Abstract
Daily, systemic injections of a positive AMPA-type glutamate receptor modulator (ampakine) have been shown to reduce synaptic plasticity defects in rodent models of aging and early-stage Huntington’s Disease (HD). Here we report that long-term ampakine treatment markedly slows the progression of striatal neuropathology and locomotor dysfunction in the R6/2 HD mouse model. Remarkably, these effects were produced by an ampakine, CX929, with a short half-life. Injected once daily for 4–7 weeks, the compound increased protein levels of brain-derived neurotrophic factor (BDNF) in neocortex and striatum of R6/2 but not wild-type mice. Moreover, ampakine treatments prevented the decrease in total striatal area, blocked the loss of striatal DARPP-32 immunoreactivity and reduced the area of intranuclear huntingtin aggregates in R6/2 striatum by 36%. The CX929 treatments also markedly improved motor performance of R6/2 mice on several measures (rotarod, vertical pole descent) but did not influence body weight or lifespan. These findings describe a minimally invasive, pharmacologically plausible strategy for treatment of HD and, potentially, other neuropathological diseases.
Keywords: therapeutic, DARPP-32, rotarod, huntingtin, BDNF, neurotrophin, striatum, neuropathology
Introduction
Ampakines are centrally active, small molecule compounds that slow deactivation and desensitization kinetics of AMPA-type glutamate receptors (AMPARs). Thus, the compounds prolong the opening of ligand bound AMPARs and increase the size and duration of fast, excitatory post-synaptic potentials (EPSPs) (Lynch, 2006). Recent work has shown that a relatively short series of daily ampakine injections rescues hippocampal synaptic plasticity in rodent models of aging (Rex et al., 2006), low estrogen levels (Kramar et al., 2010) and Huntington’s Disease (HD) (Simmons et al., 2009). In the latter case, treatment of CAG140 knock-in mice with a short half-life ampakine (CX929) also reduced memory deficits associated with early stage HD. Since testing in these experiments was performed one day after the last injection, the positive results can be ascribed to long-term changes initiated by the ampakine. Related to this point, excitatory drive is known to modulate the expression of several neurotrophic factors including BDNF (Gall and Lauterborn, 2000), a releasable neurotrophin that plays a pivotal role in long-term potentiation, a form of synaptic plasticity thought to underlie memory (Bramham and Messaoud, 2005; Lynch et al., 2007a; Rex et al., 2007). Through effects on AMPAR-mediated fast EPSPs, ampakines increase BDNF levels (Lauterborn et al., 2003) and thereby establish conditions that promote synaptic plasticity.
The above findings raise the question of whether prolonging the transcriptional effects of ampakine treatment can slow the progression of HD. While mechanisms underlying the onset and development of HD phenotypes are uncertain, evidence suggests that transcriptional dysregulation and BDNF deficits are important contributors (Canals et al., 2004; Cha, 2000; Sugars and Rubinsztein, 2003; Zuccato and Cattaneo, 2007, 2009). This view is reinforced by evidence that BDNF transgene expression reduces motor impairments and striatal neuropathology in HD-model R6/1 mice (Gharami et al., 2008). The above described short-term ampakine treatments that rescued synaptic plasticity did not improve the modest motor abnormalities (e.g., movement speed) found in young adult CAG140 mice (Simmons et al., 2009), but this result may not be predictive of outcomes using longer drug treatments or HD mice with more severe motor impairments. The present studies addressed these issues and, specifically, tested if chronic ampakine treatments can sustain elevated BDNF levels and slow the progression of HD pathology.
The R6/2 mouse model of HD was employed because it has rapidly developing, well-characterized pathological features and easily measured functional endpoints (Menalled et al., 2009; Menalled and Chesselet, 2002) against which drug effects could be tested. To maximize clinical relevance, we used a minimally invasive treatment regimen and a short half-life ampakine that nonetheless elevates forebrain BDNF levels (Rex et al., 2006; Simmons et al., 2009). Because ampakines have few side-effects, are well tolerated with longer term treatments (Murray et al., 2003) and are in clinical development for various indications (Greer and Ren, 2009; Lynch, 2006), tests of their effects on degenerative diseases are potentially important for the development of novel therapeutic strategies. Results described here provide the first evidence that chronic ampakine treatments can be used to slow the progression of an inherited neurodegenerative disease.
Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine. This includes efforts to minimize animal suffering and numbers of mice used.
Mouse husbandry
Breeding pairs of R6/2 mice were purchased from Jackson Laboratories [Bar Harbor, ME; B6CBA-TgN (HD exon1)62; JAX stock number: 006494]. Male R6/2 mice, which are transgenic for the 5′ end of the human HD gene carrying about 100–150 glutamine (CAG) repeats (for actual CAG sizes see below), and their wild-type (WT) littermates were used starting at 3 or 6 weeks of age. Mice were maintained with a 12 hr light/dark cycle (on 2 am, off 2 pm) and received rodent chow ad libitum. Genotypes were identified before weaning using polymerase chain reaction (PCR) of tail-tip DNA. Tail DNA taken at the time of sacrifice was used to confirm genotypes via real-time PCR and CAG repeat numbers were measured with ABI GeneMapper 4.0 by Laragen, Inc. (Los Angeles, CA). R6/2 mice used in this study had an average of 104 ± 6.5 (mean ± SD) CAG repeats.
Study design and drug treatments
Male R6/2 and WT mice were divided into two groups/genotype (total of 4 groups, n = 8–17/group): (1) Controls received vehicle [10% (2-Hydroxypropyl)-β-cyclodextrin (Sigma, St. Louis, MO) in 0.45% saline, 10 ml/kg intraperitoneal (IP)] and (2) Experimentals received the ampakine CX929 (5 mg/kg in vehicle; 10 ml/kg IP); in all cases mice received one injection per day, at about 9 am. The ampakine CX929 (provided by Cortex Pharmaceuticals, Irvine, CA) and the dose used was chosen for the present studies because it was previously found to elevate forebrain BDNF levels in rat and mouse in vivo (Rex et al., 2007; Simmons et al., 2009). In rat, blood values following IP injections rose quickly to a maximum and then fell back towards baseline with a calculated half-life of 15 minutes. Mice were weighed bi-weekly and assessed for morbidity and mortality twice daily.
Three different cohorts of the 4 groups of mice were run. Cohort 1 received ampakine (or vehicle) treatment for 4 weeks (from 6 to 10 weeks of age) and was tested for rotarod performance and BDNF levels. Cohort 2 was given CX929 or vehicle for 7 weeks (from 3 to 10 weeks of age), received all behavioral tests, and was processed for neuropathology and BDNF measures. Finally, Cohort 3 received treatments for their entire lifespan starting from 3 weeks of age, was tested for rotarod performance, and was used to test survivability. For the latter, survival cohort, mice were euthanized if they could no longer right themselves within 30 seconds after being placed on their sides. Deaths that occurred overnight were recorded the following morning.
Rotarod and clasping performance
Beginning 4 days after the first injection, mice were tested for motor impairment once a week using a Rotarod (Med Associates Inc., St. Albans, VT). Rotarod test sessions consisted of three 120 sec trials at 24 rpm, as employed elsewhere (Apostol et al., 2008; Carter et al., 1999; Pallier et al., 2009). For each trial, the latency to fall from the apparatus was recorded; daily results represente an average of the three trials. Clasping behavior was tested at the time of sacrifice: mice were suspended by the tail for 60 sec and the latency for the hindlimbs or all 4 paws to clasp was recorded.
Vertical Pole Descent Test
The vertical pole test was performed at 9 weeks of age as described previously (Hickey et al., 2008; Matsuura et al., 1997). For testing, mice were placed on the top of a coarse, vertical wooden pole (diameter: 1 cm; height: 55 cm) with head up; mice typically turn and descend. The total time to descend to the floor over 5 trials was recorded; the final 4 trials were analyzed. If the mouse did not turn downwards, dropped or slipped down, a default value of 60 sec was recorded.
Western Blotting
The parietal neocortex and striatum were dissected from brains of mice from Cohorts 1 and 2 the day after their last injection (i.e., ≥18 hrs later). Tissue was processed for western blot analysis of BDNF levels using rabbit anti-BDNF (1:1,000; #SC546; Santa Cruz, CA) as described (Lynch et al., 2007b; Simmons et al., 2009). Recombinant human BDNF (Chemicon) was loaded on the same gels as the samples. Blots were stripped and reprobed with anti-actin (1:2,000; Sigma) to control for loading variations. Immunoreactive bands were measured from films using NIH Image software: bands at 14–15 kDa were measured to assess mature (m) BDNF monomer levels (Lee et al., 2001; Matsumoto et al., 2008). Densities of BDNF-immunoreactive bands were expressed as a fraction of the actin-immunoreactive band in the same lane; for individual animals samples were run 2–3 separate times and results averaged. Statistical analyses used the one tailed Students t test to conduct specific planned comparisons with predicted direction of change.
Immunocytochemistry
Mice were deeply anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were postfixed (1–2 hours), cryoprotected in 20% sucrose/PB and sectioned (30 μm, coronal) using a freezing microtome. Free-floating sections were processed for immunohistochemical localization of dopamine- and cyclic AMP-regulated phosphoprotein with molecular weight of 32 kDa (DARPP-32; 1:1,000; Chemicon AB1656) or huntingtin protein (1:200; clone EM48, Chemicon) using the Vectastain Elite ABC kit (Vector Labs) and the DAB Peroxidase Substrate Kit (Vector Labs) as described (Simmons et al., 2007).
Microscopy and Quantitative Analyses
The area of the striatum was measured bilaterally in 2 coronal sections, spaced by about 12 sections (~360 μm) and matched for rostrocaudal level (~0.86 to 0.50 mm Bregma), that were processed for DARPP32 immunocytochemistry. The perimeter of the striatum was traced manually using a Zeiss Axioskop 2 microscope, an AxioCam Hrc camera, and Neurolucida v.7 image analysis software (MBF Bioscience). The anatomical landmarks of the corpus callosum, external capsule, and lateral ventricle were used to define the top, lateral and medial sides of the dorsal striatum, respectively. The bottom of the anterior commissure was used to define the ventral boundary of the structure. For quantification of immunostaining, striatal sections (2–3 sections per mouse in each hemisphere; therefore, 4–6 sample fields per mouse) were viewed and digitized using a Zeiss Axioskop 2 microscope, an AxioCam Hrc camera, and Axiovision software. Within the striatum, fixed size sample fields (see below for specifics) were placed at rostral to mid-striatal levels (+1.18 to +0.62 mm relative to Bregma) and dorsally (beneath and abutting the corpus callosum) for all animals, since HD pathology progresses from dorsal to ventral, anterior to posterior, and medial to lateral within this brain area (Vonsattel et al., 1985).
For DARPP-32 immunostaining, images of the 0.36 × 0.46 mm2 sample field were collected using a 20X microscope objective. To assess total nuclear huntingtin staining (diffuse and aggregate), a sample area of 0.85 × 0.70 mm2 was photographed using a 10X objective. For both diffuse and aggregate measures, immunostaining was quantified from the digital images using the histogram thresholding command of Image Pro Plus v6.3 software (Media Cybernetics). The threshold was manually set to identify densely immunolabeled elements (cells and/or nuclei) that were distinct from low density labeling within the neuropil, and then the proportionate are and number of elements labeled above threshold was quantified. The same image exposure times and threshold settings were used for sections from all treatment groups within a given comparison.
To assess nuclear huntingtin aggregates only, all densely immunoreactive puncta within a sample field of 75 × 67 μm2 (on each side of the brain) were manually traced while viewing with a 63X oil objective for 2 sections (i.e., 4 fields) per mouse using Neurolucida v.7 image analysis software (MBF Bioscience); the mean number of aggregates traced per mouse was 17 ± 7 (SD).
Statistical analyses employed Statview (SAS Institute, Inc., Cary, NC) or GraphPad Prism v.5 (GraphPad Software, San Diego California) software. Significance was determined using an analysis of variance (ANOVA) with repeated measures (RM) for rotarod analyses; Student’s t-tests were used for planned comparisons in this and other behavioral analyses. Analyses of histological measures used the two-way ANOVA and/or a Student’s t-test for paired comparisons (both with adjustments for unequal variance when needed). For western blots, statistical analyses were run on actin-normalized measures using the two-way ANOVA and, for planned comparisons with explicit predictions as to direction of difference, the one-tailed Student’s t-test. Results are expressed as group mean ± SEM (unless otherwise stated) and statistical significance was set at p ≤ 0.05.
Results
CX929 increases BDNF protein levels in neocortex and striatum
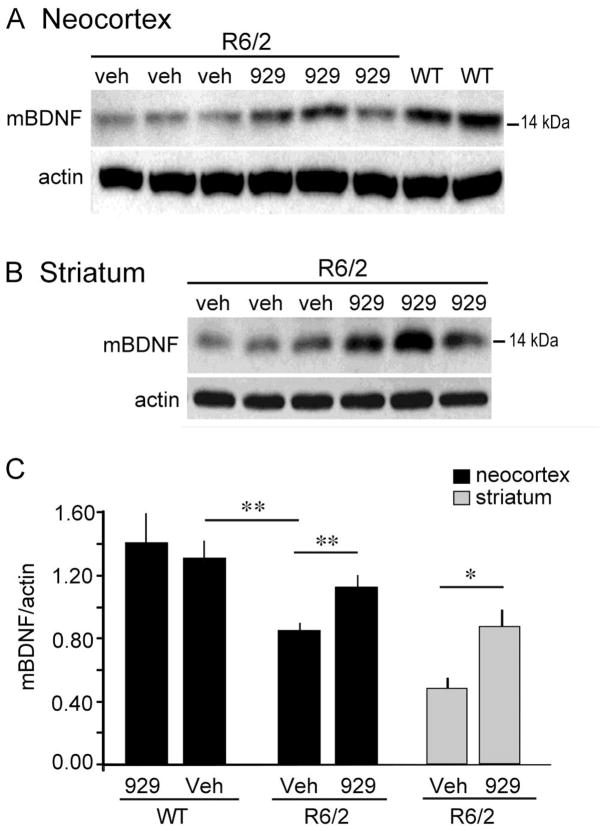
Western blots were used to determine if daily ampakine treatments influenced BDNF levels in parietal neocortex of R6/2 mice used for behavioral testing. Values were obtained for subgroups of transgenic and WT mice given four to seven weeks of daily ampakine or vehicle injections (Cohorts 1 and 2). In all cases, the brains were collected ≥hours after the last injection of vehicle or CX929. The analysis tested the explicit predictions that cortical BDNF expression in the R6/2 mice would be depressed relative to WTs, and increased by ampakine treatment, as observed in other HD mouse models (Simmons et al., 2009). In blots from both genotypes, BDNF immunoreactivity (ir) was distributed across several bands corresponding to larger pro-BDNF forms and proteolytic fragments, and to the 14–15 kDa mature (m) BDNF protein (Matsumoto et al., 2008)(Fig. 1A). Measures of mBDNF-ir bands determined that cortical BDNF protein levels were 35 ± 5% lower in vehicle-treated R6/2 (n = 8) than in WT mice (n = 10; p = 0.002, one-tail Student’s t-test; Fig. 1C). This agrees with previous studies showing that BDNF mRNA levels are reduced in neocortex of R6/2 mice as early as at 6 weeks of age (Zuccato et al., 2005). In contrast, in cortical samples from R6/2 mice given CX929 (n = 9), mBDNF levels were significantly greater than levels in R6/2s given vehicle (p=0.01, one-tail t-test), and were comparable to levels in vehicle-treated WTs (p = 0.11). CX929 did not significantly alter mBDNF levels in WT mice (p=0.66, two-tail t-test). These results demonstrate that CX929-treated R6/2 mice had normalized cortical BDNF levels at the time during which the behavioral tests were conducted.
Figure 1.
Cortical BDNF protein levels are elevated in R6/2 mice that received ampakine treatments. (A) Representative western blot from Cohort 2 mice (treated for 7 weeks beginning at 3 weeks of age), shows that mature (m) BDNF (~14–15 kDa) protein levels are lower in parietal cortex of vehicle (veh)-treated R6/2 mice compared WTs at 10 weeks of age and that the difference was attenuated in R6/2s given CX929 (929) (mice were killed 18 hours after the last injection). Corresponding actin immunobands from the stripped and re-probed blot is shown at bottom (n=8 for WT-CX929, n=10 for WT-Veh, n=8 for R6/2-Veh, n=9 for R6/2-CX929). (B) Representative western blot of striatal samples from a subset of Cohort 2 mice treated with vehicle or ampakine (n=3/group). (C) Densitometric analysis of western blots showed that for neocortex (black bars) mBDNF protein levels were lower in vehicle-treated R6/2 mice relative to WTs (**p = 0.002) and that CX929 treatment elevated BDNF protein levels in the R6/2s (**p = 0.01) to WT levels (p>0.05): Group mean striatal BDNF levels (grey bars) were 40% higher in R6/2 mice given CX929 versus those given vehicle (*p = 0.019). All p-values are from one-tail Student’s t-tests.
Evidence for ampakine-induced increases in cortical BDNF expression suggested that striatal BDNF protein levels, which largely reflect anterograde transport via cortical afferents (Altar et al., 1997; Conner et al., 1997; Fumagalli et al., 2007), would be increased as well. Western blots confirmed this prediction for mice treated for 7 weeks (Fig. 1B). BDNF protein levels were lower in R6/2s than in WTs given vehicle (p<0.04, one-tail t-test); CX929 treatment increased R6/2 striatal BDNF protein levels by 40% (p<0.02, one-tail t-test)(Fig. 1C).
Neuropathological changes in striatum are reduced by CX929
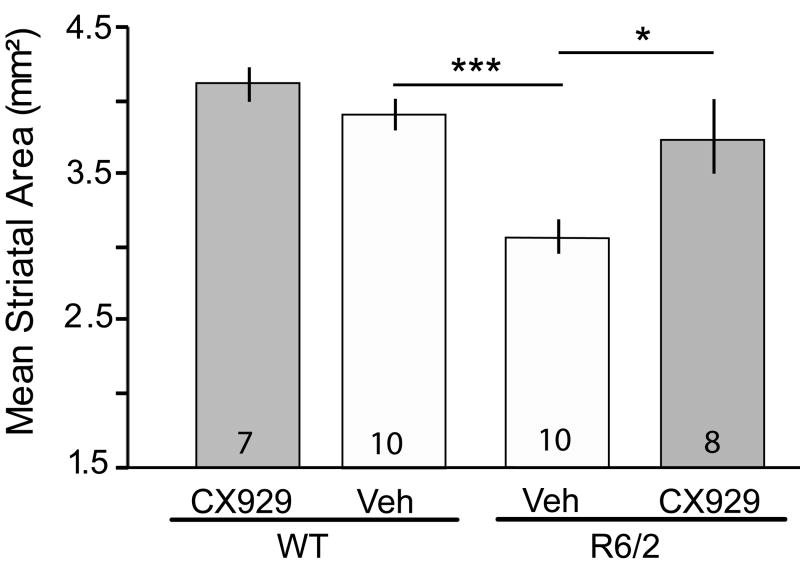
Marked atrophy of the striatum occurs in human HD patients (Vonsattel et al., 1985) as well as in R6/2 mice (Chopra et al., 2007; De March et al., 2008; Hockly et al., 2002; Stack et al., 2006). Thus, we examined whether the area of striatum was decreased in the R6/2 mice used in this study and if CX929 treatment influences this measure. There were significant effects of genotype (p= 0.0004, F=15.54) and treatment (p=0.008, F=8.17) on striatal area (two-way ANOVA; Fig. 2). R6/2 mice given vehicle exhibited a 22 ± 3% decrease in striatal area compared to vehicle-WTs (p<0.001, Student’s t-test) and this reduction was prevented by treatment with CX929 (p=0.02 vs. R6/2-vehicle mice). The decreases in striatal area in vehicle-treated R6/2s were similar to the reductions in volume seen by others (Chopra et al., 2007; De March et al., 2008; Peng et al., 2008).
Figure 2.
Striatal area is preserved in R6/2 mice given CX929 treatment. Bar graph shows that the mean cross sectional striatal area is decreased in vehicle (Veh)-treated R6/2 mice compared to similarly treated WTs (**p<0.001, Student’s t-test). This decrease was prevented by treatment with CX929 (*p=0.02 for Veh- vs. CX929-treated R6/2 mice, Student’s t-test; numbers in bars denote animals per group).
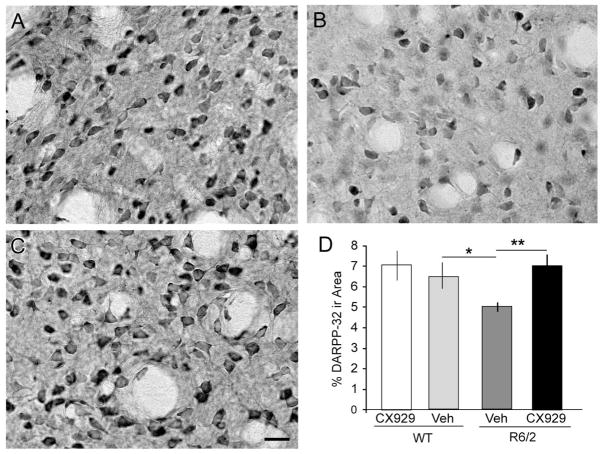
Levels of DARPP-32, a dopamine D1 receptor-activated molecule that regulates phosphatase and kinase activity, are substantially reduced in striatal medium spiny neurons in multiple HD mouse models (Bibb et al., 2000; Hickey et al., 2008; van Dellen et al., 2000). In agreement with this, DARPP-32 immunostaining was noticeably lighter in striatum of 10 week-old R6/2 mice compared to their WT littermates (Fig. 3A,B). To quantify the effects of genotype and ampakine treatment on DARPP-32-ir neurons, the proportionate area occupied by dense DARPP-32 immunolabeling was assessed for a sample region within the dorsolateral striatum. For these measures, the detection-threshold was set to capture perikaryal, but not neuropil, labeling in tissue from vehicle-treated WT mice and the parameters of image capture and analysis were held constant for tissue from all groups in the comparison. Measures demonstrated that there was a significant interaction between genotype and treatment (p=0.003, F=10.18, two-way ANOVA). The area occupied by DARPP-32-ir neurons was 23% lower in vehicle-treated R6/2 mice (p=0.02 vs. WT; Fig. 3D) as was the number of DARPP-32-positive cells (394 ± 27 vs. 344 ± 13 for vehicle-treated WT and R6/2 mice, respectively), however the latter difference did not reach statistical significance (p=0.06). Seven weeks of daily ampakine treatment (starting during the 3rd post-natal week) caused a marked increase in DARPP-32-ir neurons in the R6/2s (Fig. 3C,D): Measures of the proportion of the sample field occupied by dense immunostaining were increased by 41% over vehicle-R6/2 mice (p=0.003; Fig. 3D). Moreover, R6/2s given CX929 had significantly more immunolabeled cells (418 ± 24) than those given vehicle (p=0.01). The same 7-week ampakine treatment regimen had no detectable effect on DARPP-32 immunostaining in striatum of WT mice (vehicle vs. CX929: p=0.4 for both area and labeled cell number; Fig. 3D). It thus appears that a daily regimen of short-acting ampakine treatments potently interacts with HD-related changes in the metabolism of a critical target of dopamine signaling.
Figure 3.
Ampakine treatments offset the loss of striatal DARPP-32 in R6/2 mice. (A–C) Photomicrographs of DARPP-32 immunostaining in dorsolateral striatum of a vehicle-treated WT mouse (A) and R6/2 mice that received daily vehicle (B) or ampakine (C) injections for 7 weeks (Cohort 2). As shown, DARPP-32 immunostaining was less dense in both the neuropil and neuronal perikarya of the vehicle-treated R6/2 mouse (B) as compared to WT (A) and R6/2-CX929 treated (C) mice. Scale bar = 20 μm. (D) Bar graph shows that the proportion of the sample area occupied by dense DARPP-32 immunoreactivity (ir) was 23% lower in vehicle (Veh)-treated R6/2 mice relative to WTs and that ampakine (929) treatments eliminated the deficit in the mutants (*p=0.02 and **p=0.003 compared to R6/2 Veh, Student’s t-test; n=16 for WT-Veh, n=14 for R6/2-Veh, n=11 for R6/2-CX929, n=11 for WT-CX929).
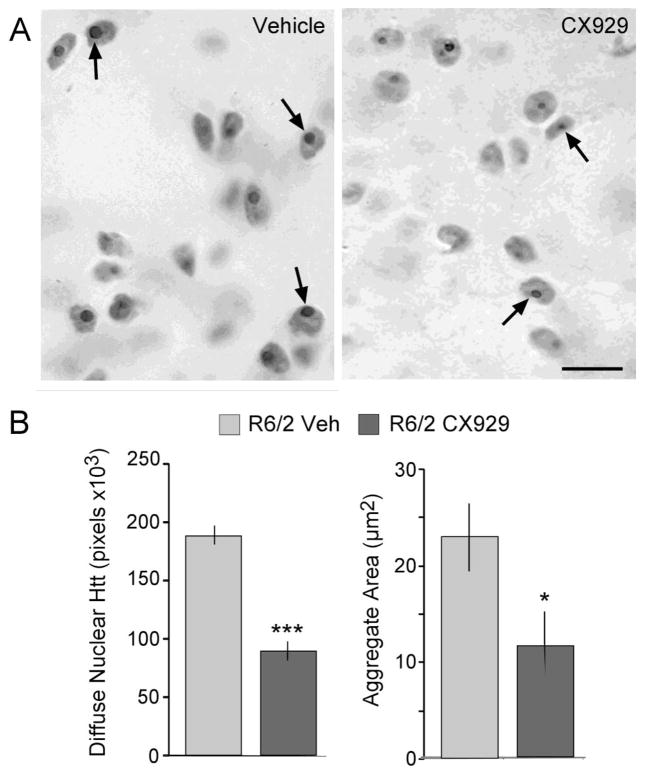
Nuclear aggregates of huntingtin (Htt) protein are hallmark pathologies of HD (Davies et al., 1997; DiFiglia et al., 1997) although their role in disease progression is not clear (Arrasate et al., 2004; Gutekunst et al., 1999; Hatters, 2008; Kuemmerle et al., 1999; Landles and Bates, 2004). Diffuse nuclear mutant htt protein and intranuclear Htt aggregates are not present in WTs but are prominent features of the R6/2 striatum starting from 3–4 weeks of age (Davies et al., 1997; Mangiarini et al., 1996). To assess effects of ampakine treatment on nuclear Htt immunoreactivity, the proportion of the dorsolateral striatum sample field occupied by (i) diffuse nuclear Htt-ir and (ii) intranuclear Htt-ir aggregates was measured in sections from R6/2 mice sacrificed after 7 weeks of daily CX929 or vehicle treatment. In transgenics treated with CX929, the area occupied by diffuse nuclear Htt-ir was 52% less than in vehicle-treated transgenics (p=0.00003; Student’s t-test; Fig. 4B, left). Similarly, immunolabeled intranuclear Htt aggregates were smaller and less intensely stained in tissue from R6/2 mice given CX929 as compared to those receiving vehicle (Fig. 4A): quantitative analyses established that in CX929-treated transgenics the mean Htt-ir aggregate area was reduced by 36% (2.39 ± 0.3 vs. 1.52 ± 0.2 μm2, for vehicle- and CX929-treated R6/2 mice, respectively; p=0.03, Student’s t-test) and the sample field area occupied by aggregates was reduced by 49% (p=0.02, Student’s t-test; Fig. 4B, right).
Figure 4.
Chronic ampakine treatment reduced intra-nuclear huntingtin levels in R6/2 mice. (A) Photomicrographs show nuclear huntingtin (Htt) immunostaining in striatum of R6/2 mice that received daily vehicle (Veh, left panel) or ampakine (right panel) injections for 7 weeks (Cohort 2). Immunostaining of intra-nuclear Htt aggregates (arrowheads) appeared smaller and less densely labeled in CX929- vs. vehicle-treated R6/2 mice (Scale bar = 5 μm). (B) Bar graphs show that in ampakine-treated, as compared to vehicle-treated, R6/2 mice the area (pixels) occupied by diffuse nuclear Htt immunoreactivity (ir) was decreased by 52% (left) whereas the area within the sample field occupied by intra-nuclear Htt aggregates was reduced by 49% (right; *p=0.02 and ***p=0.00003 for comparison to Veh-R6/2s, Student’s t-test; n=14 for Veh-R6/2, n=11 for CX929-R6/2 groups).
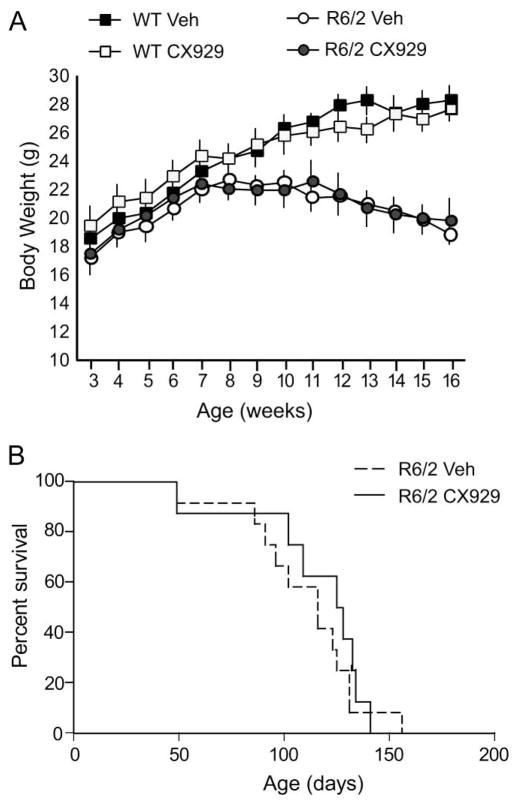
CX929 does not affect body weight or long-term survival
Body weight and survival were assessed in a subset of R6/2 mice (Cohort 3) given daily CX929 or vehicle treatment (n = 8 and 12, respectively) starting at weaning (3 weeks of age) and continuing throughout their lifespan (Fig. 5). Vehicle-treated R6/2 mice weighed significantly less than WTs by 10 weeks of age (p=0.01, Student’s t-test) and the weight difference increased thereafter. Ampakine treatment did not affect the body weight of mice of either genotype (Fig. 5A), indicating that the dose of CX929 used was well tolerated. Similarly, the ampakine did not affect the survival duration of R6/2 mice (Fig. 5B): mice given CX929 lived 16.4 ± 1.5 weeks (mean ± SD) compared to 15.7 ± 1.4 weeks for those that received vehicle (p=0.36, Student’s t-test).
Figure 5.
Body weight and survival are not affected by ampakine treatment. (A) The body weight of vehicle (Veh)-treated R6/2 mice significantly differed from WTs starting at 10 weeks of age (n=12 and 13, respectively; p=0.01, Student’s t-test) and progressively declined throughout their lifespan. CX929 treatment did not affect body weight in R6/2 or WT mice (n=15 and 11, respectively). (B) The lifespan of R6/2 mice treated with CX929 did not significantly differ from those given vehicle (Cohort 3). See text for details of statistical comparisons.
R6/2 motor performance is improved by CX929 treatment
Rotarod
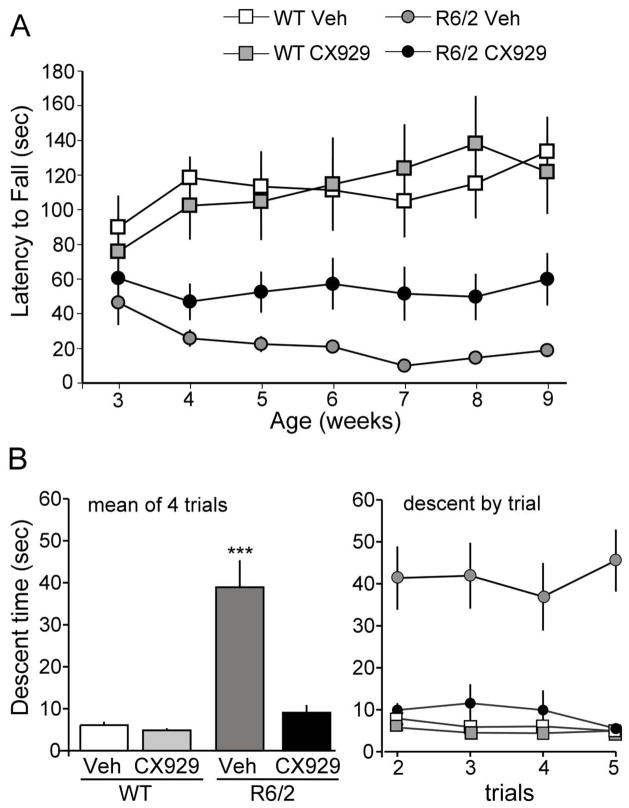
Motor performance was evaluated in separate cohorts of mice given CX929 beginning at 6 weeks of age, to evaluate effects in symptomatic mice, and at 3 weeks of age, to assess effects prior to the onset of major motor impairments. In the first group tested (Cohort 1), CX929 was administered to 6 week old R6/2 and WT mice (n=7–8/group) for a period of 4 weeks. Motor performance was assessed using a rotarod one week before (baseline) and once per week during treatment; the latter trials occurred 18 hours after the preceding ampakine injection. Vehicle-treated R6/2 mice exhibited substantial rotarod impairment by 6 weeks post-natal: pre-treatment latency-to-fall scores for WT and R6/2 mice were 69.8 ± 14.4 sec and 34.1 ± 7.7 sec, respectively (p=0.04; RM-ANOVA). After 4 weeks of treatment and testing, scores for R6/2 mice treated with CX929 were significantly higher than scores for R6/2s given vehicle (16.1 ± 6.4 sec vs. 8.5 ± 1.5 sec, respectively; p=0.011, Student’s t-test) but were well below WT values (65.9 ± 15.1 sec). This pattern also held for comparisons of mean scores for each of the 4 weeks of testing (not shown). Thus, 4 weeks of ampakine treatment, initiated after the onset of locomotor deficits, improved scores in a test of motor coordination and balance in R6/2 mice.
Following the above 4-week treatment evaluation, we conducted a larger study to determine if initiating the CX929 treatment earlier, before motor symptoms were well developed, and continuing it for a longer period, would produce more robust improvements in motor function (Cohort 2). As assessed prior to the start of treatments at 3 weeks postnatal, R6/2 mice tended to have shorter falling latencies than WTs (baseline: 54.0 ± 9.4 vs 77.1 ± 12.1 sec., respectively) but this difference did not reach statistical significance (p=0.11, Student’s t-test). Moreover, before treatment, the performance of the two groups of R6/2 mice (selected to receive vehicle or CX929 treatment) was comparable (p=0.46, Student’s t-test). As assessed over treatment weeks 5 to 9, CX929 had no detectable effect on WT performance (p>0.8). However, in R6/2 mice the ampakine treatments markedly improved rotarod times (RM-ANOVA p<0.0001, F= 9.48). In particular, by the third week of treatment, R6/2 mice given CX929 (n = 17) stayed on the rotarod 2–5 times longer than those given vehicle (n=14; RM-ANOVA p=0.02, F=5.94; Fig. 6A). Mean scores for the 5 week treatment, encompassing post-natal weeks 5–9, were 52.7 ± 15.7 sec and 16.7 ± 2.9 sec for R6/2 mice given CX929 and vehicle, respectively (p=0.007, Student’s t-test). Over the same period WT mice had a mean falling latency of 101.5 ± 12.8 sec. The enhanced motor ability of CX929- versus vehicle-treated R6/2 mice continued through the seventh weak of treatment, after which both groups of R6/2 mice began exhibiting premorbid signs.
Figure 6.
Ampakine treatments improve motor performance by R6/2 mice. (A) Rotarod scores (time on apparatus) from 3rd to 9th postnatal week in WT and R6/2 mice given vehicle (Veh) or ampakine (CX929) injections beginning during week 3 (n ≥ 10/group; Cohorts 2 and 3). Pre-morbid changes in behavior were first evident at week 10. The mean score for tests on weeks 5–9 were significantly higher in the R6/2 mice given CX929 compared to the vehicle group (52.7 ± 3.8 sec vs. 16.7 ±0.7 sec; p=0.007, Student’s t-test). However, values for the CX929-treated R6/2 group were still well below WT levels (101.5 ± 12.8 sec; p=0.01, Student’s t-test). (B) Time to descend a vertical pole in WT and R6/2 mice: both graphs show group mean descent times (± SEM) in seconds. Left plot: Mean descent times for 4 trials were far greater for vehicle-treated R6/2 mice than for WTs (p=0.0006, Student’s t-test). Seven weeks of ampakine treatment (Cohort 2) brought R6/2 performance to WT levels (R6/2-CX929 vs. combined WT, p>0.05); ***p<0.001 for comparison to both WT-Veh and R6/2-CX929 values (Student’s t-test). Right graph shows mean pole descent time per trial. Performance of vehicle-treated R6/2 mice did not improve over successive vertical pole trials; scores for ampakine-treated transgenics were comparable to scores for WTs on the last trial (p>0.05, Student’s t-test).
Clasping test
R6/2 mice exhibit a characteristic limb clasping phenotype when suspended by the tail and this response worsens with age (Mangiarini et al., 1996). Mice were tested for their latency to clasp their hindlimbs or all 4 paws when suspended at 10 weeks of age, after 4 and 7 weeks of treatment in the rotarod experiments. After 4 weeks, the latency to clasp was prolonged by about 61% in ampakine- versus vehicle-treated R6/2 mice (20.4 ± 3.6 and 12.5 ± 2.8 sec, respectively; p = 0.05, Student’s t-test). Comparable results were obtained in tests of mice treated with CX929 for 7 weeks (Cohort 2): clasping occurred after 23.7 ± 4.1 sec in the CX929 group and after 13.8 ± 2.8 sec in the vehicle group (p=0.03, Student’s t-test).
Vertical pole descent test
Recent work suggests that the sensorimotor vertical pole descent test may be particularly appropriate for assessing motor problems in mouse models of HD. Deficits on this test are evident prior to the deterioration of rotarod scores or grip strength (Hickey et al., 2008) and climbing performance is particularly sensitive to striatal damage (Matsuura et al., 1997); moreover, practiced mice rapidly complete the task with moderate intra-group variability. Accordingly, we ran additional groups of mice using 7 weeks of daily CX929 treatment beginning in the third post-natal week with testing conducted during week 9. Vehicle-treated R6/2 mice had a severe impairment in the pole descent task: WTs descended with a mean time (over 4 tests) of 6.1 ± 0.8 sec whereas vehicle-treated transgenics required 38.9 ± 6.5 sec (p=0.0006, Student’s t-test; Fig. 6B). Furthermore, 70% of vehicle-treated R6/2 mice failed to complete the task on the final trial, while all of the WTs did. Scores for ampakine-treated WTs (4.8 ± 0.5 sec) were not reliably different from those given vehicle (p=0.26, Student’s t-test). However, for R6/2 mice, the CX929 treatments had dramatic effects on the performance: Mean pole-descent times were reduced to 9.1 ± 1.8 sec, a value that was significantly less than that of matched vehicle-injected R6/2 mice (p<0.001, Student’s t-test; Fig. 6B) and not reliably different from WTs. Indeed, the only difference between WT and ampakine treated R6/2 mice was that some animals in the latter group failed to complete the task on trial numbers 3 and 4; thus, for the majority (92.5%) of the transgenics, chronic treatment with CX929 completely offset the severe impairment in vertical descent behavior.
Discussion
Successful pharmacological strategies for treating slowly developing neuropathological diseases require compounds that are not only efficacious but that also do not produce significant disturbances to brain functions when administered over long periods. The present results describe a possible route for satisfying these difficult requirements in the treatment of HD. We began with a class of compounds, ampakines, which have no known peripheral targets and that are not reported to have significant side effects in preclinical and clinical studies (Ingvar et al., 1997; Lynch, 2004). In an effort to minimize cumulative effects of drug actions over several weeks of daily treatments, these studies used a variant that has a short half-life but nevertheless influences brain BDNF expression (Rex et al., 2006; Simmons et al., 2009; Kramar et al., 2010). Thus, the treatment strategy took advantage of the short latency and temporally extended response of neurotrophin expression to excitatory activity to create circumstances in which the test drug has minimal direct effects, but produces sustained elevations in naturally occurring neurotrophin levels and signaling (Lauterborn et al., 2003; Lauterborn et al., 2009).
As assessed after four or seven weeks of daily ampakine treatments, and always ≥ 18 hours after the last injection, cortical and striatal BDNF protein levels were increased in CX929- compared to vehicle-treated R6/2 mice. Previous studies obtained comparable increases in hippocampus after twice-daily injections for 4 days with the same compound in middle-aged rats (Rex et al., 2006) and CAG140 mice (Simmons et al., 2009). We therefore conclude that this treatment regimen causes a rapidly developing, stable increase in BDNF levels in the R6/2 mouse model of HD. These results provide a first demonstration that increases in BDNF levels can be sustained for a significant portion of the lifespan, a point that is critically important with regard to strategies involving the use of enhanced BDNF trophic effects to treat chronic central nervous system disorders. Interestingly, ampakine treatment did not increase cortical BDNF protein levels in the WT mice. Although the basis for the differential genotypic response is not known, the results encourage speculation that the higher resting BDNF levels in behaviorally tested WT (as compared to R6/2) mice are near a ceiling beyond which regulatory mechanisms may limit production or levels of the neurotrophin.
Quantification of histological preparations demonstrated that semi-chronic ampakine treatments spanning 7 weeks reduced or blocked three of the most characteristic neuropathological features of HD including striatal atrophy, increases in huntingtin protein aggregates, and decreases in DARPP-32 immunoreactivity (Davies et al., 1997; Li, 1999; Gharmi, 2008). In ampakine-treated R6/2 mice, the cross sectional area of individual immunolabeled Htt aggregates was reduced by 36% whereas the proportion of the sample field encompassed by more diffuse Htt-ir was reduced by 52% (relative to vehicle-treated R6/2s). The latter value is likely to be an overestimate because CX929 treatment prevented the 22% reduction in striatal area present in vehicle-treated R6/2 mice whereas measures of aggregate size should not have been influenced by this volumetric change. A more precise quantification of the effect of treatment on overall Htt load would require measures of striatal volume, and perhaps more importantly, of both neuron volume and number to generate an index of the incidence of Htt aggregates among the surviving neuronal populations. Nevertheless, the present results indicate that the ampakine reduced the production or nuclear accumulation of toxic Htt oligomers. While the role of huntingtin nuclear inclusions in HD pathogenesis is unclear (Luo et al., 2008), these aggregates are indicators of pathological processes such as aberrant huntingtin cleavage, disrupted proteasomal activity, and protein misfolding (Gutekunst et al., 1999; Hatters, 2008; Kuemmerle et al., 1999; Landles and Bates, 2004). Furthermore, decreased aggregate formation is reported to be neuroprotective in vitro and to accompany motor improvement in in vivo HD models (Chopra et al., 2007; Dedeoglu et al., 2002; Ehrnhoefer et al., 2006; Ferrante et al., 2000). Thus, the observed protection of striatal cross-sectional area and positive effects on motor function could be related to ampakine treatment effects on huntingtin pathology.
A dramatic effect of treatment was also obtained for DARPP-32 immunostaining in striatum. Daily CX929 injections fully offset the reductions in DARPP-32 immunoreactivity otherwise evident in R6/2 mice (Bibb et al., 2000; Mangiarini et al., 1996; van Dellen et al., 2000). This effect is likely to be secondary to increases in BDNF protein content. BDNF up-regulates DARPP-32 in medium-spiny striatal neurons by activating phosphatidylinositide 3-kinase and two downstream signaling pathways (Akt/mTOR, Cdk5/p35) (Bogush et al., 2007). Moreover, BDNF transgene expression is reported to normalize striatal DARPP-32 levels, prevent reductions in striatal volume and reduce Htt inclusions in R6/1 HD model mice (Gharami et al., 2008). Thus, chronic, CX929-induced increases in BDNF protein content and trophic activity likely account for the reductions in these HD pathologies in the present work.
Most notably, ampakine treatments initiated prior to, or soon after, the appearance of significant locomotor impairments markedly reduced deficiencies in motor function in R6/2 mice. After the third postnatal week, rotarod performance deteriorated steadily in vehicle- but not ampakine-treated transgenics so that differences in group scores were pronounced after 2–3 weeks of treatment. Overall, the R6/2 mice receiving CX929 remained on the rotarod about 3 times longer than their vehicle-treated counterparts. An even more dramatic effect of ampakine treatment was obtained in the vertical pole descent test. Vehicle-treated R6/2 mice had a severe deficit on this measure at 9 weeks of age, as evidenced by the failure of 70% of the group to complete the task on the final trial. In contrast, CX929 treatment rescued task performance in the transgenics: by the final trial CX929-treated R6/2 mice performed equally well as both of the WT groups. It bears repeating that for both rotarod and vertical pole descent tests, trials were run ≥18 hours after the last injection, a time point at which the short half-life ampakine had been metabolized hours earlier but BDNF levels were still elevated. Why the ampakine had a greater positive effect on performance in the pole descent test than the rotarod is unclear. Performance on the pole descent test has been shown to reflect striatal dopamine content in Parkinson Disease model mice (Matsuura et al., 1997). The marked protective effects of the ampakine on DARPP-32 levels seen here suggests that CX929 treatments may have also attenuated the severe dopamine depletion otherwise present in R6/2 mice (Hickey et al., 2002) and, thereby, improved performance on the descent task. Moreover, the rotarod test depends on stamina and rapid motor coordination to a greater degree than the pole descent test that is primarily sensorimotor. In CAG140 knock-in HD-model mice, we found that ampakine treatment normalized the distribution of movements within an open field but did not normalize the speed or length of spontaneous movements (Simmons et al., 2009). Thus, systems coordinating more rapid movements may be less affected by ampakine treatments and increases in BDNF content. It is also possible that there are differences in stress induced by the motor tasks employed, and that differential effects of ampakine treatment reflect, in part, the anti-anxiety effects of increases in BDNF expression (Deltheil et al., 2008).
Finally, ampakine treatments had no effect on body weight or long term survival in R6/2 mice. In this regard, the effects of ampakine treatment are similar to results obtained using several other treatment regimens in which significant reductions in neuropathology and improvements in motor performance are noted despite the lack of effect on weight or lifespan (Chopra et al., 2007; Chou et al., 2005; Li et al., 2009). In exception to this pattern, an increase in lifespan has been observed with semi-chronic, systemic FGF2 treatment (Jin et al., 2005). For the ampakines, the absence of an effect on lifespan is not surprising in light of the almost exclusive action of the compounds on central glutamatergic synapses. Body weight loss in HD patients and R6/2 mice has been traced to increased metabolism. While the origins of this effect are still uncertain, R6/2 mice have abnormalities in brown adipose tissue and blood borne signaling factors that influence metabolic rate (van der Burg et al., 2008) and a tendency to develop diabetes (Hunt and Morton, 2005). It is thus most likely that the weight loss and other signs of generalized deterioration are of peripheral origin and not responsive to ampakine-driven increases in neurotrophin signaling in brain regions controlling complex movements. It is of interest in this regard that weight loss does not correlate with various motor and cognitive scores in HD patients.
In summary, daily treatments with a short-lived ampakine are sufficient to markedly reduce HD-related pathologies in striatum and these changes are accompanied by equally pronounced improvements in a striatum-dependent motor task. The treatments did not offset the body weight losses characteristic of R6/2 mice, which may explain, in part, their incomplete restoration of motor coordination. Future studies will extend the analysis of ampakine effects to other measures of HD pathology (e.g., neuropeptide expression changes, striatal volume) and test if newer, more potent ampakines can further alleviate motor impairments and normalize spontaneous movements in both R6/2 mice and in mouse models expressing full-length mutant huntingtin which have more slowly emerging pathology (Menalled and Chesselet, 2002). Finally, it remains to be determined if the short-acting drug strategy used here can be employed in other models of neurodegenerative diseases and if there are development problems associated with clinical use of very short half-life ampakines. More generally, the evolution of this strategy will require additional evidence on the extent to which BDNF or its relatives are responsible for the neuroprotection described here and, if so, how they produce such effects.
Acknowledgments
The authors thank Cortex Pharmaceuticals Inc. for providing CX929, Dr. Malcolm Casale for helpful discussions and Jihua Liu for assistance with Western blots. We also thank Brittany Parker, Yong Park, Maria Sanchez, Lindsay Peltz, and Son Do for help with behavioral experiments. Drs. Lauterborn, Gall and Lynch hold a patent for the use of ampakines to increase BDNF expression in vivo. This research was supported by grants from the National Institute of Neurological Disorders and Stroke (NS051823 and NS045260) and Cortex Pharmaceuticals, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, Nairn AC, Messer A, Greengard P. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc Natl Acad Sci U S A. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogush A, Pedrini S, Pelta-Heller J, Chan T, Yang Q, Mao Z, Sluzas E, Gieringer T, Ehrlich ME. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem. 2007;282:7352–7359. doi: 10.1074/jbc.M606508200. [DOI] [PubMed] [Google Scholar]
- Bramham C, Messaoud E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Lione L, Humby T, Mangiarini L, Mahal A, Bates G, Dunnett S, Morton A. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JH. Transcriptional dysregulation in Huntington's disease. Trends Neurosci. 2000;23:387–392. doi: 10.1016/s0166-2236(00)01609-x. [DOI] [PubMed] [Google Scholar]
- Chopra V, Fox JH, Lieberman G, Dorsey K, Matson W, Waldmeier P, Housman DE, Kazantsev A, Young AB, Hersch S. A small-molecule therapeutic lead for Huntington's disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse. Proc Natl Acad Sci U S A. 2007;104:16685–16689. doi: 10.1073/pnas.0707842104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, Lee YC, Chen HM, Chiang MC, Lai HL, Chang HH, Wu YC, Sun CN, Chien CL, Lin YS, Wang SC, Tung YY, Chang C, Chern Y. CGS21680 attenuates symptoms of Huntington's disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and messenger RNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Turmaine M, Cozens B, DiFiglia M, Sharp A, Ross C, Scherzinger E, Wanker E, Mangiarini L, Bates G. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Reperant C, Guilloux JP, Coudore F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharm. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- De March Z, Zuccato C, Giampà C, Patassini S, Bari M, Gasperi V, De Ceballos ML, Bernardi G, Maccarrone M, Cattaneo E, Fusco FR. Cortical expression of brain derived neurotrophic factor and type-1 cannabinoid receptor after striatal excitotoxic lesions. Neuroscience. 2008;152:734–40. doi: 10.1016/j.neuroscience.2007.11.044. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, Davies S, Bates G, Vonsattel J, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Genet. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Gall C, Lauterborn J. Regulation of BDNF Expression: Multifaceted, region-specific control of a neuronal survival factor in the adult CNS. In: IM, editor. Neurobiology of the Neurotrophins. Johnson City, TN: FP Graham Publishing Co; 2000. pp. 541–579. [Google Scholar]
- Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol. 2009;168:153–157. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters DM. Protein misfolding inside cells: the case of huntingtin and Huntington's disease. IUBMB Life. 2008;60:724–728. doi: 10.1002/iub.111. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Reynolds GP, Morton AJ. The role of dopamine in motor symptoms in the R6/2 transgenic mouse model of Huntington's disease. J Neurochem. 2002;81:46–59. doi: 10.1046/j.1471-4159.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157:280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockly E, Cordery PM, Woodman B, Mahal A, van Dellen A, Blakemore C, Lewis CM, Hannan AJ, Bates GP. Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann Neurol. 2002;51:235–42. doi: 10.1002/ana.10094. [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Morton AJ. Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington's disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res. 2005;166:220–229. doi: 10.1007/s00221-005-2357-z. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington's disease. Ann Neurol. 1999;46:842–849. [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Lauterborn JS, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington's disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5:958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn J, Truong G, Baudry M, Bi X, Lynch G, Gall C. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience. 2009;159:283–295. doi: 10.1016/j.neuroscience.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Li M, Huang Y, Ma AA, Lin E, Diamond MI. Y-27632 improves rotarod performance and reduces huntingtin levels in R6/2 mice. Neurobiol Dis. 2009;36:413–420. doi: 10.1016/j.nbd.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Li XJ. The early cellular pathology of Huntington's disease. Mol Neurobiol. 1999;20:111–124. doi: 10.1007/BF02742437. [DOI] [PubMed] [Google Scholar]
- Luo S, Mizuta H, Rubinsztein DC. p21-activated kinase 1 promotes soluble mutant huntingtin self-interaction and enhances toxicity. Hum Mol Genet. 2008;17:895–905. doi: 10.1093/hmg/ddm362. [DOI] [PubMed] [Google Scholar]
- Lynch G. AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6:82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. LTP consolidation: substrates, explanatory power, and functional significance. Neuropharmacology. 2007a;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J Neurosci. 2007b;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S, Bates G. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Menalled L, El-Khodor BF, Patry M, Suarez-Farinas M, Orenstein SJ, Zahasky B, Leahy C, Wheeler V, Yang XW, MacDonald M, Morton AJ, Bates G, Leeds J, Park L, Howland D, Signer E, Tobin A, Brunner D. Systematic behavioral evaluation of Huntington's disease transgenic and knock-in mouse models. Neurobiol Dis. 2009;35:319–336. doi: 10.1016/j.nbd.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Chesselet MF. Mouse models of Huntington's disease. Trends Pharmacol Sci. 2002;23:32–39. doi: 10.1016/s0165-6147(00)01884-8. [DOI] [PubMed] [Google Scholar]
- Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, Vandergriff JL, Baumbarger P, Siuda E, Gates M, Ogden AM, Skolnick P, Zimmerman DM, Nisenbaum ES, Bleakman D, O'Neill MJ. LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2003;306:752–762. doi: 10.1124/jpet.103.049445. [DOI] [PubMed] [Google Scholar]
- Pallier PN, Drew CJ, Morton AJ. The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington's disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull. 2009;78:347–355. doi: 10.1016/j.brainresbull.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, Duan W. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol. 2008;210:154–63. doi: 10.1016/j.expneurol.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington's disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Smith KM, Ryu H, Cormier K, Chen M, Hagerty SW, Del Signore SJ, Cudkowicz ME, Friedlander RM, Ferrante RJ. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington's disease mice. Biochim Biophys Acta. 2006;1762:373–80. doi: 10.1016/j.bbadis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19:233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington's in mice. Nature. 2000;404:721–722. doi: 10.1038/35008142. [DOI] [PubMed] [Google Scholar]
- van der Burg JM, Bacos K, Wood NI, Lindqvist A, Wierup N, Woodman B, Wamsteeker JI, Smith R, Deierborg T, Kuhar MJ, Bates GP, Mulder H, Erlanson-Albertsson C, Morton AJ, Brundin P, Petersen A, Bjorkqvist M. Increased metabolism in the R6/2 mouse model of Huntington's disease. Neurobiol Dis. 2008;29:41–51. doi: 10.1016/j.nbd.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Vonsattel J, Myers R, Stevens T, Ferrante R, Bird E, Richardson EJ. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, Valenza M, Cattaneo E. Progressive loss of BDNF in a mouse model of Huntington's disease and rescue by BDNF delivery. Pharmacol Res. 2005;52:133–139. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]