Abstract
We report herein that oxidation of a mitochondria-specific phospholipid tetralinoleoyl cardiolipin (L4CL) by cytochrome c and H2O2 leads to the formation of 4-hydroxy-2-nonenal (4-HNE) via a novel chemical mechanism which involves cross-chain peroxyl radical addition and decomposition. As one of the most bioactive lipid electrophiles, 4-HNE possesses diverse biological activities ranging from modulation of multiple signal transduction pathways to the induction of intrinsic apoptosis. However, where and how 4-HNE is formed in vivo is much less understood. Recently a novel chemical mechanism has been proposed that involves inter-molecular dimerization of fatty acids by peroxyl bond formation; but the biological relevance of this mechanism is unknown because a majority of the fatty acids are esterified in phospholipids in the cellular membrane. We hypothesize that oxidation of cardiolipins, especially L4CL, may lead to the formation of 4-HNE via this novel mechanism. We employed L4CL and di-linoleoyl-phosphatidylcholine (DLPC) as model compounds to test this hypothesis. Indeed, in experiments designed to assess the intramolecular mechanism, more 4-HNE is formed from L4CL and DLPC oxidation than 1-palmitoyl-2-linoleoyl-phosphatydylcholine (PLPC). The key products and intermediates that are consistent with this proposed mechanism of 4-HNE formation have been identified using liquid chromatography – mass spectrometry (LC-MS) methods. Identical products from cardiolipin oxidation were identified in vivo in rat liver tissue after carbon tetrachloride treatment. Our studies provide the first evidence in vitro and in vivo for the formation 4-HNE from cardiolipin oxidation via cross-chain peroxyl radical addition and decomposition, which may have implications in apoptosis and other biological activities of 4-HNE.
Keywords: 4-hydroxy-2-nonenal (4-HNE), cardiolipin, cytochrome c, LC-MS, lipid peroxidation, free radicals, mitochondria, apoptosis
Introduction
Free radical-induced oxidation products of polyunsaturated fatty acids (PUFAs) have been implicated as a major upstream component in signal transduction cascades in many cellular functions including apoptosis, proliferation, inflammatory responses, stimulating adhesion molecules, and chemoattractant production [1–3]. Fatty acid hydroperoxides are the primary products in enzymatic and non-enzymatic oxidation of fatty acids. These lipid hydroperoxides not only have potent biological activities related to human physiology and pathophysiology, but also can serve as precursors to form other highly oxidized lipid oxidation products or decompose to generate an array of reactive lipid electrophiles [4]. Over the past two decades, 4-hydroxy-2-nonenal (4-HNE) has become one of the most studied reactive lipid electrophiles [5–9]. As a highly reactive α, β-unsaturated aldehyde, 4-HNE exhibits a variety of biological activities including inhibition of protein and DNA synthesis and inactivation of enzymes. 4-HNE can also form protein adducts with amino acid residues such as cysteine, histidine, arginine, and lysine due to its strong electrophilic character [10–12]. Moreover, the presence of these protein adducts can serve as biomarkers for lipid peroxidation and oxidative stress [5]. 4-HNE can also trigger multiple signaling events in a physiological context [8].
In contrast to the biology of 4-HNE, the mechanisms for its formation are much less understood [13–15]. Several mechanisms have been proposed that may account for the formation of 4-HNE, which includes free radical-induced decomposition of lipid hydroperoxides such as 13-hydroperoxyoctadecadienoic acid (13-HpODE) or 15-hydroxyeicosatetraenoic acid (15-HETE) [16, 17]. Ferrous iron (Fe2+) or vitamin C induced decomposition of lipid hydroperoxides have also been proposed [18, 19]. Recently, an alternative mechanism was suggested, which involves an intermolecular cross-linking of a peroxyl radical [13, 17]. This mechanism has precedence in styrene-oxygen polymerization and depolymerization [20]. There is also evidence to support the decomposition of these cross-linked peroxides of linoleic acid as initiators of free radical lipid oxidation and formation of reactive lipid aldehydes in vitro [21–25]. It should be noted that all these studies were carried out using linoleic acid or methyl ester as models and thus the biological relevance of this mechanism remains unclear because a majority of linoleic acids are esterified on phospholipids in cellular membranes.
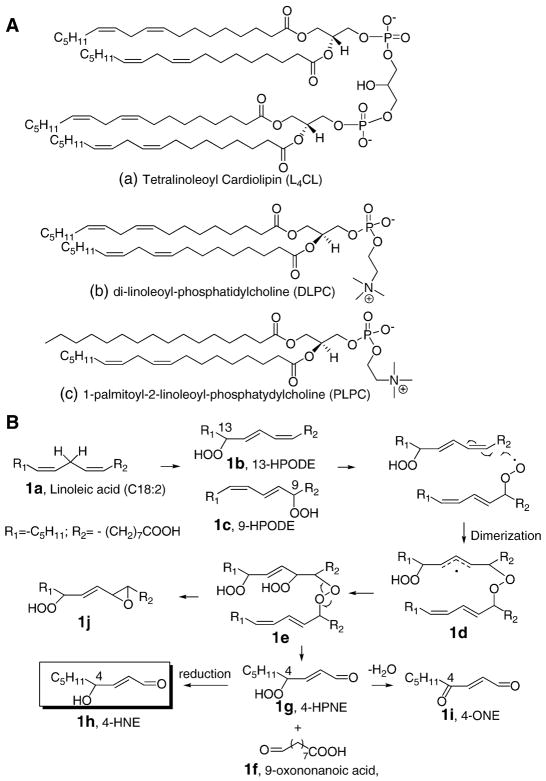
Cardiolipin (CL) is a unique class of phospholipids containing four fatty acyl side chains and three glycerol moieties (Figure 1A). In most mammalian tissues the predominant form of CL is tetralinoleoyl CL (L4CL), and typically the distribution of linoleate mitochondrial CL is around 85–90 % [26–28]. Recently, cardiolipin oxidation has attracted much attention due to the fact that it is involved in regulation of programmed cell death (apoptosis) initiated in mitochondria [29]. During apoptosis, CL interacts with cytochrome c to form a peroxidase complex that catalyzes CL oxidation and accumulating evidence indicates that the oxidation products of CL play a critical role in the mitochondrial stage of the execution of the cell death program [30, 31].
Fig. 1. Chemical structures of linoleoyl-phospholipids and proposed chemical mechanism for 4-HNE formation.
(A) Chemical structures of L4CL, DLPC, and PLPC. (B) Formation of 4-HNE from dimeric peroxides of two linoleic acids under free radical conditions. Note: other isomers of 1e can be formed during the process, but only the structures that are relevant to the proposed mechanism are illustrated for simplicity.
We hypothesize that L4CL is the best model compound with biological relevance to test the idea of inter-chain peroxyl radical addition and decomposition to form 4-HNE. In this case, the addition of a peroxyl radical on one linoleic acid to another fatty acid side chain occurs intra-molecularly in L4CL instead of inter-molecular addition in the model systems that have been studied previously. Moreover, the intra-molecular addition of a peroxyl radical in L4CL is favored kinetically over the inter-molecular addition. Due to the complexity of the peroxidation of L4CL, we first use DLPC as a simplified model to test this idea. A number of mass spectrometry (MS) - based techniques have been employed to study the formation of 4-HNE from free radical oxidation of DLPC and L4CL, decomposition products and their postulated dimeric precursor have also been identified using state-of–the-art MS methods. Furthermore, we identified the key products with epoxyalcohol on one side chain and truncated aldehyde on the adjacent side chains in vivo in rat liver tissue after treatment with carbon tetrachloride. Our data are consistent with the concept that intramolecular cross-chain peroxyl radical addition and decomposition may contribute significantly to the enhanced peroxiditon of CL and 4-HNE formation. These findings link CL oxidation and 4-HNE formation to mitochondrial dysfunction, apoptosis, and other biological activities in mitochondria caused by oxidative stress and free radical lipid peroxidation.
EXPERIMENTAL PROCEDURES
Reagents
Phospholipids, 1-palmitoyl-2-oleoyl-3-phosphatidylcholine (POPC), 1-palmitoyl-2-linoleoyl-3-phosphatidylcholine (PLPC), 1,2-dilinoleoyl-3-phosphatidylcholine (DLPC), tetraoleoyl cardiolipin (O4CL) were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Bovine heart cardiolipin was purchased as a chloroform solution and purified by HPLC method described below. Bovine heart cytochrome c, diethylenetriaminepentaacetic acid (DTPA), dinitrophenylhydrazine (DNPH), and soy bean lipoxygenase were purchased from Sigma-Aldrich Chemical Company (Milwaukee, WI). HPLC quality solvents, such as methanol, water, 2-propanol, and acetonitrile were purchased from either Fisher Chemical (Phillipsburg, NJ) or EM Science (Gibbstown, NJ). The free radical azo initiator 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (MeOAMVN) and C-0 (VA-044) were generously donated by Wako Chemicals USA Inc. (Richmond, VA). 4-HNE, 4-oxo-2-nonenal (4-ONE), 4-hydroperoxy-2-nonenal (4-HPNE), and 4-HNE-d3 were purchased from Cayman Chemicals (Ann Arbor, MI) and used without further purification. All animal procedures were performed in accordance with institutional guidelines after approval from the Animal Care and Use Committee of Vanderbilt University.
Purification of L4CL from bovine heart cardiolipin
Bovine heart cardiolipin contains about 80% L4CL. HPLC was carried out on a Waters Model 600E pump with a Waters 996 Photodiode array detector. Millenium32 chromatography software (Waters Corp., Milford, MA) was used to control the array detector and to collect and process data. Purification of L4CL was performed on a Phenomenex (Torrence, CA) Luna C8 column (3μm, 4.6 × 150 mm) with a flow rate of 1.0 ml/min. Mobile phase A contained MeOH: CH3CN: NH4Ac (2mM) = 60:20:20 (v:v:v) while mobile phase B was methanol. The gradient started with 100% A and increased to 100% B in 15 min and held for 5 min before it returned to 100% A. L4CL eluted from 18 to 20 min under this condition. The purity of L4CL was analyzed by LC-MS method described below. The stocked solution of the purified L4CL was stored at −80ºC under argon.
Oxidation of PLPC, DLPC, and L4CL in liposomes with free radical azo initiator C-0
PLPC, DLPC, L4CL, and POPC were dissolved in chloroform as stock solutions. Appropriate volumes of the stock solution were added into separate glass vials to make final solution of 0.6, 0.3, and 0.15 mM of PLPC, DLPC and L4CL respectively. A constant concentration of 14.4 mM of POPC was added to each vial before the chloroform solutions were evaporated by a stream of nitrogen. PBS (50 mM, pH 7.4) with 100 μM of DTPA was added to make the above concentrations for the phospholipids. After the lipid mixture was vortexed and sonicated for 10 min under nitrogen in water, 0.06 mM of C-0 in PBS was added to the mixture immediately to initiate oxidation. The reaction mixture was incubated at 37 °C under air.
Oxidation of L4CL with cytochrome c and hydrogen peroxide
Oxidation of CL by cytochrome c and H2O2 was carried out based on a published protocol [29]. CL and POPC stored in chloroform were added to a glass vial and the solvent was removed by a flow of nitrogen. PBS (50 mM, pH 7.4) with 100μM DTPA was added and the lipid mixture was vortexed and sonicated for 10 min under nitrogen in water. Then to the lipid mixture were added cytochrome c and H2O2 at 37°C under air. H2O2 was added four times (every 15 min) during the incubation period. The final reaction mixture contained 250 μM CL, 250 μM POPC, 20 μM cytochrome c, and 100 μM H2O2. Oxidation of PLPC and DLPC using the cyt c/H2O2 system was carried out in the presence of O4CL.
Synthesis of phospholipid hydroperoxides
A previously published protocol was used to synthesize PLPC and DLPC hydroperoxides [32]. Soybean lipoxygenase (20 mg) was dissolved in 30 ml of borate buffer that contained 10 mM deoxycholate at pH = 9.0. PLPC (25 mg) or DLPC (20 mg) was taken up in 3 ml of the same buffer and added to the enzyme solution. The reaction was stirred at room temperature for 30–60 min. The reaction was monitored by measuring UV absorbance at 234 nm. The reaction mixtures were extracted with chloroform: methanol (2:1, v/v) three times. The combined organic phase was evaporated and reconstituted with methanol. The hydroperoxide was further purified by reverse phase HPLC using a Discovery C18 HPLC column (Supelco, Bellefonte, PA; 25 cm × 21.2 mm, 5 μm) equilibrated with methanol/water (90/10) at a flow rate of 15 ml/min. The detection was monitored by the absorbance at 234 nm.
Mass Spectrometry
Oxidized phospholipids were separated online by UPLC using a Waters Acquity UPLC system (Waters Corp., Milford, MA) with a Phenomenex Luna 3μ C8 column (150 × 4.6 mm) at a flow rate of 1.0 ml/min and a flow splitter which sent approximately 20% of the mobile phase to the mass spectrometer. LC separation was performed using the same gradient that was used to purify L4CL from bovine heart cardiolipin. Mass spectrometry analysis was carried out on Finnigan LTQ Iontrap mass spectrometer or Thermo Quantum Ultra triple quadrupole mass spectrometer. The ESI source was fitted with a stainless steel capillary (100 μm inner diameter). Mass spectrometer was operated in the negative ion mode for structural identification while quantitative analysis was carried out using selective reaction monitoring (SRM) in positive mode for some oxidized PC. Analysis of cardiolipins was performed in the negative ion mode. Nitrogen was used as the sheath gas at 38 p.s.i. The capillary temperature was 350 °C. The spray voltage was 4.5 kV, and the tube lens voltage was 100 V. Spectra were displayed by averaging scans across chromatographic peaks. Data acquisition and analysis were performed using Xcaliber software, version 2.0.
Analysis of 4-HNE, 4-HPNE, and 4-ONE by LC-MS method
Analysis of 4-HNE, 4-HPNE, and 4-ONE was carried out using a modified literature method [33]. Briefly, phospholipid oxidation mixtures were extracted by CHCl3/MeOH (2:1, v/v) and 4-HNE-d3 was added as the internal standard. After the solvent was removed by a flow of nitrogen, 0.5 ml of saturated DNPH solution containing 1N HCl was added to the residue and stored in the dark at room temperature for 2 h. DNPH derivatives were extracted twice from the mixture with CH2Cl2 and evaporated to dryness under a stream of nitrogen. The sample was reconstituted with MeOH/water (3:1, v/v). LC-MS/MS was conducted using a Thermofinnigan TSQ Quantum Ultra equipped with a Finnigan Surveyor Autosampler Plus. Reverse phase HPLC was performed using a Phenomenex Luna C18 column (250 × 4.6 mm, 5 μm) with an isocratic elution of MeOH/water (3:1, v/v) at a flow rate of 1 ml/min. The MS was operated in the negative ion mode using electrospray ionization (ESI) in the selective reaction monitoring (SRM) mode. MS parameters were optimized for 4-HNE-DNPH derivative and were as follows: auxiliary gas pressure was set at 45 psi, sheath gas pressure was 50 psi, utilizing nitrogen for both. The vaporizer temperature was set at 400 °C. Collision induced dissociation (CID) for the 4-HNE-DNPH, 4-ONE-DNPH and 4-HpNE-DNPH were optimized at 25, 24 and 24 eV respectively under 1.0 mTorr of argon. Data acquisition and analysis were performed using Xcaliber software, version 2.0 (San Jose, CA).
Identification of cadiolipin oxidation products that are consistent with this novel pathway in vivo
After intragastric administration of CCl4 (2 mg/kg) in corn oil to Sprague-Dawley rats for two hours, the animals were anesthetized with pentobarbital (60 mg/kg) intraperitoneally and sacrificed, and the livers were harvested. Around 1 gram of liver tissue was homogenized in a mixture of chloroform and methanol (2:1) with triphenylphosphine and BHT. The organic phase was evaporated and reconstituted in methanol for LC-MS analysis.
Statistical Analysis
Data are presented as the mean ± SEM. Statistical analysis was performed using analysis of variance followed by Student’s t test. Differences were considered significant when p < 0.05.
RESULTS
Proposed mechanism of 4-HNE formation: Peroxyl radical addition to conjugated dienes and peroxide decomposition
Despite the substantial efforts to study the biological activities of 4-HNE, there is a lack of understanding of the mechanisms of its formation in vivo. Evidence provided here suggests that free radical-induced 4-HNE formation from PUFA may be due to peroxyl radical addition to a conjugated diene followed by the decomposition of the cross-linked peroxides due to the instability of these intermediates [17]. As shown in Figure 1B, linoleic acid 1a is one of the major fatty acids that serve as the precursor for 4-HNE. 13-HPODE 1b and 9-HPODE 1c are the two primary oxidation products formed under free radical conditions. In this mechanism, a peroxyl radical from one of the hydroperoxides adds to the conjugated double bond of another hydroperoxide to form compound 1e after addition of O2 and hydrogen atom abstraction. This dimeric hydroperoxide has limited stability and is proposed to decompose to give 9-oxononanoic acid 1f and 4-HPNE 1g; the latter can be reduced to form 4-HNE 1h or dehydrated to generate 4-ONE 1i. The epoxide hydroperoxide 1j is also formed on the other fatty acid in this mechanism. It is of some interest that the linoleic acid-containing L4CL may be the best source for the 4-HNE formation by this mechanism because this CL contains four linoleic acids in the same molecule. The peroxyl radical addition reaction is therefore intra-molecular in this instance since it occurs between pendant chains of the caridolipin.
In order to simplify a model study, we first compared oxidation of DLPC, where peroxyl radical addition could occur intramolecularly with PLPC, a compound that requires that addition occur intermolecularly. These studies were carried out in liposomes diluted with an inert phospholipid such that the concentration of linoleate in the liposome was comparable for the two substrates. That is, the mole fraction of PLPC used in these test reactions was twice that of DLPC used in parallel experiments.
More 4-HNE is formed from the oxidation of DLPC and L4CL than PLPC
Based on the mechanism illustrated in Figure 1B, more 4-HNE is expected to be formed from DLPC and L4CL than from PLPC in the oxidation of liposomes containing equivalent mole fractions of linoleate ester. We developed an LC-MS method to simultaneously quantify the levels of 4-HNE, 4-HPNE and 4-oxononenal (4-ONE) from the oxidation of different phospholipids. As shown in supplement Figure S1, SRM experiments were carried out on the derivatives of 4-ONE, 4-HPNE and 4-HNE after derivatization with 2,6-dintrophenylhydrazine (DNPH). A deuterated internal standard of 4-HNE was used to quantify these compounds. As shown in Figure 2, significantly higher levels of 4-HNE are formed in the oxidation of DLPC and L4CL than from PLPC using a free radical initiator. Similar results are obtained for the formation of 4-HPNE and 4-ONE (data not shown). It is noteworthy that when the oxidation was carried out with either an azo-free radical initiator or cytochrome c/H2O2 systems, similar results were observed, i.e., the amounts of 4-HNE, L4CL > DLPC > PLPC. These data suggest that phospholipids containing two or more linoleate fatty acids in the same molecule generate more 4-HNE and related compounds under free radical conditions, consistent with the mechanism proposed in Figure 1B.
Fig. 2. Levels of 4-HNE from free radical-induced oxidation of PLPC, DLPC and L4CL.
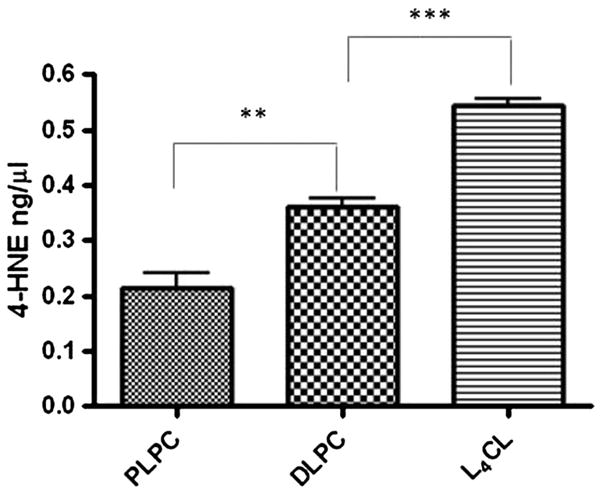
The reaction was carried out with 0.15, 0.3, 0.6 mM of L4CL, DLPC and PLPC respectively in 14.4 mM of POPC. Analysis of 4-HNE was performed by LC-MS after derivatization with DNPH. **, p< 0.01; ***, p< 0.001. The phospholipids were oxidized using free radical initiator but similar results were observed when the oxidation was carried out using cytochrome c and H2O2.
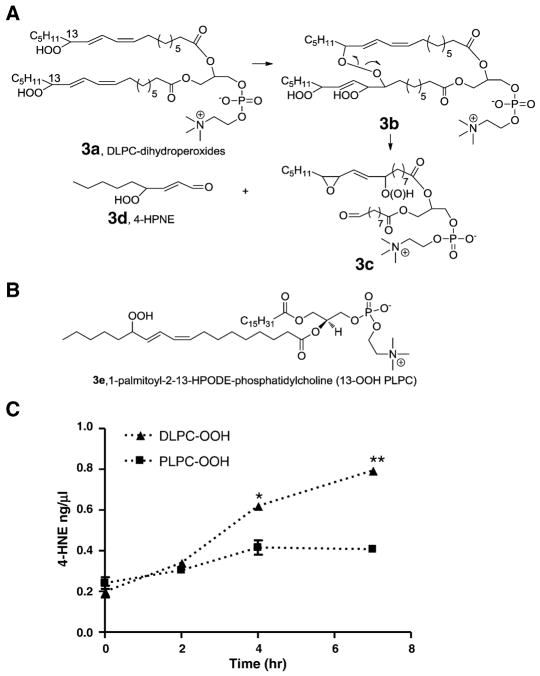
DLPC dihydroperoxide is a valid model to identify the key products and precursors for 4-HNE formation
In addition to the formation of 4-HNE and related compounds, epoxy-alcohol or epoxyhydroperoxides are predicted products from the decomposition of the dimeric intermediates [17]. We propose that DLPC dihydroperoxides 3a (Figure 3) can be used as a simplified model to test this mechanism. As shown in Figure 3A, dihydroperoxides DLPC 3a form a cross-linked peroxide 3b which readily decomposes to form two alkoxyl radicals. The alkoxyl radical on sn-1 cyclizes to form epoxy-hydroperoxide or hydroxide 3c whereas the alkoxyl radical on sn-2 undergoes β-cleavage to form an aldehyde on C9 and 4-HPNE. It should be noted that other isomers can be formed during the process, but only the structures that are relevant to the proposed mechanism are illustrated for simplicity.
Fig 3. Formation of 4-HNE from phospholipid hydroperoxides.
(A) Pathways for 4-HNE formation from oxidation of DLPC hydroperoxides via cross-linked two side chains. (B) Chemical structure of 13-HPODE-PLPC. (C) Time course of 4-HNE formation from decomposition of DLPC and PLPC hydroperoxides. *, p< 0.05; **, p< 0.01, relative amounts of 4-HNE from DLPC vs PLPC. The phospholipid hydroperoxides were synthesized by soy bean lipoxygenase and further oxidation was carried out using free radical initiator.
In order to show that DLPC dihydroperoxide 3a can be used as a valid model to test this mechanism, we synthesized DLPC dihydroperoxide 3a and PLPC hydroperoxide 3e by the use of soybean lipoxygenase. These two precursor hydroperoxides were diluted in an unoxidizable phospholipid POPC (2% for DLPC dihydroperoxide and 4% for PLPC hydroperoxides respectively) and decomposed as a thin film in the presence of an azo free radical initiator MeOAMVN. Time-dependent formation of 4-HNE was observed and significantly more 4-HNE was generated from decomposition of DLPC dihydroperoxide 3a than was formed from PLPC hydroperoxide 3e (Figure 3C). Similar results were obtained for 4-HPNE and 4-ONE (Figure S2). Even though the hydrogen atoms in OOH are more easily abstracted than those at the bis-allylic positions of un-oxidized phospholipids, the data shown in Figure 3c suggest that DLPC-dihydroperoxide 3a offers a valid model for study of the proposed mechanism when the levels of 4-HNE from these two hydroperoxides are compared.
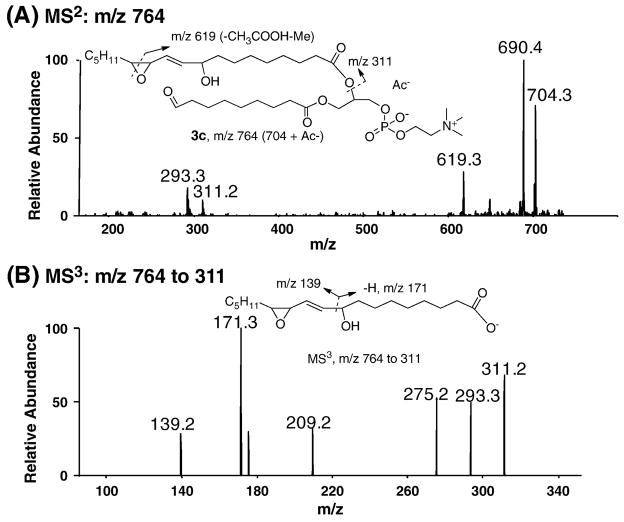
Identification of the critical decomposition products epoxy-alcohol and epoxy-hydroperoxide 3c from DLPC dihydroperoxide
The reaction mixture of DLPC dihydroperoxide taken at the 7hr time point was treated with PPh3 and analyzed by LC-Iontrap MS. PC species are readily ionized in the positive ion mode MS but only limited structural information can be obtained in this way because the fragmentation from CID only leads to a prominent ion of the choline head group with m/z 184. Thus, the acetate (Ac−) adduct of compound 3c (m/z 764) was subjected to CID in the negative ion mode. The results are illustrated in Figure 4. MS2 of m/z 764 gives a fragment of m/z 704 which represents the loss of acetic acid whereas a fragment of m/z 690 corresponds to the further loss of a methyl group on the choline head group [34, 35]. A characteristic fragment of m/z 311 is consistent with the carboxylate of the epoxyalcohol side chain. Dehydration of m/z 311 gives rise to m/z 293. MS3 of m/z 311 is shown in Figure 4B. The fragments with m/z 171 and 139 are indicative of the epoxyalcohol moiety in compound 3c. Taken together, these data support the notion that decomposition of DLPC dihydroperoxide 3a generates the key product epoxyalcohol 3c, a conclusion that is consistent with the formation 4-HNE through the dimerization mechanism.
Fig 4. Mass spectra of epoxyalcohol 3c from decomposition of DLPC hydroperoxides analyzed by LC-Iontrap MS.
(A) MS2 of acetate adduct of compound 3c, m/z 764. (B) MS3 of m/z 764 to 311. The oxidation mixture used was from the experiment described in Figure 3.
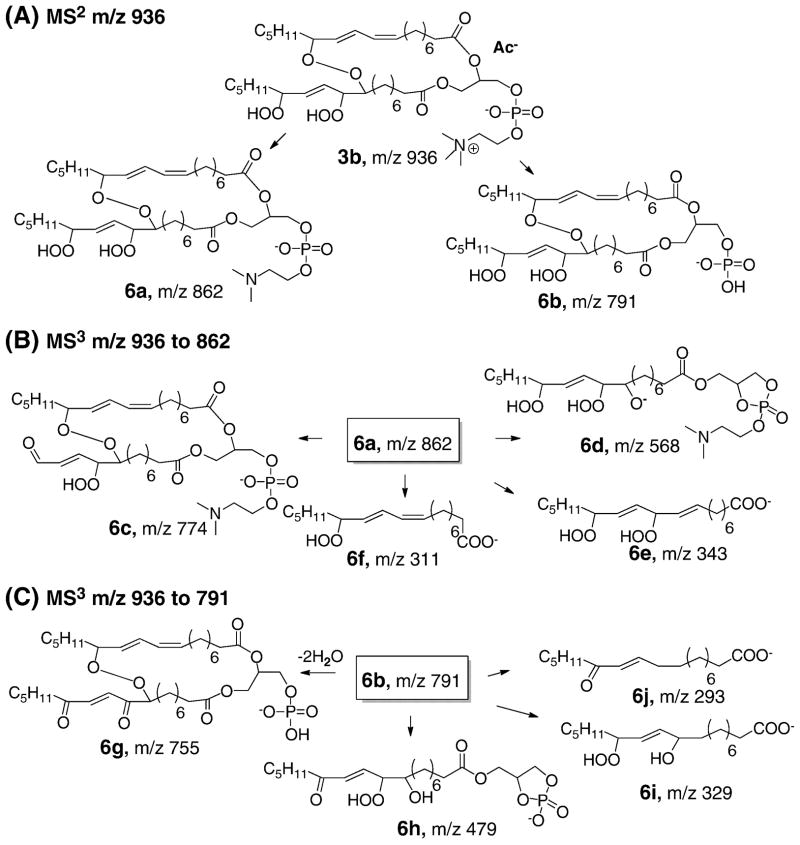
Identification of the intact cross-linked peroxides 3b
In order to identify the cross-linked peroxide precursors 3b, we fractionated the reaction mixture derived from oxidation of DLPC dihydroperoxide by HPLC. The separated fractions were evaporated and let stand as a thin film for two hours to determine whether they contained 4-HNE precursors. Six fractions were collected and the levels of 4-HNE and epoxyalcohol 3c were analyzed before and after the two-hour incubation. The results are summarized in supplement Figure S3. Two fractions had elevated levels of 4-HNE and 3c after further decomposition, which suggested that cross-linked peroxides 3b eluted in these fractions. Structural elucidation of this compound was carried out by LC-ESI-Iontrap MS experiments. The analyses are summarized in Figure 5 and the proposed fragmentation pathways are shown in Figure 6. Multi-stage CID experiments were employed to characterize the proposed structure 3b in the negative mode of MS. MS2 carried out on the acetate adduct of 3b with m/z 936 and further MS3 experiments were performed on the two major fragments of m/z 862 and 791 respectively. Based on the MS data, the intact cross-linked peroxide 3b was unambiguously identified by the LC-MS technique.
Fig. 5. Mass spectra of intact cross-linked peroxide 3b analyzed by LC-Iontrap MS.
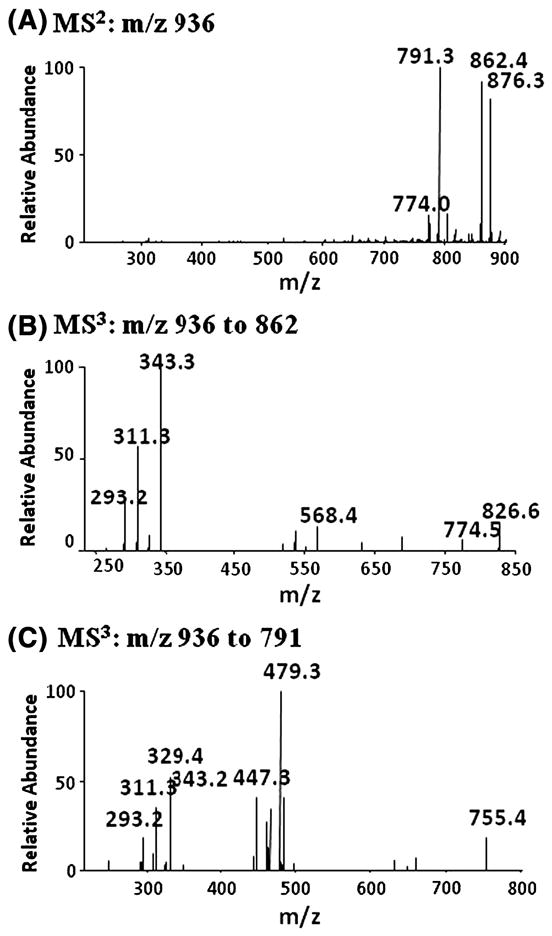
(A) MS2 of acetate adduct of compound 3b, m/z 936. (B) MS3 of m/z 936 to 862. (C) MS3 of m/z 936 to 791. The oxidation mixture used was from the experiment described in Figure 3.
Fig 6. Fragmentation pathways of multi-stage CID experiments for the intact cross-chain peroxide 3b.
(A) MS2 of m/z 936: m/z 876 represents the loss of acetic acid whereas fragments with m/z 862 and 791 are further loss of methyl group on the choline head group (6a) and complete loss of the head group (6b) respectively. (B) MS3 of m/z 862: m/z 774 (6c) is derived from the cleavage of the tail end of the fatty acid whereas a peak with m/z 568 (6d) is consistent with a fragment having one of the side chain and head group attached; m/z 343 (6e) and m/z 311(6f) are consistent with the cleavage of the cross-linked peroxyl bond. (C) MS3 on m/z 936 to 791: m/z 755 may be due to the loss of two molecules of water from 6b; m/z 479 (6h) is derived from the loss of one of the side chain when the phosphate anion cyclizes and removes one of the side chains followed by dehydration; m/z 329 and 293 are indicative of the proposed side chains. The oxidation mixture used was from the experiment described in Figure 3.
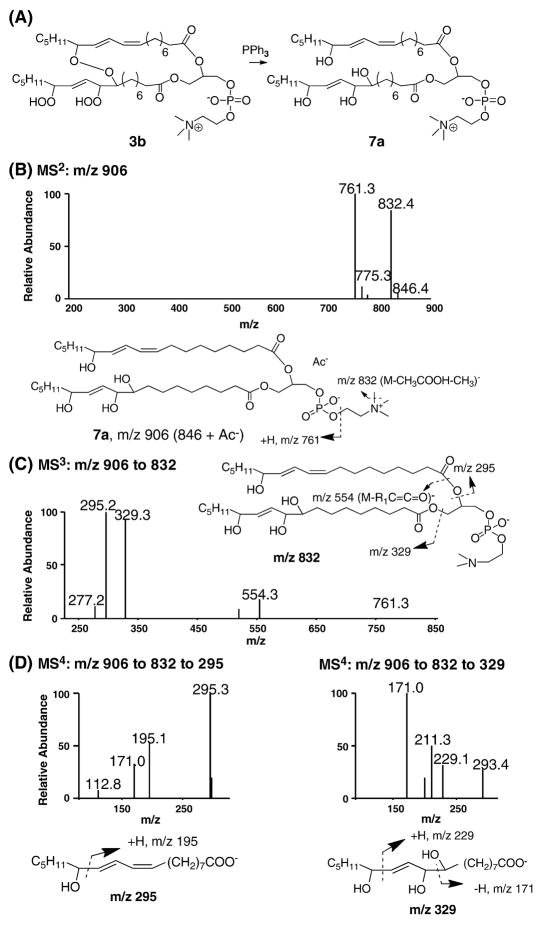
Derivatization of the intact cross-linked peroxides 3b and characterization of the resulting products
Due to the limited stability of the cross-linked peroxides, characterization of these precursors proved to be difficult. Reaction of the peroxides 3b with PPh3 led to compounds that are more stable and can be characterized by MS methods. Reduction of the cross-linked peroxide 3b leads to compound 7a as shown Figure 7. Successful conversion of 3b to 7a was confirmed by MS analysis and the data were summarized in supplement Figure S4.
Fig. 7. Characterization of the reduced cross-chain peroxides 7a by LC-Iontrap MS.
(A) Reduction of compound 3b by PPh3 leads to a more stable compound 7a. (B) MS2 of acetate adduct of compound 7a, m/z 906. (C) MS3 of m/z 906 to 832. (D) MS4 of m/z 906 to 832 to 295; MS4 of m/z 906 to 832 to 329. In addition to the characteristic fragments of m/z 229 and 171, m/z 293 represents loss of two molecules of water from m/z 329 while m/z 211 is from dehydration of m/z 229. The oxidation mixture used was from the experiment described in Figure 3.
Characterization of compound 7a was performed by Iontrap MS in the negative ion mode and the results are shown in Figure 7. MS2 of the acetate adduct of compound 7a with m/z 906 (Figure 7B) produced two major fragments: a peak with m/z 832 was consistent with the loss of acetic acid and a methyl group on the choline head group; the fragment of m/z 761 represented the loss of choline head group. MS3 on m/z 832 clearly indicated the two acyl chains with m/z 329 and m/z 295 respectively (Figure 7C). The unambiguous structural support of these two oxidized acyl chains was obtained in the MS4 spectra (Figure 7D). Taking these data together, the chemical structure of compound 7a was unambiguously confirmed by the Iontrap MS.
Formation of 4-HNE from L4CL via the similar mechanism that involves decomposition of intramolecular cross-linked peroxide
Using DLPC as a model compound, we have provided evidence that 4-HNE and other reactive aldehydes are derived from the novel chemical mechanism, and we show here that L4CL is the precursor for these reactive aldehydes in vivo with biological relevance.
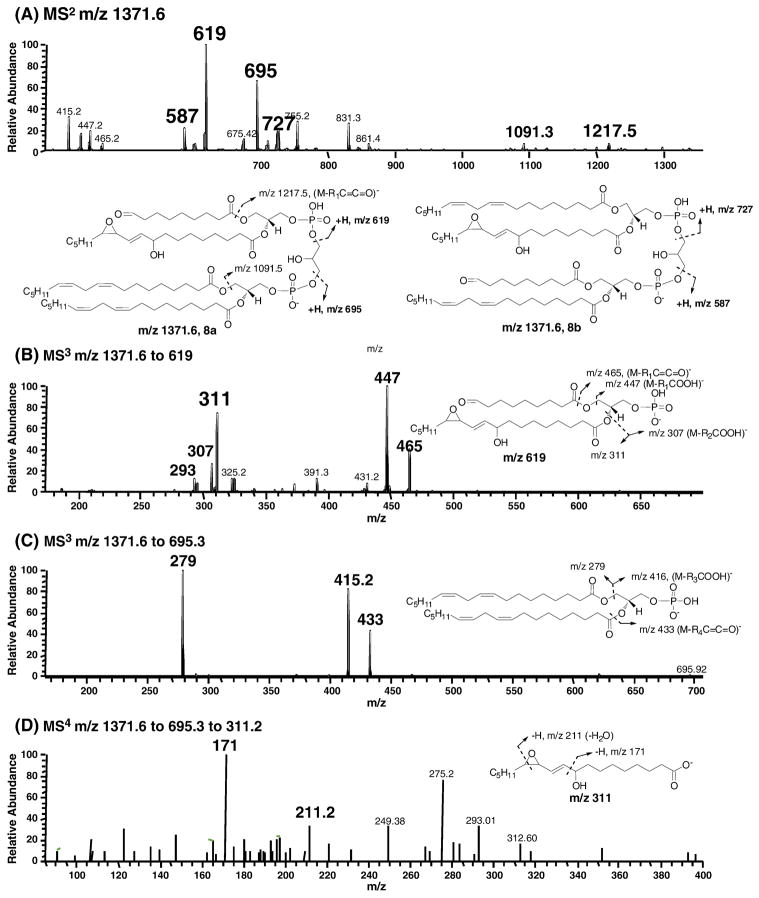
The proposed key products consistent with the peroxyl radical addition mechanism are epoxyalcohol on one acyl side chain and an aldhyde at C9 on the other side chain. The putative structure as shown in Figure 8 has m/z 1371 and it lacks a conjugated diene structure as shown in supplementary Figure S5. Cardiolipin is an anionic phospholipid which is readily detected in the negative ion mode MS. The two oxidized side chains can be on the same (8a) or different (8b) glycerol backbone. As shown in MS2 of m/z 1371.6 (Figure 8A), in addition to the common fragments of m/z 1217 and 1091 for these different regioisomers, fragments with m/z 619 and 695 are consistent with structure 8a whereas m/z 727 and 587 are indicative of the structure 8b. The assignment of these two critical fragments m/z 619 and 695 was further supported by respective MS3 experiments. MS3 on m/z 1371.6 to 619 generated characteristic fragments with m/z 311, 307, 447 and 293 (Figure 8B). A simpler fragmentation pattern of MS3 on m/z 1371.6 to 695 was obtained as shown in Figure 8C. Fragments with m/z 279, 415, and 433 were consistent with the two intact linoleoyl side chains on the same glycerol backbone. The presence of the key epoxy-alcohol in the structure was evident in MS4 of m/z 1371.6 to 619.3 to 311.2 (Figure 8D). In addition to the fragments of m/z 293 and 275, two fragments with m/z 171 and 211 were consistent with the presence of epoxyalcohol moiety in this compound. MSn experiments were carried out for ions m/z 727 and 587 and the data are consistent with the proposed structure 8b (data not shown).
Fig. 8. Characterization of the key product of epoxyalcohol from L4CL oxidation by LC-Iontrap MS.
(A) MS2 of m/z 1371.6. The oxidized two side chains can be on the same glycerol backbone (8a) or different glycerol backbone (8b). Besides the common fragments such as m/z 1217 and 1091, fragments m/z 619 and 695 are characteristic of 8a while m/z 587 and 727 are from 8b. (B) MS3 of m/z 1371.6 to 619. (C) MS3 of m/z 1371.6 to 695. (D) MS4 of m/z 1371.6 to 619 to 311. L4CL was oxidized using cytochrome c and H2O2 as described in details in the experimental section.
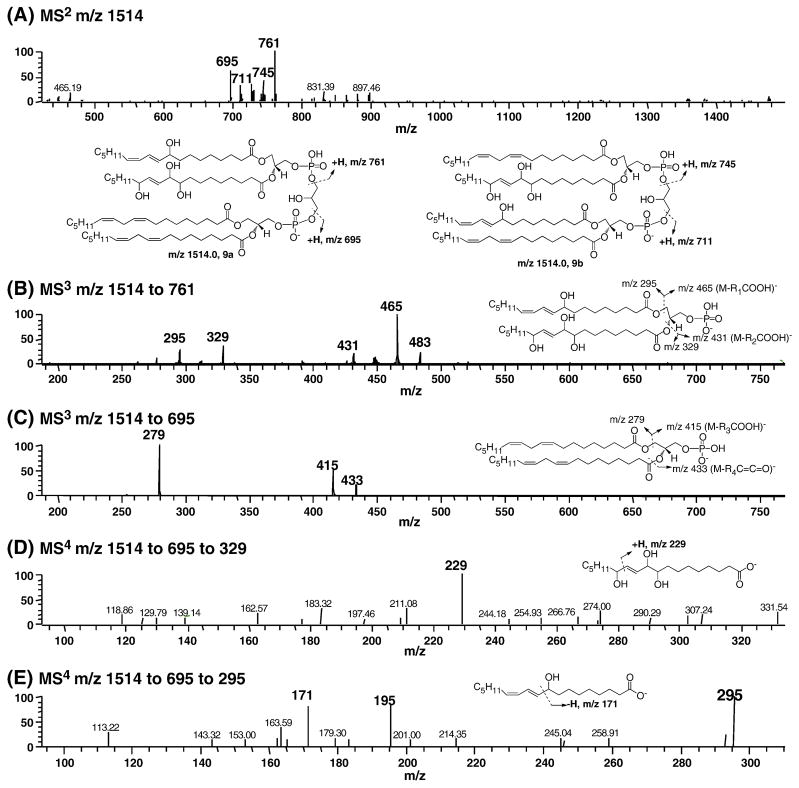
We also attempted to identify the cross-linked peroxide precursors that would lead to 4-HNE. The stabilized precursor by PPh3 reduction had an alcohol moiety on one side chain and three hydroxyl groups on the neighboring acyl chain. The LC-MS data of the reduced oxidation mixture are summarized in Figure 9. As shown in Figure 9A, the two oxidized side chains can be on the same (9a) or different (9b) glycerol bones. MS2 of m/z 1514 showed two characteristic fragments of m/z 761 and 695 for 9a while m/z 711 and 745 are derived from 9b. Further structural characterization was performed by MS3 and MS4. MS3 on m/z 1514 to 761 generated m/z 465 and 295. In the same way, fragments with m/z 431 and 329 were consistent with the presence of three hydroxyls on the other side chain. MS3 on m/z 1514 to 695 demonstrated that the compound had two intact linoleate side chains on the same glycerol moiety because of the fragments with m/z 279, 415 and 433. MS4 on m/z 1514 to 761 to 329 clearly showed the proposed structure due to the presence of the characteristic fragment m/z 229. MS4 on m/z 1514 to 761 to 295 indicated that there was a mixture of regioisomeric hydroxide on one of the side chain: m/z 171 resulted from 9-HODEs while m/z 195 was indicative of 13-HODE. Similar data were obtained for structure 9b. Therefore, the precursor with cross-chain peroxides is unambiguously identified based on MS data.
Fig. 9. Characterization of reduced cross-chain peroxide derived from oxidation of L4CL by LC-Iontrap MS.
(A) MS2 of m/z 1514. The oxidized two side chains can be on the same glycerol backbone (9a) or different glycerol backbone (9b). Fragments m/z 761 and 695 are characteristic of 9a while m/z 711 and 745 are from 9b. (B) MS3 of m/z 1514 to 761, the fragments of m/z 465, 431, 295, and 329 indicate the presence of a hydroxyl group on one side chain and three hydroxyl groups on a neighboring side chain. (C) MS3 of m/z 1514 to 695, the fragments with m/z 279, 415, and 433 suggest two intact linoleoyl side chains. (D) MS4 of m/z 1514 to 761 to 329. (E) MS4 of m/z 1514 to 761 to 295, m/z 171 and 195 are characteristic of 9- and 13-HODE respectively. L4CL was oxidized using cytochrome c and H2O2 as described in details in the experimental section.
Taken together, these data indicate that the oxidation of L4CL generates 4-HNE through a mechanism similar to that of DLPC based on the presence of the key products as well as the putative cross-linked peroxide precursors.
Identification of the key products of cardiolipin oxidation via the novel mechanism in vivo
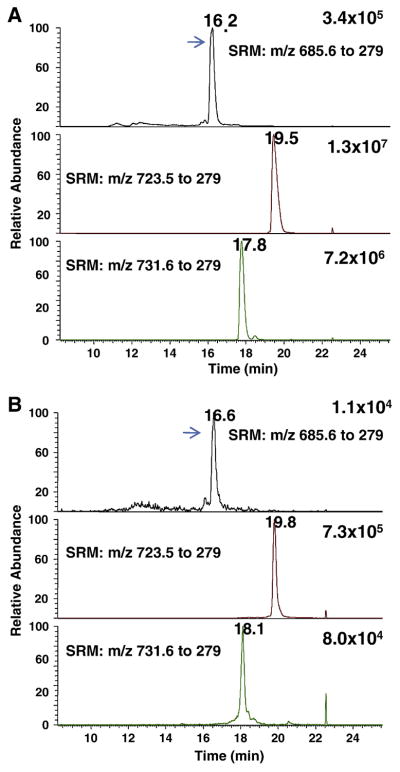
Carbon tetrachloride-induced liver injury is a well-established animal model for in vivo free radical generation and oxidative stress [36]. Elevated levels of lipid peroxidation products, such as F2-IsoPs, malondialdehde (MDA), TBARS, and 4-HNE, have been detected in this model. We performed experiments to identify the key products of cardiolipin oxidation in vivo in rat liver tissue after carbon tetrachloride treatment. The results were shown in Figure 10. We identified characteristic fragments for compound 8a in CID experiments on m/z 1371.8 (singly charged ions) or m/z 685.6 (doubly charged ion) from in vitro oxidation of L4CL and extracts of rat liver tissue (data not shown). A peak at 16.2 min in Figure 10A and 10B demonstrated the formation of this key product in vitro and in vivo. It is noted that unoxidized L4CL and the major oxidation product with addition of one oxygen atom were also detected. These data indicate that the novel mechanism for 4-HNE also operates in vivo and it may play an important role in 4-HNE formation in vivo.
Fig. 10. Identification of the key oxidation product (compound 8a) of cardiolipin from the novel mechanism in vivo.
(A) In vitro oxidation of L4CL by cytochrome c/H2O2. (B) Rat liver extracts after CCl4 treatment. SRM transitions: m/z 685.6 to 279, Compound 8a; L4CL, m/z 723.5 to 279; primary oxidation product with the addition of one oxygen, m/z 731.6 to 279. The experiments were performed on a triple quadruple MS in the negative ion mode coupled to reverse phase LC separation. The arrows indicate compound 8a. See experimental section for details.
DISCUSSION
Free radical-induced oxidation of PUFAs has been extensively studied for more than half a century while 4-HNE was first discovered in vivo almost three decades ago [37]. Investigation of the toxicological and physiological actions of this reactive lipid electrophile has been extensive, but the mechanism for its formation is much less understood. A novel mechanism for the formation of 4-HNE by intermolecular peroxyl radical addition and decomposition was recently proposed [13, 17]. We provide evidence here that this mechanism may play an important role for the oxidation of the mitochondria-specific phospholipid cardiolipin, especially L4CL. Formation of 4-HNE and other reactive aldhehydes from this process may have significant implications in apoptosis and other functions of cardiolipins in mitochondria.
Substantial efforts have been made to understand the mechanism of 4-HNE formation in vivo since the discovery of 4-HNE [14, 15]. PUFAs containing ω-6 chains, such as linoleic acid (C18:2) and arachidonic acid (C20:4), are precursors for 4-HNE and much attention has been paid to the decomposition of lipid hydroperoxides to generate 4-HNE and related lipid electrophiles. We have utilized different lipid hydroperoxides as precursors to study the formation of 4-HNE. Our previous studies using 15-HETE and 15-HPETE identified epoxyalcohol and epoxyhydroperoxide derivatives as major products during the formation of 4-HNE from these precursors [17]. These results prompted us to propose a novel mechanism of 4-HNE formation via intermolecular addition of a peroxyl radical to form a dimeric peroxide, followed by decomposition of this unstable precursor (Figure 1B). There is precedence for the dimerization of linoleic acid during the free radical oxidation in vitro and these resulting peroxides can be used as initiators for further free radical processes [21]. Successful separation and characterization of several dimeric lipid hydroperoxides were carried out and their decomposition gave rise to a number of reactive aldehydes. These studies also discovered the instability of these peroxide dimers [22–25, 38]. However, the biological relevance of the intermolecular cross reaction mechanism is unknown because a majority of the fatty acids are esterified on phospholipids in cellular membranes and the linoleate acyl chain is often separated by saturated acyl side chains. The data we presented here suggest that CL is one of the major sources for 4-HNE because it has four linoleate acyl chains in the same molecule and the formation of these important peroxyl dimers occurs intramolecularly. Thus, it is this unique chemical structure of CL that is responsible for the enhanced oxidation of CL (Figure S1B) and formation of 4-HNE under free radical conditions (Figure 2). It is noted that intramolecular peroxyl radical addition to a conjugated diene is favored kinetically over the intermolecular addition. Similar chemistry has been reported in vitro in other lipids that have two or more linoleate side chains in the same molecule. For example, kinetic studies showed that triglycerides containing two or more linoleate side chains underwent enhanced autooxidation due to the arm-to-arm hydrogen atom abstraction under free radical conditions [39, 40].
We also detected the characteristic product compound 8a that are postulated to be formed via this novel mechanism in vivo in rat liver tissue after carbon tetrachloride treatment. As a well-established rodent model for in vivo oxidative stress, CCl4 treatment induces free radical generation in the liver that leads to massive lipid peroxidation, protein modification, and DNA damage [36]. Recent studies also demonstrate that CCl4 treatment inhibits hepatic mitochondrial aldhyde dehydrogenases through JNK-mediated phosphorylation and further exacerbates the liver damage by 4-HNE and other reactive lipid aldehydes [41].
The MS techniques that we employed here have proved indispensible to characterize the proposed oxidation products of phospholipids [42–44]. Free radical oxidation of PUFA-containing phospholipids generates an array of oxidation products and most of them are unstable peroxides. It is extremely difficult to isolate these oxidation products and characterize them by the conventional techniques such as NMR. MS coupled with LC separation has advantages in providing the molecular weight and structural information for the proposed compounds. Iontrap MS has been widely employed for this purpose due to the capability of multi-stage CID [45–47]. In the negative ion mode, the chemical structures that are consistent with the proposed mechanism were unambiguously identified using consecutive CID experiments. On the other hand, the iontrap technique has limitations in efficient trapping of the ions with lower m/z ratios. We also employed triple quadruple MS to conform the formation of these characteristic smaller fragments that did not show in MS2 of iontrap instruments (data now shown). These two complimentary techniques enable us to identify the key products that are consistent with our proposed mechanisms from a complex oxidation mixture of phospholipids.
The mechanism presented here links 4-HNE formation to CL oxidation in mitochondria where substantial amount of ROS, such as super oxide anion and hydrogen peroxides, are generated. Lipid peroxidation and subsequent formation of 4-HNE in mitochondria have been suggested to play an important role in mitochondrial dysfunction [48]. Paradies et al showed a direct correlation between lipid peroxidation, decreased CL and decreased cytochome c oxidase activity when they exposed rat heart mitochondria to t-butyl hydroperoxides [49]. Sen et al oxidized rat brain mitochondria using iron-ascorbate and found that increased lipid peroxidation as measured by MDA/TBARS correlated with decreased levels of cardiolipin [50]. A seminal work carried out by Kagan et al demonstrated that oxidation of CL by the peroxidase function of cytochrome c due to its association with CL was required to trigger intrinsic apoptotic pathways [29, 31]. Using a state-of-the-art oxidative lipidomics approach they showed that CLs in mitochondria were preferentially oxidized in the presence of other highly oxidizable phospholipids, such as arachidonic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) containing phosphatidylcholine (PC) and phosphatidylethanolamine (PE). Previous studies also established that hydroperoxides of CL were important in the induction of apoptotic events [51–53]. Our studies suggest that 4-HNE formation from these CL-OOHs may also play a significant role in these events because 4-HNE has been shown to induce apoptosis [8, 54].
This novel mechanism for 4-HNE formation from CL oxidation in mitochondria may potentially have some physiological and pathophysiological significance. Generation of 4-HNE in vivo has been implicated in cardiovascular diseases, such as atherosclerosis [55], and neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease [5, 56–60]. L4CL is the major CL species in the mitochondria of most mammalian tissues and it constitutes more than 70% in the heart [61]. Thus formation of 4-HNE via this novel mechanisms is likely important. 4-HNE alters multiple essential functions of brain mitochondria, which plays a pivotal role in initiation and progression of neurodegenerative diseases [62]. 4-HNE also induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy [63].
Modulation of the formation of 4-HNE and other lipid electrophiles from CL oxidation in mitochondria may lead to novel approaches to control the cytotoxic activities of these compounds. A number of strategies have been explored to modulate the oxidation of CL in mitochondria [64]. The peroxidase activity of cytochrome c and CL can be activated by hydrogen peroxide or lipid hydroperoxides [65]. Thus disruption of this interaction by scavenging H2O2 or reducing lipid hydroperoxides may lead to the inhibition of apoptosis. Overexpression of phospholipid hydroperoxide glutathione peroxidase (PHGPX) in mitochondria of RBL2H3 cells prevented the release of cyt c, activation of caspase-3, and apoptosis caused by 2-deoxyglucose (2DG), whereas cells overexpressing nonmitochondrial PHGPx were prone to apoptosis [51, 66]. Furthermore, liver apoptosis and cytochrome c release were suppressed in transgenic mice overexpression of this enzyme when mice were treated with diquat to induce apoptosis. Levels of cardiolipin oxidation products in these transgenic animals was also suppressed compared to the wild type animals [67]. Reduction of mitochondrial hydrogen peroxide by overexpressing mitochondria specific peroxiredoxin 3 (Prdx3/Prx3) led to the reduced levels of F2-IsoPs, a widely accepted standard for oxidative stress in vivo [68], and 4-HNE [69]. Lastly, some antioxidants may also have inhibitory effects on the formation of these reactive lipid aldehydes if they can be incorporated into the cytochrome c/CL peroxidase complex and compete with the intramolecular hydrogen atom abstraction or addition to the double bonds.
Alternative approach to mitigate the detrimental effects of 4-HNE and related compounds may involve in stimulation of the deactivation mechanism for these compounds [70]. Aldehyde dehydrogenase-2 (ALDH2) is a mitochondria-specific enzyme that is involved in oxidation of 4-HNE to its acid analogue. A recent study demonstrated that ischemic damage to the heart was significantly reduced by activation of ALDH2 using a small molecule agonist Alda-1 [71, 72]. Moreover, increasing evidence suggested that mitochondrial detoxification pathways for 4-HNE play a significant role in delay of the development of AD and other neurodegenerative diseases [62].
In summary, we have provided evidence that 4-HNE and related reactive lipid electrophiles can be generated through a novel chemical mechanism of cardiolipin oxidation under free radical conditions in vitro and in vivo. This mechanism links 4-HNE generation to cardiolipin oxidation in mitochondria where abundant ROS are generated. Biological studies are under way to investigate the role of CL oxidation and 4-HNE formation in mitochondria function.
Supplementary Material
Fig. S1. (A) Analysis of 4-HNE, 4-ONE and 4-HPNE by LC-MS. 4-HNE-d3 was used as internal standard. 4-ONE and 4-HPNE were monitored with the same transition in the SRM experiment because dehydration of 4-HPNE gives rise to 4-ONE. The peak representing 4-HPNE disappeared with the increase in size of the peak due to 4-HNE when the sample was treated with PPh3. (B) Time course of conjugated diene formation during the oxidation of these three phospholipids. The analysis was performed using LC-MS and UV at 234 nm was detected.
Fig. S2. Time course of PLPC and DLPC hydroperoxides decomposition. (A) UV absorbance of parent hydroperoxides (conjugated diene has characteristic absorption at 234 nm). (B) Levels of 4-HPNE. (C) Levels of 4-ONE.
Fig. S3. Fractionation of decomposition mixture of DLPC hydroperoxides. (A) HPLC chromatogram of oxidation mixture of DLPC dihydroperoxides. UV absorbance of 234 nm is characteristic of conjugated diene; 220 nm is for α,β-unsaturated aldehyde such as 4-HNE; 210 nm is for isolated double bonds. (B) Levels of 4-HNE for the six collected fractions before and after further decomposition. *, p < 0.05, student t test. Fraction 1 has less 4-HNE after further decomposition because 4-HPNE is in this fraction and it decomposes upon further incubation. Fraction 5 and 6 had higher levels of 4-HNE after 2 hr decomposition, suggesting that the precursor that leads to the formation of 4-HNE elutes in these two fractions. (C) Levels of epoxyhydroxide (m/z 706) and epoxyhydroperoxide (m/z 722) derived from the decomposition of collected fractions. These data further suggested that fraction 5 and 6 contained the precursors.
Fig. S4. Derivatization of the cross-chain peroxides 3b to a more stable compound 7a. LC-MS analysis indicated that PPh3 successful reduced the peroxide bond in compound 3b. The difference on the retention times is due to fact that the samples were analyzed on separate dates.
Fig. S5. Key product of L4CL oxidation that is consistent with the proposed mechanism. (A) UV 234 nm and extracted ion of m/z 1371.8. The target compound does not have conjugated diene in the structure. (B) MS spectrum of the epoxyhydroxide with m/z 1371.8.
Acknowledgments
This work is supported by NIH grants GM15431, DK48831, and ES13125.
Abbreviations
- ALDH
aldehyde dehydrogenase
- AD
Alzheimer’s disease
- C-0
a water soluble azo initiator
- CID
collision-induced dissociation
- CL
cardiolipin
- DHA
docosahexaenoic acid
- DLPC
1,2-dilinoleoyl-sn-glycero-3-phosphocholine
- DNPH
2,6-dinitrophenylhydrazine
- EPA
eicosapentaenoic acid
- ESI
electrospray
- GST
glutathione S-transferase
- 4-HNE
4-hydroxy-2-nonenal
- 4-HPNE
4-hydroperoxy-2-nonenal
- HPLC
high performance liquid chromatography
- HODE
hydroxyoctadecadienoic acid
- HPODE
hydroperoxyoctadecadienoic acid
- HETE
hydroxyeicosatetraenoic acid
- IsoP
isoprostanes
- L4CL
tetralinoleoyl cardiolipin
- MeOAMVN 2
2′-azobis(4-methoxy-2,4-dimethylvaleronitrile
- MS
mass spectrometry
- O4CL
tetraoleoyl cardiolipin
- 4-ONE
4-oxo-2-nonenal
- PLPC
1-palmitoyl-2-linoleoyl-phosphatidylcholine
- POPC
1-palmitoyl-2-oleoyl-phosphatidylcholine
- PC
phosphatidylcholine
- PE
phosphotidylethanolamine
- PHGPX
phospholipid hydroperoxide glutathione peroxidase
- Prdx
peroxiredoxin
- PD
Parkinson disease
- PPh3
triphenylphosphine
- ROS
reactive oxygen species
- SRM
selective reaction monitoring
- UPLC
ultra pressure liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niki E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50:S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 4.Yin H, Porter NA. New Insights Regarding the Autoxidation of Polyunsaturated Fatty Acids. Antioxid Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 5.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 6.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H, Zollner H, Schauer RJ. Aldehydes formed by lipid peroxidation: mechanisms of formation, occurence, and determination. In: Vigo-Pelfrey C, editor. membrane Lipid Oxidaiton. Boca Raton, FL: CRC Press, Inc; 1991. pp. 239–268. [Google Scholar]
- 8.West JD, Marnett LJ. Endogenous Reactive Intermediates as Modulators of Cell Signaling and Cell Death. Chem Res Toxicol. 2006;19:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph TK, Freeman BA. Transduction of Redox Signaling by Electrophile-Protein Reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- 11.Doorn JA, Hurley TD, Petersen DR. Inhibition of Human Mitochondrial Aldehyde Dehydrogenase by 4-Hydroxynon-2-enal and 4-Oxonon-2-enal. Chem Res Toxicol. 2006;19:102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 12.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Schneider C, Porter NA, Brash AR. Routes to 4-Hydroxynonenal: Fundamental Issues in the Mechanisms of Lipid Peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C, Tallman KA, Porter NA, Brash AR. Two Distinct Pathways of Formation of 4-Hydroxynonenal: Mechanisms of nonenzymatic trnsformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C, Boeglin WE, Yin H, Stec Donald F, Porter DLHA, Brash AR. Synthesis of Dihydroperoxides of Linoleic and Linolenic Acids and Studies on Their Transformation to 4-Hydroperoxynonenal. Lipids. 2005;40:1155–1162. doi: 10.1007/s11745-005-1480-3. [DOI] [PubMed] [Google Scholar]
- 17.Schneider C, Boeglin WE, Yin H, Porter NA, Brash AR. Intermolecular Peroxyl Radical Reactions during Autoxidation of Hydroxy and Hydroperoxy Arachidonic Acids Generate a Novel Series of Epoxidized Products. Chem Res Toxicol. 2008;21:895–903. doi: 10.1021/tx700357u. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Oe T, Blair IA. Vitamin C-Induced Decomposition of Lipid Hydroperoxides to Endogenous Genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 19.Gu X, Zhang W, Salomon RG. Fe2+ Catalyzes Vitamin E-Induced Fragmentation of Hydroperoxy and Hydroxy Endoperoxides That Generates γ-Hydroxy Alkenals. J Am Chem Soc. 2007;129:6088–6089. doi: 10.1021/ja0689785. [DOI] [PubMed] [Google Scholar]
- 20.Mayo FR, Miller AA. Oxidation of Unsaturated Compounds. II. Reactions of Styrene Peroxide1. J Am Chem Soc. 1956;78:1023–1034. [Google Scholar]
- 21.Morita M, Tokita M. The real radical generator other than main-product hydroperoxide in lipid autoxidation. Lipids. 2006;41:91–95. doi: 10.1007/s11745-006-5075-9. [DOI] [PubMed] [Google Scholar]
- 22.Miyashita K, Hara N, Fujimoto K, Kaneda T. Formation of Dimers during the Initial Stage of Autoxidation in Methyl Linoleate. Agric Biol Chem. 1982;46:751–755. [Google Scholar]
- 23.Miyashita K, Hara N, Fujimoto K, Kaneda T. Structural Studies of Polar Dimers in Autoxidized Methyl Linoleate during the Initial Stages of Autoxidation. Agric Biol Chem. 1984;48:2511–2515. [Google Scholar]
- 24.Miyashita K, Hara N, Fujimoto K, Kaneda T. Decomposition products of Dimers Arising from Secondary Oxidation of Methyl Linoleate Hydroperoxides. Agric Biol Chem. 1985;49:2633–2640. [Google Scholar]
- 25.Miyashita K, Hara N, Fujimoto K, Kaneda T. Dimers formed in oxygenated methyl linoleate hydroperoxides. Lipids. 1985;20:578–587. [Google Scholar]
- 26.Hauff KD, Hatch GM. Cardiolipin metabolism and Barth Syndrome. Prog Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–1027. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalvez F, Gottlieb E. Cardiolipin: Setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 31.Kagan VE, BayIr HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne GL, Seal JR, Havrilla CM, Wijtmans M, Porter NA. Identification and analysis of products formed from phospholipids in the free radical oxidation of human low density lipoproteins. J Lipid Res. 2005;46:307–319. doi: 10.1194/jlr.M400311-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Uchida T, Gotoh N, Wada S. Method for analysis of 4-hydroxy-2-( E )-nonenal with solid-phase microextraction. Lipids. 2002;37:621–626. doi: 10.1007/s11745-002-0941-z. [DOI] [PubMed] [Google Scholar]
- 34.Harrison KA, Davies SS, Marathe GK, McIntyre T, Prescott S, Reddy KM, Falck JR, Murphy RC. Analysis of oxidized glycerophosphocholine lipids using electrospray ionization mass spectrometry and microderivatization techniques. J Mass Spectrom. 2000;35:224–236. doi: 10.1002/(SICI)1096-9888(200002)35:2<224::AID-JMS933>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 35.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 36.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, IILJR, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Thiel DHV, Wellner D, Walter PB, Tome KB, Mason RP, Barrett JC. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 38.Miyashita K, Hara N, Fujimoto K, Kaneda T. Structural of Dimers Produced from Methyl Linoleate during the Initial Stages of Autoxidation. Agric Biol Chem. 1982;46:2293–2297. [Google Scholar]
- 39.Cosgrove J, Church D, Pryor W. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22:299–304. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]
- 40.Bowry VW. Arm-to-Arm Autoxidation in a Triglyceride: Remote Group Reaction Kinetics. J Org Chem. 1994;59:2250–2252. [Google Scholar]
- 41.Moon KH, Lee YM, Song BJ. Inhibition of hepatic mitochondrial aldehyde dehydrogenase by carbon tetrachloride through JNK-mediated phosphorylation. Free Radic Biol Med. 2010;48:391–398. doi: 10.1016/j.freeradbiomed.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin H, Cox BE, Liu W, Porter NA, Morrow JD, Milne GL. Identification of intact oxidation products of glycerophospholipids in vitro and in vivo using negative ion electrospray iontrap mass spectrometry. J Mass Spectrom. 2009;44:672–680. doi: 10.1002/jms.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 44.Domingues MRM, Reis A, Domingues P. Mass spectrometry analysis of oxidized phospholipids. Chemistry and Physics of Lipids. 2008;156:1–12. doi: 10.1016/j.chemphyslip.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Tyurin VA, Tyurina YY, Kochanek PM, Hamilton R, DeKosky ST, Greenberger JS, Bayir H, Kagan VE, Roya Khosravi-Far ZZRAL, Mauro P. Methods in Enzymology. Academic Press; 2008. Oxidative Lipidomics of Programmed Cell Death; pp. 375–393. [DOI] [PubMed] [Google Scholar]
- 46.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of [gamma]-irradiation-induced intestinal injury. Free Radical Biology and Medicine. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Tyurina YY, Tyurin VA, Kapralova VI, Amoscato AA, Epperly MW, Greenberger JS, Kagan VE. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol. 2009;580:153–183. doi: 10.1007/978-1-60761-325-1_9. [DOI] [PubMed] [Google Scholar]
- 48.Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: Mechanistic significance of 4-hydroxynonenal. Environmental and Molecular Mutagenesis. 2010;51:380–390. doi: 10.1002/em.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS LETT. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- 50.Sen T, Sen N, Tripathi G, Chatterjee U, Chakrabarti S. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem Int. 2006;49:20–27. doi: 10.1016/j.neuint.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa Y. Initiation of Apoptotic Signal by the Peroxidation of Cardiolipin of Mitochondria. Ann NY Acad Sci. 2004;1011:177–184. doi: 10.1007/978-3-662-41088-2_18. [DOI] [PubMed] [Google Scholar]
- 52.Orrenius S, Zhivotovsky B. Cardiolipin oxidation sets cytochrome c free. Nat Chem Biol. 2005;1:188–189. doi: 10.1038/nchembio0905-188. [DOI] [PubMed] [Google Scholar]
- 53.Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 54.Ji C, Amarnath V, Pietenpol JA, Marnett LJ. 4-Hydroxynonenal Induces Apoptosis via Caspase-3 Activation and Cytochrome c Release. Chemical Research in Toxicology. 2001;14:1090–1096. doi: 10.1021/tx000186f. [DOI] [PubMed] [Google Scholar]
- 55.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 56.Uchida K. Future of Toxicology-Lipid Peroxidation in the Future: From Biomarker to Etiology. Chem Res Toxicol. 2007;20:3–5. doi: 10.1021/tx600304n. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q, Raina AK, Smith MA, Sayre LM, Perry G. Hydroxynonenal, toxic carbonyls, and Alzheimer disease. Mol Aspects Med. 2003;24:305–313. doi: 10.1016/s0098-2997(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 58.Lin D, Lee H-g, Liu Q, Perry G, Smith MA, Sayre LM. 4-Oxo-2-nonenal Is Both More Neurotoxic and More Protein Reactive than 4-Hydroxy-2-nonenal. Chem Res Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- 59.Sayre LM, Perry G, Smith MA. Oxidative Stress and Neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 60.Sayre LM, Smith MA, Perry G. Chemistry and Biochemistry of Oxidative Stress in Neurodegenerative Disease. Curr Med Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 61.Wang H-YJ, Jackson SN, Woods AS. Direct MALDI-MS Analysis of Cardiolipin from Rat Organs Sections. J Am Soc Mass Spectrom. 2007;18:567–577. doi: 10.1016/j.jasms.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Picklo SMJ, Montine TJ. Mitochondrial Effects of Lipid-Derived Neurotoxins. J Alzheim Dis. 2007;12:185–193. doi: 10.3233/jad-2007-12209. [DOI] [PubMed] [Google Scholar]
- 63.Akude E, Zherebitskaya E, Roy Chowdhury S, Girling K, Fernyhough P. 4-Hydroxy-2-Nonenal Induces Mitochondrial Dysfunction and Aberrant Axonal Outgrowth in Adult Sensory Neurons that Mimics Features of Diabetic Neuropathy. Neurotox Res. 2010;17:28–38. doi: 10.1007/s12640-009-9074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting Mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 65.Belikova NA, Tyurina YY, Borisenko G, Tyurin V, Samhan Arias AK, Yanamala N, Furtmuller PG, Klein-Seetharaman J, Obinger C, Kagan VE. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J Am Chem Soc. 2009;131:11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- 66.Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang H, Ran Q, Jang YC, Holstein D, Lechleiter J, McDonald-Marsh T, Musatov A, Song W, Van Remmen H, Richardson A. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic Biol Med. 2009;47:312–320. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milne GL, Yin H, Morrow JD. Human Biochemistry of the Isoprostane Pathway. J Biol Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L, Na R, Gu M, Salmon AB, Liu Y, Liang H, Qi W, Remmen HV, Richardson A, Ran Q. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008;7:866–878. doi: 10.1111/j.1474-9726.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brichac J, Ho KK, Honzatko A, Wang R, Lu X, Weiner H, Picklo MJ. Enantioselective Oxidation of trans-4-Hydroxy-2-Nonenal Is Aldehyde Dehydrogenase Isozyme and Mg2+ Dependent. Chem Res Toxicol. 2007;20:887–895. doi: 10.1021/tx7000509. [DOI] [PubMed] [Google Scholar]
- 71.Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, Mochly-Rosen D. Activation of Aldehyde Dehydrogenase-2 Reduces Ischemic Damage to the Heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu S-Q, Bhatnagar A, Srivastava S. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J. 2009;417:513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) Analysis of 4-HNE, 4-ONE and 4-HPNE by LC-MS. 4-HNE-d3 was used as internal standard. 4-ONE and 4-HPNE were monitored with the same transition in the SRM experiment because dehydration of 4-HPNE gives rise to 4-ONE. The peak representing 4-HPNE disappeared with the increase in size of the peak due to 4-HNE when the sample was treated with PPh3. (B) Time course of conjugated diene formation during the oxidation of these three phospholipids. The analysis was performed using LC-MS and UV at 234 nm was detected.
Fig. S2. Time course of PLPC and DLPC hydroperoxides decomposition. (A) UV absorbance of parent hydroperoxides (conjugated diene has characteristic absorption at 234 nm). (B) Levels of 4-HPNE. (C) Levels of 4-ONE.
Fig. S3. Fractionation of decomposition mixture of DLPC hydroperoxides. (A) HPLC chromatogram of oxidation mixture of DLPC dihydroperoxides. UV absorbance of 234 nm is characteristic of conjugated diene; 220 nm is for α,β-unsaturated aldehyde such as 4-HNE; 210 nm is for isolated double bonds. (B) Levels of 4-HNE for the six collected fractions before and after further decomposition. *, p < 0.05, student t test. Fraction 1 has less 4-HNE after further decomposition because 4-HPNE is in this fraction and it decomposes upon further incubation. Fraction 5 and 6 had higher levels of 4-HNE after 2 hr decomposition, suggesting that the precursor that leads to the formation of 4-HNE elutes in these two fractions. (C) Levels of epoxyhydroxide (m/z 706) and epoxyhydroperoxide (m/z 722) derived from the decomposition of collected fractions. These data further suggested that fraction 5 and 6 contained the precursors.
Fig. S4. Derivatization of the cross-chain peroxides 3b to a more stable compound 7a. LC-MS analysis indicated that PPh3 successful reduced the peroxide bond in compound 3b. The difference on the retention times is due to fact that the samples were analyzed on separate dates.
Fig. S5. Key product of L4CL oxidation that is consistent with the proposed mechanism. (A) UV 234 nm and extracted ion of m/z 1371.8. The target compound does not have conjugated diene in the structure. (B) MS spectrum of the epoxyhydroxide with m/z 1371.8.