Subjective Responses
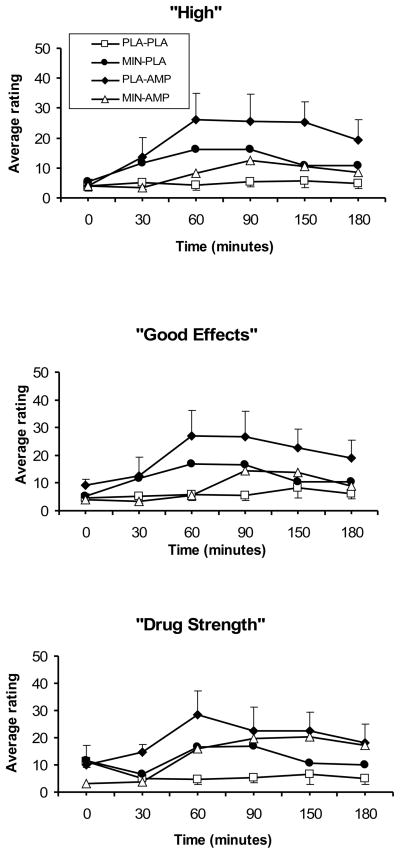
DAMP increased the rating of “feel the drug strength” (DAMP main effect; F (1, 225) = 25.4; p<0.0001), “feel good drug effects” (DAMP main effect; F (1, 225) = 18.4; p<0.0001), “feel high” (DAMP main effect; F (1, 225) = 18.8; p<0.0001), and “want more drug” (DAMP main effect; F (1, 225) = 4.5; p<0.05) items of the DEQ (Figure 2). Minocycline+DAMP, compared to DAMP, significantly reduced the rating of “feel good drug effects” (minocycline-by-DAMP; F (1, 225) = 11.5; p<0.001), “feel high” (minocycline-by-DAMP; F (1, 225) = 13.4 p<0.001).
Fig. 2.
Selected average (SEM), subjective responses to 20 mg/70 kg DAMP (AMP) or placebo (PLA) under 200 mg/day minocycline (MIN) or placebo (PLA) conditions (from 0 to 100). Measurements were taken at baseline, then at 30, 60, 90, 120, 150, and 180 minutes after DAMP administration. A significant minocycline-by-DAMP interaction was observed for “feel good drug effects” and “feel high” (p<0.05).
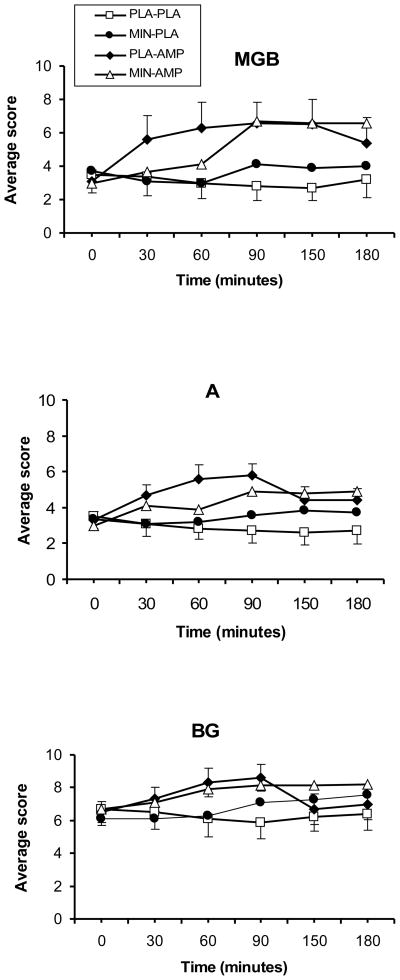
DAMP significantly increased the scores of the BG (DAMP main effect; F (1, 221) = 24.9; p<0.0001), MBG (DAMP main effect; F (1, 221) = 24.9; p<0.0001), and A (DAMP main effect; F (1, 221) = 50.4; p<0.0001) subscales of the ARCI (Figure 3). Minocycline + DAMP reduced the scores on the A subscale, compared to DAMP (minocycline-by-DAMP; F (1, 221) = 3.7; p=0.05).
Figure 3.
The A (Amphetamine), BG (Benzedrine Group), and MBG (Morphine-Benzedrine Group) subscales of the ARCI in response to a 20 mg/70 kg DAMP (AMP) or placebo (PLA) under 200 mg/day minocycline (MIN) or placebo (PLA) conditions. Measurements were taken at baseline, then at 30, 60, 90, 120, 150, and 180 minutes after DAMP administration. For the A subscale, a significant DAMP-by-minocycline was observed (p=0.05).
DAMP reduced scores for the fatigue (DAMP main effect; F (1, 221) = 7.8; p<0.01), and increased scores for the vigor (DAMP main effect; F (1, 221) = 11.4; p<0.001), subscales of the POMS. DAMP-minocycline reduced the scores for fatigue, (DAMP-by-minocycline interaction; F (1, 221) = 6.4; p<0.05), compared to the DAMP-placebo.
Endocrine Responses
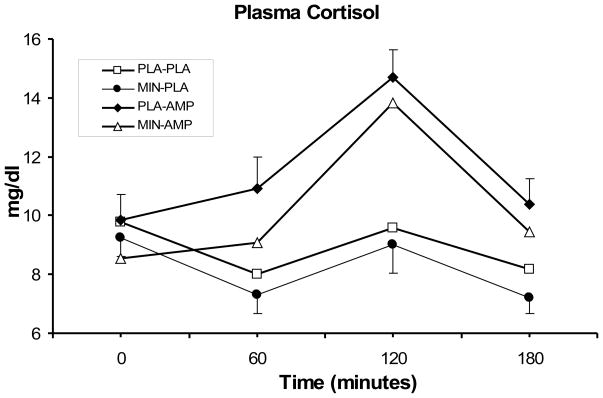
As shown in Figure 4, DAMP reduced cortisol responses, relative to placebo DAMP (main effect of DAMP; F (1, 140) = 27.5; p<0.0001), while minocycline reduced them, (main effect of minocycline; F (1, 138) = 5.1; p<0.05). [INSERT FIGURE 4 ABOUT HERE]
Fig. 4.
Average (SEM) plasma cortisol (μg/dL) responses to 20 mg/70 kg DAMP (AMP) or placebo (PLA) under 200 mg/day minocycline (MIN) or placebo (PLA) conditions. Measurements were taken at baseline, then at 60, 120, and 180 minutes after DAMP or placebo administration. A significant treatment effects for minocycline was observed (p<0.05).
Sustained Attention to Response Test (SART)
Summary statistics on the SART are summarized in Table 1. Under minocycline treatment, the reaction times were faster (main effects of minocycline; F (1, 26) = 4.52, p < 0.05).
Table 1.
Sustained Attention to Response Test (SART)
| PLA+PLA. | DAMP + PLA | PLA+MIN | DAMP + MIN | |
|---|---|---|---|---|
| Errors on GO trials (/25) | 13.4 (9.2) | 10.7 (9.0) | 14.2 (6.7) | 12.7 (8.1) |
| Errors on NO-GO trials (/200) | 6.2 (8.4) | 5.4 (9.9) | 6.6 (8.5) | 1.9 (2.1) |
| Mean RT on GO trials (ms) | 403.2 (106.0) | 435.6 (150.1) | 387.5 (78.7) | 342.7 (78.1) |
Note. M (SD) on the SART (n = 10). PLA = placebo; DAMP = dextroamphetamine; MIN = minocycline.
Drug Choice Task
Minocycline did not affect DAMP choice behavior, with 60 percent of subjects chose no drug under minocycline or placebo condition. Under placebo condition, 40 % of subject chose one or more doses of DAMP. In comparison, under minocycline treatment, 10 % chose one or more doses of DAMP and 30 chose placebo (p > 0.05).
Discussion
DAMP produced typical subjective psychostimulant effects including increase in drug-induced euphoria (MBG), stimulant-like effects (A), and intellectual efficiency and energy (BG) subscales of ARCI, and rating of “good drug effects,” “high,” and “want more drug,” items of the DEQ. Minocycline attenuated some of DAMP’s subjective-rewarding effects including the “high” and “feel good drug effects” items of the DEQ and the stimulant-like effects (A) subscale in the ARCI. In a recent study minocycline did not change the subjective effects from intravenous nicotine (Sofuoglu et al. 2009), suggesting that minocycline does not attenuate the subjective effects of all dopamine releasing agents, like nicotine. Consistent with our findings, acute minocycline treatment attenuated DAMP-induced locomotor activity in rats (Kofman et al. 1990; Kofman et al. 1993) and prevented development of behavioral sensitization to methamphetamine (Mizoguchi et al. 2008; Zhang et al. 2006) or cocaine (Chen et al. 2009). Further, using in vivo microdialysis technique, Zhang et al (Zhang et al. 2006) have shown that minocycline reduced methamphetamine-induced striatal dopamine release in mice. Dopamine release in striatum is a critical step for the rewarding effects of stimulants (Volkow et al. 1997) and its reduction by minocycline may explain attenuation of DAMP’s subjective effects by minocycline.
Minocycline also improved reaction time in the SART test. Previous preclinical and clinical studies have demonstrated the cognitive-enhancing effects of minocycline. In preclinical studies, minocycline improved methamphetamine-induced impairment on an object recognition task in mice (Fujita et al. 2008). It also reversed deficits in visuo-spatial memory and sensorimotor-gating induced by the NMDA antagonist PCP in rats (Levkovitz et al. 2007). In schizophrenic individuals 200 mg/day of minocycline also improved executive functions including working memory, cognitive shifting, and cognitive planning functions (Levkovitz et al. 2009).
Minocycline did not change self-administration of DAMP in our choice procedure although it attenuated the subjective-rewarding effects of DAMP. Consistent with our findings, previous studies reported dissociation between subjective drug effects and drug self-administration in humans (Dudish-Poulsen and Hatsukami 1997; Haney and Spealman 2008). One caveat was that our subjects were healthy volunteers who had a low rate of drug administration and chose no drugs for 60 percent of the options. In future studies, minocycline’s effects on DAMP choice behavior can be examined in those with a history of stimulant use. Minocycline also reduced the cortisol levels following DAMP or placebo administration in healthy volunteers, suggesting direct inhibitory effects of minocycline on cortisol release. Reduction of either dopamine or glutamate input to the paraventricular area of the hypothalamus may result in reduced CRH release (Patchev et al. 1994; Ziegler et al. 2005). Minocycline increased DAMP-induced systolic blood pressure elevations. We are not aware of previous studies showing blood pressure increases induced by minocycline.
The mechanism by which minocycline attenuates the subjective and cortisol responses to DAMP are unknown. One possibility is enhancing AMPA transmission by increasing phosphorylation and surface expression of the AMPA receptor subunit GluR1 (Imbesi et al. 2008). Increased GluR1 phosphorylation facilitates learning and memory and is a medication development target for cognitive disorders, schizophrenia, and depression. Studies also indicate that activation of GluR1 receptors may prevent sensitization to stimulants. However, not consistent with this possibility, another medication with similar GluR1 activating effects, riluzole, enhanced the subjective effects of DAMP and increased errors of commission on the SART test in healthy controls (Sofuoglu et al. 2008b). Additionally, clinically used doses of minocycline inhibit nitric oxide (NO) synthesis (Kim et al. 2004; Sadowski and Steinmeyer 2001). NMDA-type glutamate receptor activation releases NO as a second messenger that facilitates glutamate release and increased dopamine release into the nucleus accumbens (Itzhak and Ali 2006). This inhibition of the NO second messenger system opens possibilities for further medication development with other compounds.
Our study has several other treatment implications. First, minocycline’s attenuation of the subjective-rewarding effects of DAMP, together with the promising preclinical studies summarized above, supports its use for stimulant addiction. Recently, Tanibuchi et al. (2010) reported a case showing that minocycline is effective in a patient with a methamphetamine-related psychotic disorder. Thus, controlled efficacy studies of minocycline in cocaine or methamphetamine addicted individuals are warranted. Second, improvement of the reaction time in the SART is consistent with the minocycline’s cognitive-enhancing effects that were previously observed in both preclinical and clinical studies. Minocyline is currently under investigation for neurocognitive disorders, although a multicenter study with Amyotrophic Lateral Sclerosis (ALS) patients found a more rapid cognitive decline in minocycline (up to 400 mg/day) treated patients (Gordon et al. 2007). Other studies suggested that a lower dose of 200 mg/day is safer in this population (Leigh et al. 2008). Ongoing clinical trials should help to establish the safety of minocycline in different patient populations.
This study had several limitations. First, this study did not examine the dose-dependent effects of minocycline since only 200 mg/day dose was used. Second, similarly the study did not examine minocycline’s effects on dose-dependent dextroamphetamine responses since only 20 mg dose of dextroamphetamine was used. Lastly, our subjects were healthy controls and the generalization of these findings to stimulant users need to be demonstrated.
In summary, 200 mg/day minocycline attenuated some of the subjective-rewarding responses to DAMP and reduced cortisol levels in healthy controls. Minocycline also speeded reaction times on a sustained attention task. Further studies are warranted to examine the therapeutic utility of minocycline as a treatment medication for stimulant addiction and as a cognitive enhancer.
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and National Institute on Drug Abuse (NIDA) grants P50-DA12762, K05-DA0454 (TK), K02-DA021304 (MS), and K01-DA-019446 (MM).
We would like to thank Ellen Mitchell, R.N. and Stacy Minnix for technical assistance. Dr. Kosten is in the Speakers Bureau of Reckitt; served as a consultant for Celtic, Alkermes, Synosia, Catalyst, and Gerson Lerman; and has ownership interests with Pfizer and Johnson & Johnson. Dr. Mooney has received research grants from Pfizer. None of the products of these companies are associated with this work.
Footnotes
The other authors have no conflicts of conflict of interest.
References
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Chaves C, Marque CR, Trzesniak C, Machado de Sousa JP, Zuardi AW, Crippa JA, Dursun SM, Hallak JE. Glutamate-N-methyl-D-aspartate receptor modulation and minocycline for the treatment of patients with schizophrenia: an update. Braz J Med Biol Res. 2009;42:1002–14. doi: 10.1590/S0100-879X2009001100002. [DOI] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H. Minocycline affects cocaine sensitization in mice. Neurosci Lett. 2009;452:258–61. doi: 10.1016/j.neulet.2009.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–60. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentations. Drug Alcohol Depend. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–52. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Ishima T, Kunitachi S, Hagiwara H, Zhang L, Iyo M, Hashimoto K. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocycline. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:336–9. doi: 10.1016/j.pnpbp.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Gonzalez JC, Egea J, Del Carmen Godino M, Fernandez-Gomez FJ, Sanchez-Prieto J, Gandia L, Garcia AG, Jordan J, Hernandez-Guijo JM. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca(2+) signalling in hippocampal neurons. Eur J Neurosci. 2007;26:2481–95. doi: 10.1111/j.1460-9568.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–53. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Grady TA, Broocks A, Canter SK, Pigott TA, Dubbert B, Hill JL, Murphy DL. Biological and behavioral responses to D-amphetamine, alone and in combination with the serotonin3 receptor antagonist ondansetron, in healthy volunteers. Psychiatry Res. 1996;64:1–10. doi: 10.1016/0165-1781(96)02884-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–23. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psychiatry. 2007;61:577–81. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Uz T, Manev R, Sharma RP, Manev H. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci Lett. 2008;447:134–7. doi: 10.1016/j.neulet.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Role of nitrergic system in behavioral and neurotoxic effects of amphetamine analogs. Pharmacol Ther. 2006;109:246–62. doi: 10.1016/j.pharmthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jonas M, Cunha BA. Minocycline. Ther Drug Monit. 1982;4:137–45. [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007;9:389–97. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Kong PJ, Kim BS, Sheen DH, Nam SY, Chun W. Inhibitory action of minocycline on lipopolysaccharide-induced release of nitric oxide and prostaglandin E2 in BV2 microglial cells. Arch Pharm Res. 2004;27:314–8. doi: 10.1007/BF02980066. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Klein E, Newman M, Hamburger R, Kimchi O, Nir T, Shimon H, Belmaker RH. Inhibition by antibiotic tetracyclines of rat cortical noradrenergic adenylate cyclase and amphetamine-induced hyperactivity. Pharmacol Biochem Behav. 1990;37:417–24. doi: 10.1016/0091-3057(90)90006-4. [DOI] [PubMed] [Google Scholar]
- Kofman O, van Embden S, Alpert C, Fuchs I. Central and peripheral minocycline suppresses motor activity in rats. Pharmacol Biochem Behav. 1993;44:397–402. doi: 10.1016/0091-3057(93)90481-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–10. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Leigh PN, Meininger V, Bensimon G, Cudkowicz M, Robberecht W. Minocycline for patients with ALS. Lancet Neurol. 2008;7:119–20. doi: 10.1016/S1474-4422(08)70006-1. author reply 120–1. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Levi U, Braw Y, Cohen H. Minocycline, a second-generation tetracycline, as a neuroprotective agent in an animal model of schizophrenia. Brain Res. 2007;1154:154–62. doi: 10.1016/j.brainres.2007.03.080. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2009 doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Manual for profile of mood states. Educational and industrial testing services, Educational and industrial testing services; 1971. [Google Scholar]
- Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2008;196:233–41. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:380–6. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Munzar P, Li H, Nicholson KL, Wiley JL, Balster RL. Enhancement of the discriminative stimulus effects of phencyclidine by the tetracycline antibiotics doxycycline and minocycline in rats. Psychopharmacology (Berl) 2002;160:331–6. doi: 10.1007/s00213-001-0989-7. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Karalis K, Chrousos GP. Effects of excitatory amino acid transmitters on hypothalamic corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP) release in vitro: implications in pituitary-adrenal regulation. Brain Res. 1994;633:312–6. doi: 10.1016/0006-8993(94)91554-7. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–58. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Sadowski T, Steinmeyer J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. J Rheumatol. 2001;28:336–40. [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Waters A, Sewell A, Hill K, Kosten T. Disulfiram enhances subjective effects of dextroamphetamine in humans. Pharmacol Biochem Behav. 2008a;90:394–8. doi: 10.1016/j.pbb.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and d-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007 doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and d-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2008b;32:16–22. doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O’Malley SS. Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav. 2009;92:135–40. doi: 10.1016/j.pbb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–82. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology (Berl) 2000;151:184–91. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–93. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484:43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]