Abstract
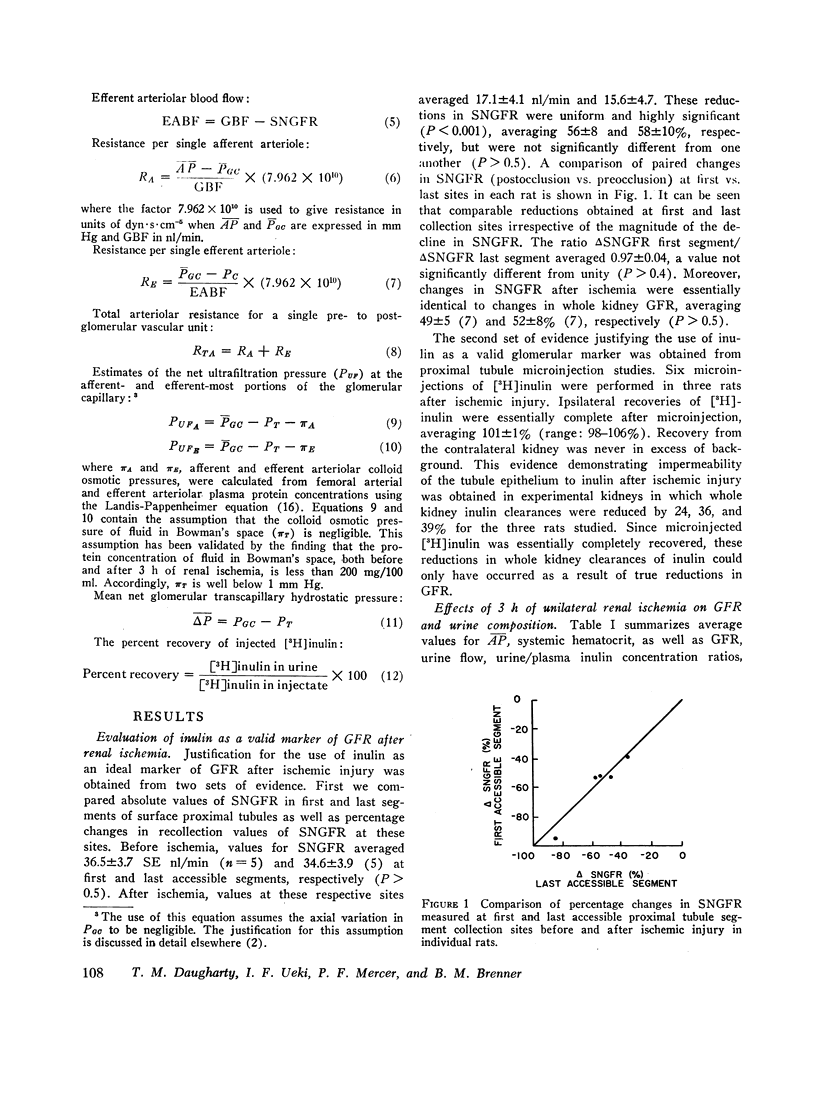
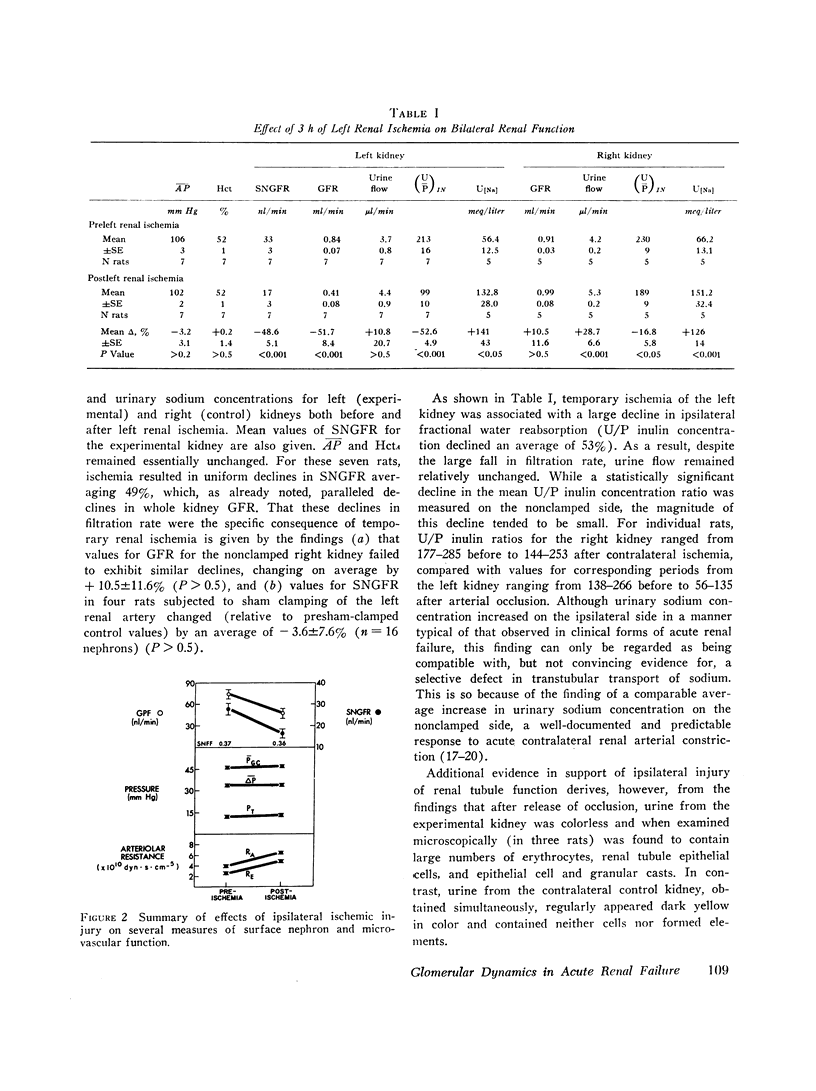
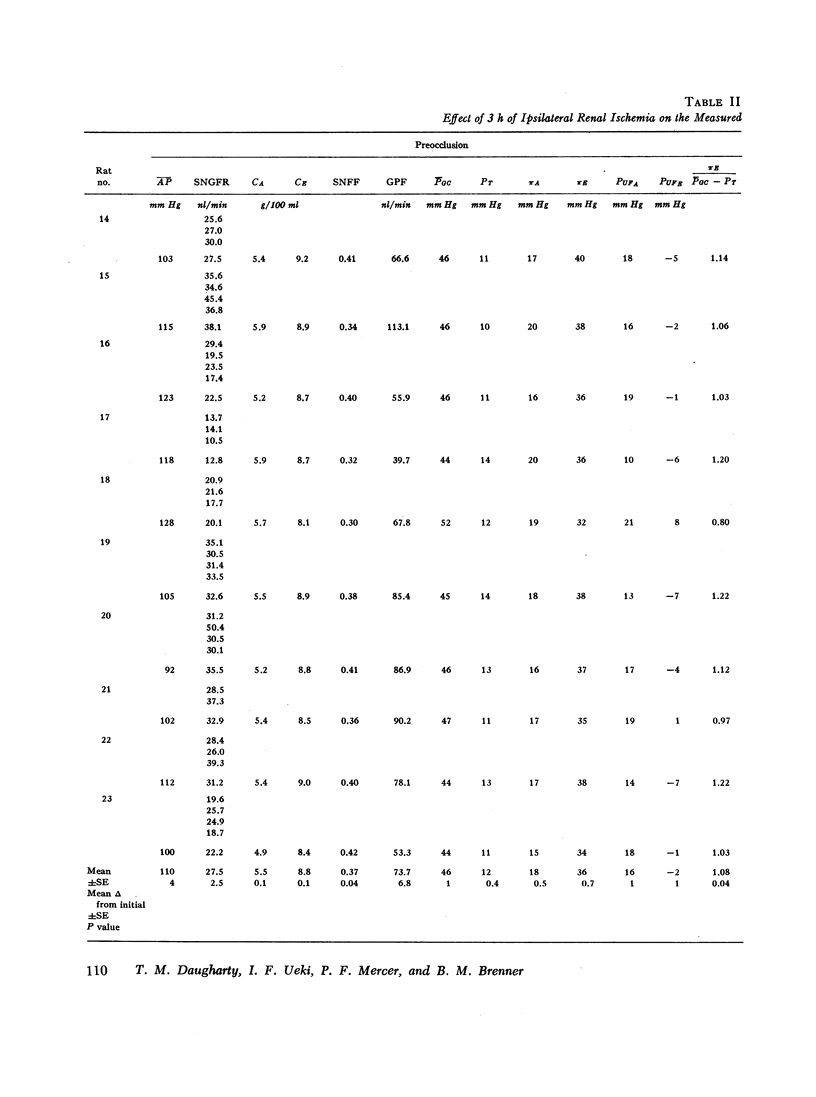
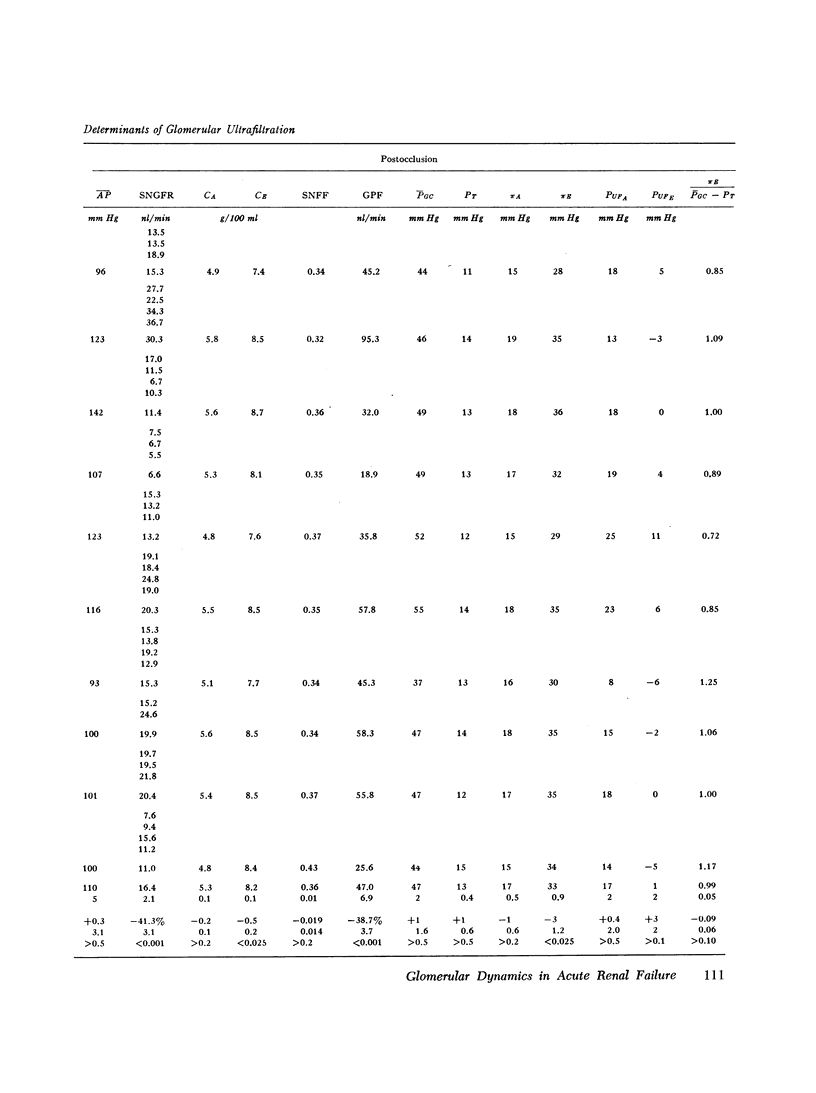
An experimental model of postischemic, acute renal failure has been developed in Wistar rats with surface glomeruli, thereby making possible a direct assessment of the mechanisms responsible for the fall in glomerular filtration rate that characterizes this disorder. Whole kidney and cortical single nephron filtration rates were reduced proportionately, on average by approximately 40%, after 3 h of nearly complete occlusion of the ipsilateral renal artery. The possibility of a significant transtubular leak of inulin was excluded. This decline in filtration rate occurred in the absence of measured changes in mean arterial pressure, mean glomerular transcapillary hydrostatic pressure, or net ultrafiltration pressure at afferent and efferent ends of the glomerular capillary. Net ultrafiltration pressure at the efferent end of the capillary approached zero both before and after ischemic injury, demonstrating that filtration pressure equilibrium was achieved throughout this study. Single nephron filtration fraction remained unchanged, indicating that the fall in filtration rate was accompanied by a proportional decline in glomerular plasma flow. The results indicate that the fall in filtration rate was solely the consequence of this fall in glomerular plasma flow. Since filtration rate per nephron is equal to the product of the ultrafiltration coefficient and mean ultrafiltration pressure, this product must also have fallen in proportion to the decline in glomerular plasma flow. Evidence is presented to indicate that a change in ultrafiltration coefficient is not required to account for the observed fall in filtration rate. The reduction in glomerular plasma flow, occuring in the absence of a concomitant decline in mean glomerular capillary hydrostatic pressure, resulted from large and proportional increases in afferent and efferent arteriolar resistances. These resistance changes appear to play a fundamental role in the pathogenesis of this form of acute renal failure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Daugharty T. M., Ueki I. F., Troy J. L. Quantitative assessment of proximal tubule function in single nephrons of the rat kidney. Am J Physiol. 1971 Jun;220(6):2058–2067. doi: 10.1152/ajplegacy.1971.220.6.2058. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Galla J. H. Influence of postglomerular hematocrit and protein concentration on rat nephron fluid transfer. Am J Physiol. 1971 Jan;220(1):148–161. doi: 10.1152/ajplegacy.1971.220.1.148. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. Pressures in cortical structures of the rat kidney. Am J Physiol. 1972 Feb;222(2):246–251. doi: 10.1152/ajplegacy.1972.222.2.246. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971 Aug;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Comparative renal effects of isoncotic and colloid-free volume expansion in the rat. Am J Physiol. 1972 Jan;222(1):225–235. doi: 10.1152/ajplegacy.1972.222.1.225. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Berliner R. W. Hydrostatic pressures in peritubular capillaries and tubules in the rat kidney. Am J Physiol. 1971 May;220(5):1422–1426. doi: 10.1152/ajplegacy.1971.220.5.1422. [DOI] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MOREL F., MYLLE M. TRACER MICROINJECTION STUDIES OF RENAL TUBULAR PERMEABILITY. Am J Physiol. 1965 Jul;209:173–178. doi: 10.1152/ajplegacy.1965.209.1.173. [DOI] [PubMed] [Google Scholar]
- Kramer P., Ochwadt B. Sodium excretion in Goldblatt hypertension. Long-term separate kidney function studies in rats by means of a new technique. Pflugers Arch. 1972;332(4):332–345. doi: 10.1007/BF00588579. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaf A. Regulation of intracellular fluid volume and disease. Am J Med. 1970 Sep;49(3):291–295. doi: 10.1016/s0002-9343(70)80019-5. [DOI] [PubMed] [Google Scholar]
- Oken D. E. Nosologic considerations in the nomenclature of acute renal failure. Nephron. 1971;8(6):505–510. doi: 10.1159/000179956. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- SHEEHAN H. L., DAVIS J. C. Renal ischaemia with failed reflow. J Pathol Bacteriol. 1959 Jul;78:105–120. [PubMed] [Google Scholar]
- Stein R. M., Abramson R. G., Bercovitch D. D., Levitt M. F. Effects of unilateral renal arterial constriction on tubular reabsorption of sodium and water during an osmotic diuresis. J Clin Invest. 1965 Oct;44(10):1720–1729. doi: 10.1172/JCI105279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. K., Jamison R. L. The no reflow phenomenon in renal ischemia. Lab Invest. 1971 Dec;25(6):635–643. [PubMed] [Google Scholar]
- WIEDERHIELM C. A., WOODBURY J. W., KIRK S., RUSHMER R. F. PULSATILE PRESSURES IN THE MICROCIRCULATION OF FROG'S MESENTERY. Am J Physiol. 1964 Jul;207:173–176. doi: 10.1152/ajplegacy.1964.207.1.173. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Powell W. J., Jr, Guiney T. E., Stark J. J., Sanders C. A., Leaf A. Improvement in myocardial function and coronary blood flow in ischemic myocardium after mannitol. J Clin Invest. 1972 Dec;51(12):2989–2998. doi: 10.1172/JCI107126. [DOI] [PMC free article] [PubMed] [Google Scholar]



