Abstract
Human brucellosis, a zoonotic disease of major public health concern in several developing countries, is primarily caused by Brucella abortus, B. melitensis, and B. suis. No brucellosis vaccine is available for human use. The aim of this study was to determine if B. neotomae, a bacterium not known to cause disease in any host, can be used for developing brucellosis vaccines. B. neotomae and its recombinant strains overexpressing superoxide dismutase and a 26 kDa periplasmic protein were rendered non-replicative through exposure to gamma-radiation and used as vaccines in a murine brucellosis model. All three vaccines induced antigen-specific antibody and T cell responses. The vaccinated mice showed significant resistance against challenge with virulent B. abortus 2308, B. melitensis 16M, and B. suis 1330. These results demonstrate that the avirulent B. neotomae is a promising platform for developing a safe and effective vaccine for human brucellosis.
Keywords: Brucellosis, B. neotomae, gamma radiation, vaccine, broad protection
Introduction
Brucellosis is a zoonotic disease caused by members of the genus Brucella, which are Gram-negative, facultatively intracellular bacteria [1]. There are six-well recognized (B. abortus, B. melitensis, B. suis, B. canis, B. ovis, and B. neotomae) and four recently added (B. ceti, B. pennipedialis, B. microti, and B. inopinata) species in genus Brucella. In domestic and wild mammals brucellosis often results in abortions and infertility. In humans, brucellosis manifests itself as a chronic infection with undulant fever and general malaise; other clinical signs vary depending on the affected organ systems [2]. Humans usually acquire the infection by consuming contaminated dairy or meat products or by coming in contact with the infected animal tissues and secretions [3]. Ingestion, inhalation, and contamination of conjunctiva or broken skin by the infected animal products are the common modes by which humans are infected. In several developing countries, brucellosis is an important public health concern [4]. Of the six well-recognized species of Brucella, B. melitensis, B. suis and B. abortus are highly virulent to humans [2]. These 3 Brucella species are considered potential bioterror agents and they belong to NIAID Category B priority pathogens list. At present there is no vaccine available for human brucellosis. Cell-mediated immunity (CMI) plays a central role in acquired resistance against brucellosis, and antibodies to the O polysaccharide (O antigen) of the lipopolysaccharide (LPS) participate in providing enhanced resistance against infections by B. abortus, B. melitensis and B. suis [5]. Attenuated, live Brucella strains such as B. abortus RB51 and 19, and B. melitensis Rev1 are being used as vaccines to control brucellosis in domestic animals [6]. However, these vaccines are not suitable for humans since they can cause disease even in individuals with healthy immune system [7-8].
B. neotomae was isolated in 1957 from desert wood rats of the western U.S. [9]. No disease, either in human or other animal species, has ever been attributed to B. neotomae. Unlike the virulent Brucella spp., B. neotomae does not establish a chronic infection in immuno-competent mouse models of brucellosis (Moustafa and Vemulapalli, unpublished observation). The overall goal of this study was to examine the feasibility of developing a safe and effective vaccine for human brucellosis using B. neotomae. We used exposure to a minimum dose of gamma-radiation as a means to increase the vaccine safety by eliminating the bacteria’s ability to replicate but retain the metabolic activity. We also tested if overexpression of Brucella Cu-Zn superoxide dismutase (SOD) and a 26 kDa periplasmic protein (Bp26) in B. neotomae would enhance its vaccine efficacy. Our results demonstrate that mice immunized with gamma-irradiated B. neotomae develop significant protective immunity against challenge with virulent B. abortus 2308, B. melitensis 16M, and B. suis 1330.
1. Materials and methods
2.1. Bacteria
B. neotomae strain 5K33 was purchased from the American Type Culture Collection, Manassas, Va. Virulent strains B. melitensis 16M, B. abortus 2308, and B. suis 1330 were from the culture collection at Virginia Tech, Blacksburg, VA. Escherichia coli strain DH5α (Invitrogen, Carlsbad, CA) was used for producing the necessary plasmid constructs and for recombinant protein production. The recombinant B. neotomae/Bp26 and B. neotomae/SOD were generated by transforming B. neotomae with recombinant plasmids pBB4Bp26 and pBB4SOD, respectively. The pBB4Bp26 plasmid was constructed by cloning the gene encoding the 26 kDa periplasmic protein along with its promoter sequences into the Kpn I and Xho I restriction sites of pBBR1MCS-4 [10]. The gene sequences were first PCR amplified from the genomic DNA of B. abortus RB51 using a custom-designed primer-pair (forward primer: 5’-aaggtaccacccgaaagaaagccgggata-3’; reverse primer: 5’-aactcgagcagatcgaaacgcgctctaat-3’) and the resulting 1.2 kb DNA fragment was digested with Kpn I and Xho I enzymes and cloned into the same sites of pBBR1MCS-4. The nucleotide sequence integrity of the cloned fragment was confirmed by sequence analysis. A 1.1 kb fragment containing the B. abortus sodC gene and its promoter sequence was excised from pBS/SOD [11] with ClaI restriction enzyme digestion and subcloned into pBBR1MCS-4 to generate pBB4SOD. B. neotomae was transformed with pBB4Bp26 and pBB4SOD by electroporation following the previously described procedures [12].
All bacteria were grown in tryptic soy broth (TSB) or tryptic soy agar (TSA) at 37°C. Bacteria harboring the plasmids were grown in the presence of 100 μg/ml concentration of ampicillin. Colony forming units (CFU) of Brucella strains were determined by plating 10-fold serial dilutions of the cultures on TSA. All experiments with B. neotomae were performed in a Biosafety level (BSL)-2 facility using BSL-3 practices. All experiments with virulent Brucella were performed in a BLS-3 facility approved for the select agents work.
2.2. Vaccine preparation
B. neotomae, B. neotomae/Bp26, and B. neotomae/SOD were grown in TSB or TSB with ampicillin to mid log phase, and aliquots containing 5×1010- 1×1011 CFU/ml were stored at -80°C until use. Two to three weeks before immunization, aliquots of the vaccines were exposed to 350 krads of gamma irradiation using a 60Co source irradiator (Gammacell 220 irraditor). The inability of the irradiated bacteria to replicate was confirmed plating on TSA and incubating for at least 7 days. The irradiated bacteria were stored at 4°C until use for immunization.
2.3. Determination of bacterial metabolic activity
Metabolic activity of the gamma irradiated bacteria was assayed using Alamar blue (BioSource International, Camarillo, CA) and LIVE/DEAD ® BacLight Bacterial viability Kit for microscopy (Molecular Probes, Eugene, OR). Alamar blue is a redox indicator and its color changes from blue to pink in response to chemical reduction. Alamar blue is reduced by FMNH2, FADH2, NADH, and NADPH, which are present in metabolically active cells. Alamar blue is extensively used for monitoring proliferation and viability of various eukaryotic and prokaryotic cells. For the Alamar blue assay, irradiated samples were washed in saline solution and resuspended in TSB to the original concentration. Ninety microliters of the irradiated suspension were mixed with 10 μl of Alamar blue and incubated at 37°C for 1 hour, and the change in color from blue to pink was monitored as described previously [13]. The manufacturer’s instructions were followed while using the LIVE/DEAD Baclight kit which utilizes a mixture of the green-fluorescent SYTO® 9 and the red-fluorescent propidium iodide to stain the bacterial nucleic acids. Following staining with the mixture, the live bacteria with intact cell membranes fluoresce green, whereas the dead bacteria with damaged membranes fluoresce red. The stained bacteria were observed using Nikon Eclipse–E400 microscope equipped with a 480/530 and 450/580 bandpass filter sets.
2.4. Quantification of irradiated bacteria in mice spleens by real-time PCR
The length of persistence of the irradiated B. neotomae in spleens of the vaccinated mice was determined by quantifying the bacterial DNA using RT-PCR. Two groups of 12 female BALB/c mice (6 weeks of age) each were inoculated intraperitonially with 108 CFU-equivalent of either heat-killed (65°C for 1 hour) or gamma-irradiated B. neotomae. Three mice from each group were euthanized at 3 hours, one, three and five days post inoculation, and their spleens were collected aseptically. Total DNA was extracted from the collected spleens using DNeasy® Blood and Tissue Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol. The amount of Brucella DNA present in the samples was determined using a previously described real-time PCR that amplifies a 178 bp region of IS711 element using primers IS421-F (5′-cgctcgcgcggtggat-3’) and IS511-R (5’- cttgaagcttgcggacagtcacc-3’) and measures the amplified product using a TaqMan probe (Cy5-acgaccaagctgcatgctgttgtcgatg-BHQ2) [14]. DNA extracted from different concentrations of the irradiated B. neotomae suspension (100 to 108 CFU-equivalent/ml) was used to construct the standard curve. After logarithmic conversion, the concentration of each dilution series was plotted against the cycle number at which the fluorescent signal increased above a threshold value (Ct value). The regression equation derived from the standard curve was used to calculate the concentration of B. neotomae present in the spleens. The PCR reactions were performed in a Stratagene MX3000P thermocycler and the data were analyzed using MxPro QPCR software (Stratagene, La Jolla, CA). All samples and standards were assayed in duplicates.
2.5. Mice immunizations
Female BALB/c mice of 4 to 6 weeks of age were vaccinated by two intraperitoneal inoculations, at day 0 and day 14, with 1×108 CFU-equivalent of the irradiated B. neotomae and its recombinants B. neotomae/Bp26 and B. neotomae/SOD. As a negative control, one group of mice was injected with saline alone. Mice were bled by puncturing the retro-orbital plexus under anesthesia at two weeks post inoculation (p.i.) (prior to the booster immunization), and at 6 weeks p.i. (4 weeks after the booster). The serum was separated from the clotted blood and stored at -20°C until use for detection of antigen-specific antibodies by enzyme-linked immunosorbent assay (ELISA). At 6 weeks p.i., all the mice were euthanized by CO2 asphyxiation followed by cervical dislocation, spleens were collected aseptically and used for determining the antigen-specific T cell immune responses by measuring cytokine production upon in vitro stimulation with specific antigens.
2.6. Preparation of antigens
2.6.1. B. neotomae crude extract
Late log phase culture of B. neotomae was centrifuged at 6,000 × g for 10 min and the pelleted bacteria were washed three times with sterile distilled water and resuspended in 0.5% sodium dodecyl sulfate (SDS). The bacteria were lysed by shaking gently for 2 hours at room temperature and then sonicating for 10 min on ice. The unlysed bacteria were removed by centrifugation at 8,000 × g for 10 min. The clear supernatants were collected, and the protein concentration was determined by the Bradford method [15]. Aliquots of the antigen extract were stored at −80°C until use for ELISA.
2.6.2. LPS extraction
Total LPS was extracted from live B. neotomae by butanol-water procedure as previously described [16-17]. Briefly, live B. neotomae organisms were harvested by centrifugation and 10g of wet pellet was resuspended in 0.85% NaCl at a concentration of 0.25 g wet weight/ml, and thoroughly dispersed by mixing with a magnetic stirrer at 4°C. An equal volume of water saturated butanol was added with constant mixing for 15 min at 4°C. After centrifugation at 35,000 × g for 20 min, the aqueous phase was collected and the insoluble precipitate was further extracted with ½ initial volume of the saline solution. The combined aqueous extracts were centrifuged in order to remove any traces of insoluble materials. LPS was precipitated using 4 volumes of methanol, and the precipitate was dissolved in 0.1M Tris buffer (pH 8) containing 2% SDS and 2% mercaptoethanol. The mixture was heated for 5 min at 100°C followed by 90 min incubation with proteinase K at 60°C. LPS was precipitated by methanol, followed by two washes with cold methanol, and dissolved in water. In order to confirm the presence of any traces of proteins in the extracted LPS, SDS-PAGE gel followed by silver staining was performed to ascertain the quality of the LPS preparation. The extracted LPS was used for ELISA.
2.6.3. Bp26 purification
The Bp26 protein of B. abortus was expressed in E. coli by using expression vector pMalC2 (New England Biolabs Inc.), and the purification was done according to manufacturer’s suggested procedure. Using pMalC2 vector, BP26 protein was expressed as a fusion protein with MBP at the amino terminus so that the recombinant protein can be purified by affinity chromatography with amylose resin. The concentration of the purified protein was determined by the Bradford method. Aliquots of the protein were stored at −80°C until use for ELISA or in vitro stimulation of splenocytes. The purified protein was also used to raise antigen-specific antibodies by hyper-immunizing mice using alum as the adjuvant.
2.6.4. Purification of SOD
Brucella SOD was expressed in E. coli DH5α and purified according to the method previously described [18]. Briefly, SOD was extracted from the E. coli cells using 10 mM phosphate buffer (pH 7.6) containing 0.1% Triton X-100. Cu/Zn SOD was purified using an equilibrated anion-exchange column (HiTrapQ; Pharmacia Biotech). By applying the extract to the column, all of the proteins except SOD were bound to the resin. SOD present in the flow-through was collected, absorbed with polymyxin B beads (Affi-Prep polymyxin support; Bio-Rad Laboratories, Hercules, CA) to remove the LPS, and dialyzed extensively against phosphate-buffered saline (PBS). The concentration of the purified protein was determined by the Bradford method. Aliquots of the protein were stored at −80°C until use for ELISA or in vitro stimulation of splenocytes.
2.7. SDS-PAGE and Western blotting
To confirm the overexpression of SOD and Bp26 in the recombinant B. neotomae strains, SDS-PAGE and Western blot analyses were performed as previously described [19]. As controls, antigen extracts of B. neotomae, the purified recombinant MBP-Bp26 fusion protein, and the purified SOD were used. For Western blotting, goat anti-B. abortus SOD sera [20], and mouse anti-MBP-Bp26 sera produced in this study were used as the primary antibodies.
2.8. Indirect ELISA
Levels of serum immunoglobulin G (total IgG), as well as IgG1 and IgG2a isotypes with specificity to Bp26, SOD and B. neotomae LPS and crude lysate were determined by indirect ELISA [19]. The levels of serum IgG2b, IgG3 and IgM against smooth LPS were also determined. The antigens were diluted in carbonate buffer, pH 9.6, 1 in 10 for B. neotomae LPS and to 10 μg/ml of protein concentration for MBP-Bp26, SOD and crude lysate. The wells of polystyrene plates (Nunc-Immunoplate with maxisorp surface) were coated with the diluted antigens (100μl/well). Following overnight incubation at 4°C, plates were washed four times in wash buffer (Tris-buffered saline at pH 7.4, 0.05 % Tween 20) and blocked with 2% bovine serum albumin (BSA) in Tris-buffered saline. After 1hour incubation at 37°C, mouse sera with appropriate dilution in blocking buffer were added to the wells (50 μl/well). Each serum sample was tested in duplicate wells; the plates were incubated for 4 hours at room temperature and washed four times. Horseradish peroxidase–labeled anti-mouse isotype specific conjugates (Southern Biotechnology Associates Inc, Birmingham, Alabama) were added (50 μl/well) at an appropriate dilution. After 1 hour incubation at room temperature, the plates were washed four times. A 100 μl of substrate solution (TMB Microwell peroxidase substrate; Kirkegaard& Perry Laboratories, Gaithersburg, MD) was applied to each well. After 20 min incubation at room temperature, the enzyme reaction was stopped by adding 100 μl of stop solution (0.185 M sulfuric acid), and the absorbance at 450 nm was recorded using microplate reader (Molecular devices, Sunnyvale, CA).
2.9. Splenocyte culture and cytokine quantifications
Splenocytes from the vaccinated mice (four mice per group) were obtained as previously described [19], and were cultured in triplicates in 96-well flat-bottomed culture plates (5×105 cells /well) in the presence of different stimulants: 10 μg/ml of SOD, 30 μg/ml of MBP-Bp26, and 107 CFU-equivalent of gamma-irradiated B. neotomae. Cells with plain medium and cells stimulated with 2.5 μg/ml of concanavalin (ConA) were used as controls. The splenocytes were cultured for 5 days, their supernatants were collected and the concentration of selected cytokines was determined using Bio-RAD Bio-Plex Pro™ Mouse cytokine Th1/Th2 Assay according to the manufacturer instructions. The following cytokines were tested in the collected supernatants: IL-2, GM-CSF, IFN-γ, TNF-α, IL-4, IL-5, IL-10, IL-12p70.
2.10. Flow cytometry analysis for IFN-γ secreting antigen-specific CD8+ and CD4+ T cells
Intracellular staining for IFN-γ was performed as previously described with some modifications [21]. After euthanizing the mice, spleens were collected from the vaccinated mice (4 mice in each group) and single cell suspensions were prepared from spleens. Using ACK solution, erythrocytes were lysed and the splenocytes were cultured in 96 well flat-bottomed plates (106 cells /well) with or without specific antigen as described above for splenocyte cultures. After 8 hours of incubation at 37°C in a humid incubator with 5% CO2, brefeldin A (Golgistop; Pharmingen) was added to the culture medium and incubated for another 8 hours. Cells from each treatment were suspended in PBS containing 1% BSA and 0.2% sodium azide (FACS buffer) and stained for surface markers CD8 and CD4 by incubating for 30 minutes at 4°C with appropriately diluted FITC conjugated anti-mouse CD8 antibody (BD Pharmigen, clone53-6.7) and APC-conjugated anti-mouse CD4 antibody (BD Pharmigen, clone L3T4-RM 4.5). After three washes with FACS buffer to remove unbound antibodies, the cells were subjected to intracellular IFN-γ staining with PE-conjugated rat anti-mouse IFN-γ antibody using the Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer’s instructions. Cell stained with PE-conjugated rat IgG1 antibody served as the isotype control. The cells were acquired on BD FACS Canto II™ Flow cytometer (BD Biosciences, CA, USA). The data were analyzed using BD FACSDIVA version 6 software (BD Biosciences, CA, USA) and the proportion of CD4+ and CD8+ T cells that secreted IFN-γ was determined.
2.11. Protection experiments
Groups of 15 mice each were vaccinated by intraperitoneal inoculation with 108 CFU-equivalent of irradiated B. neotomae, B. neotomae/Bp26 and B. neotomae/SOD. A group of mice injected with saline alone served as the control. Two weeks post inoculation the mice were given a booster immunization using the same route and dose. Six weeks after the initial vaccination, 5 mice from each group were challenged by intraperitoneal inoculation with 3 × 104 CFU/mouse of B. melitensis 16M, B. abortus 2308, or B.suis 1330. Two weeks after challenge, the mice were euthanized and the bacterial burden in their spleens was determined as previously described [22].
2.12. Statistical analyses
Absorbance values of ELISA, concentrations of cytokines, and the flow cytometry data of IFN-γ positive T cells were analyzed for differences among the groups by performing analysis of variance with post-hoc Bonferroni for pair-wise comparison using SPSS version 17.0 (SPSS inc, an IBM company, USA). For protection study, one-tailed t-test modified for unequal variances between groups was performed to compare the log transformed bacterial loads in spleens of mice from each vaccinated group with the respective saline control group. P values of ≤0.05 were considered significant.
2. Results
3.1. Overexpression of SOD and Bp26 in B. neotomae
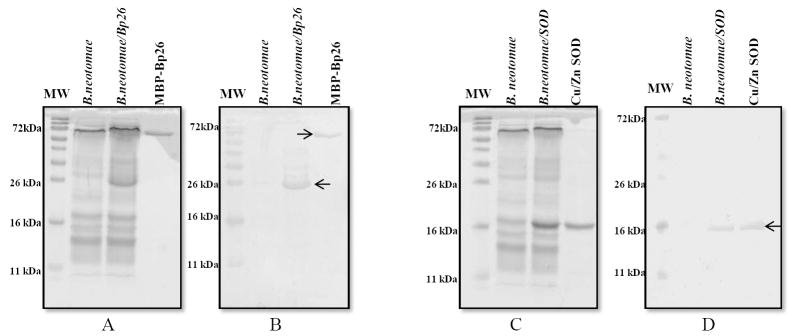
Overexpression of SOD and Bp26 proteins in B. neotomae/SOD and B. neotomae/Bp26 was detected by SDS-PAGE (Fig. 1A and 1C), which was further confirmed by Western blot analysis using the antigen-specific antibodies (Fig.1B and 1D). Compared to B. neotomae, antigen extracts of B. neotomae/Bp26 contained an overexpressed protein of 26 kDa in size which was recognized by the mouse anti-MBP-Bp26 serum (Fig. 1A and Fig. 1B). Similarly, B. neotomae/SOD contained an overexpressed protein of 16 kDa in size which reacted with the goat anti-SOD antibodies (Fig. 1C and 1D). In accordance with the previous reports [23], no detectable level of SOD expression was present in B. neotomae (Fig. 1D).
Figure 1.
Detection of expression of Bp26 and SOD proteins in B. neotomae/Bp26 and B. neotomae/SOD by SDS-PAGE and Western blot analyses. The whole antigen of B. neotomae and B. neotomae/Bp26 and B. neotomae/SOD were separated by 12.5 % SDS-PAGE and either stained with Commassie brilliant blue (A and C) or analyzed by Western blotting with mono-specific serum to MBP-Bp26 (B) or SOD (D). MBP-Bp26k and SOD are proteins purified from overexpressing E. coli. The arrow heads indicate the reacting protein. Lane MW, molecular weight marker in kilodaltons (kDa).
Stable overexpression of both Bp26 and SOD in the respective recombinant B. neotomae strains was consistently detected in all subcultures or gamma-irradiated preparations (data not shown).
2.2. Gamma irradiated B. neotomae: replication, viability and metabolic activity
Aliquots of B. neotomae were exposed to different doses gamma radiation and the CFU present in each aliquot were determined by culturing on TSA plates. As shown in Fig. 2A, gradual decrease in CFU of B. neotomae was detected with increased exposure to gamma radiation. A total loss of replicative ability of the bacteria was observed at a minimum dose of 300 kilorads (Fig 2A).
Figure 2.
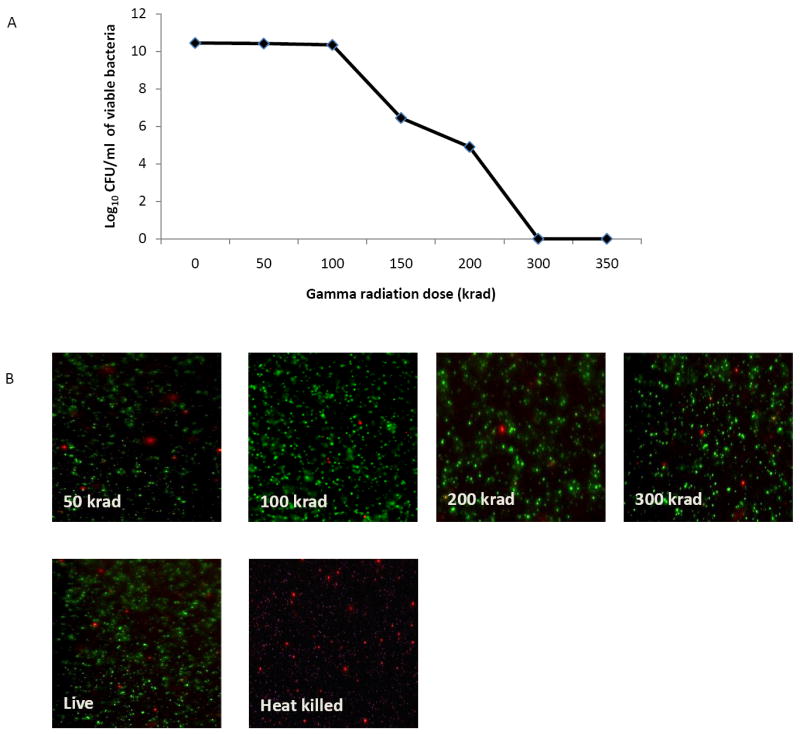
Effect of gamma-irradiation on the viability of B. neotomae. Aliquots of B. neotomae were exposed to different doses of gamma radiation, the viable bacteria in each aliquots were determined by plating 10-fold dilutions on TSA plates (A), and staining with live/dead BacLight kit for microscopy (B). As a control, an aliquot each of live and heat-killed bacteria was included for microscopy.
When stained with both green-fluorescent SYTO 9 and red-fluorescent propidium iodide, gamma-irradiated B. neotomae, similar to the live bacteria, displayed strong green fluorescence and weak red fluorescence, indicating the undamaged cell membranes (Fig 2B). As expected, the heat-killed bacteria showed strong red fluorescent reflecting the damaged cell membranes (Fig 2B).
Gamma irradiation did not impair the metabolic activity of the bacteria, as indicated by the ability of irradiated and live B. neotomae to change the color of Alamar blue dye from blue to pink (data not shown). In contrast, heat-killed B. neotomae failed to cause the color change in the Alamar Blue dye (data not shown).
3.3. Persistence of gamma-irradiated B. neotomae in mice spleens
Persistence of the irradiated bacteria in spleens of inoculated mice was determined by extracting DNA from the tissues and performing a quantitative real-time PCR specific to Brucella. As shown in Table 1, compared to the heat-killed bacteria, gamma-irradiated B. neotomae was present in higher numbers in the spleens at 3 hours, 24 hours and 3 days post-inoculation. By day 5 post-inoculation, no bacteria were detected in the spleens of mice inoculated with either of the bacterial preparations.
Table 1.
Persistence of gamma-irradiated and heat-killed B. neotomae in mouse spleens as detected by the real-time quantitative PCR.
| Post-inoculation time | log 10 CFU equivalent/spleen (mean ± stdev) | |
|---|---|---|
| Irradiated B. neotomae | Heat-killed B. neotomae | |
| 3 hours | 5.04±0.01 | 4.76±0.13 |
| 24 hours | 4.19±0.02 | 1.8±0.07 |
| 3 days | 3.34±0.02 | 0.21±0.15 |
| 5 days | - | - |
3.4. Induction of specific antibody responses
Presence of antibodies specific to B. neotomae total antigens, Bp26, SOD and LPS in serum of the mice vaccinated with the irradiated vaccines was determined by ELISA. Serum samples collected at 2 and 6 weeks after the initial immunizations were analyzed in comparison with the samples from saline inoculated group. Mice vaccinated with B. neotomae and its recombinants B. neotomae/Bp26 and B. neotomae/SOD developed significantly higher levels of IgG specific to the total antigen of B. neotomae at 2 and 6 weeks post vaccination than did mice in the saline inoculated group. Assays with IgG1 and IgG2a specific conjugates revealed that antibodies of both isotypes were present in significantly higher levels than in saline inoculated mice (data not shown).
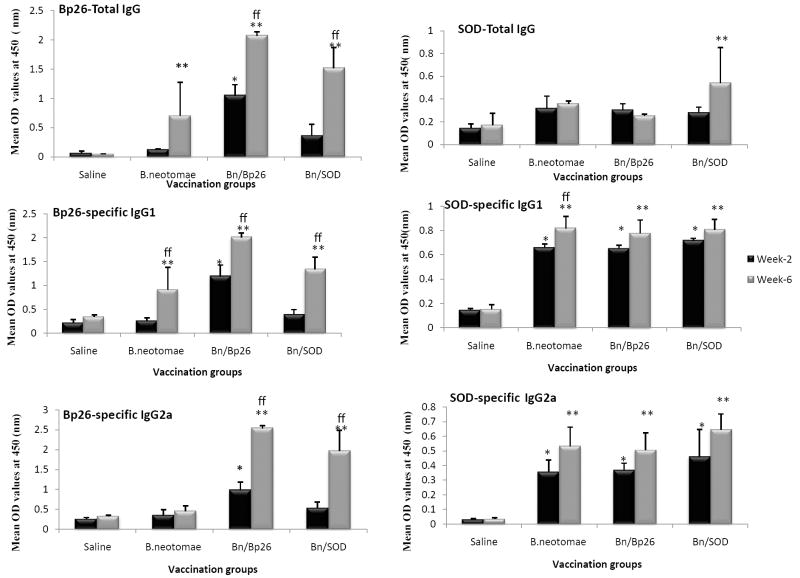
At 2-weeks post vaccination, only mice vaccinated with B. neotomae/Bp26, developed significantly higher levels of Bp26-specific IgG than saline inoculated mice (Fig. 3). However, by week 6, all vaccinated groups showed production of significant levels of Bp26-specific antibodies. Both IgG1 and IgG2a isotypes specific to Bp26 were detected in the vaccinated mice. As expected, the level of Bp26-specific antibodies in mice vaccinated with B. neotomae/Bp26 was higher compared to that detected in mice vaccinated with B. neotomae or B. neotomae/SOD (Fig. 3).
Figure 3.
ELISA detection of Bp26- and SOD-specific IgG, IgG1, and IgG2a antibodies in serum of mice vaccinated with gamma irradiated B. neotomae, B. neotomae/Bp26, and B. neotomae/SOD, or inoculated with saline. Serum samples were collected at 2 and 6 weeks after initial vaccination, and were diluted 1 in 200 and assayed for the presence of total antigen specific antibodies. Results are shown as mean ± standard deviation (n=4) of absorbance at 450 nm of the color developed.
* Significantly different from the corresponding saline group at week 2 (P<0.05).
** Significantly different from the corresponding saline group at week 6 (P<0.05).
ff Week 6 is significantly different from week 2 within each vaccine group (P<0.05).
Significant levels of IgG specific to SOD were detected in serum of all vaccinated mice (Fig. 3). The induced antigen-specific antibodies were of both IgG1 and IgG2a isotypes. No significant differences were detected in the levels of SOD-specific antibodies among the different vaccine groups (Fig. 3).
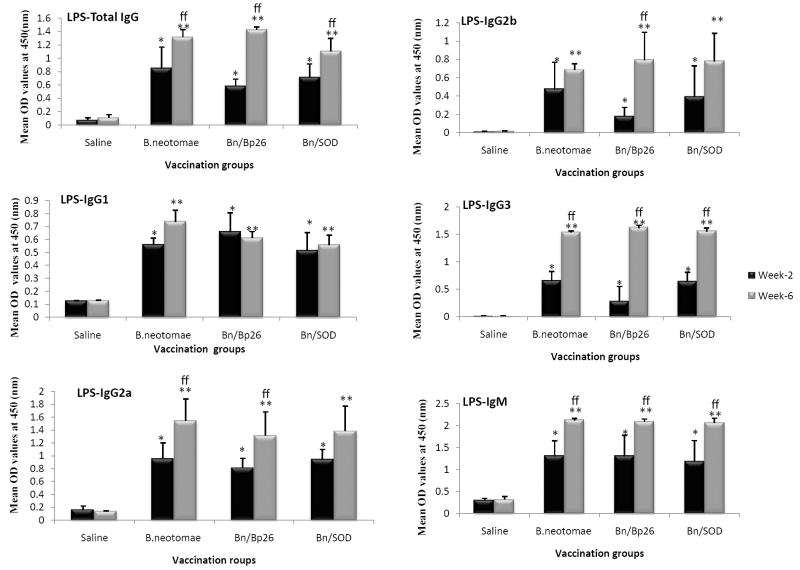
Serum of all vaccinated mice contained significant levels of IgG and IgM to B. neotomae LPS (Fig. 4). The IgG and IgM levels were higher at 6 weeks than at 2 weeks. The produced LPS-specific antibodies included IgG1, IgG2a, IgG2b, and IgG3 isotypes (Fig. 4). There was no difference in the lgG1 levels between the 2- and 6-week serum samples. In contrast, the levels of IgG2a, IgG2b, and IgG3 were higher in the 6-week samples (Fig. 4).
Figure 4.
ELISA detection of B. neotomae LPS specific antibodies IgG, IgG1, and IgG2a antibodies in serum of mice vaccinated with gamma irradiated B. neotomae, B. neotomae/Bp26, and B. neotomae/SOD, or inoculated with saline. Serum samples were collected at 2 and 6 weeks after initial vaccination, and were diluted 1 in 200 and assayed for the presence of total antigen specific antibodies. Results are shown as mean ± standard deviation (n=4) of absorbance at 450 nm of the color developed.
* Significantly different from the corresponding saline group at week 2 (P<0.05).
** Significantly different from the corresponding saline group at week 6 (P<0.05).
ff Week 6 is significantly different from week 2 within each vaccine group (P<0.05).
Antibody levels were also measured in ELISA titers by endpoint titration of the pooled serum samples of each vaccinated group (Supplementary Tables 1-3). Differences in antibody titers between the time-points or groups mirrored that of the antibody levels based on ELISA absorbance values presented in Figs. 3 and 4.
3.5 Antigen-specific cellular immune responses
Specific CMI responses of the vaccinated mice at 6 weeks post initial immunization were determined by quantification of a panel of Th1/Th2 cytokines secreted by the splenocytes and the number of IFN-γ secreting CD4+ and CD8+ T cells upon in vitro stimulation with irradiated B. neotomae, MBP-Bp26 and SOD.
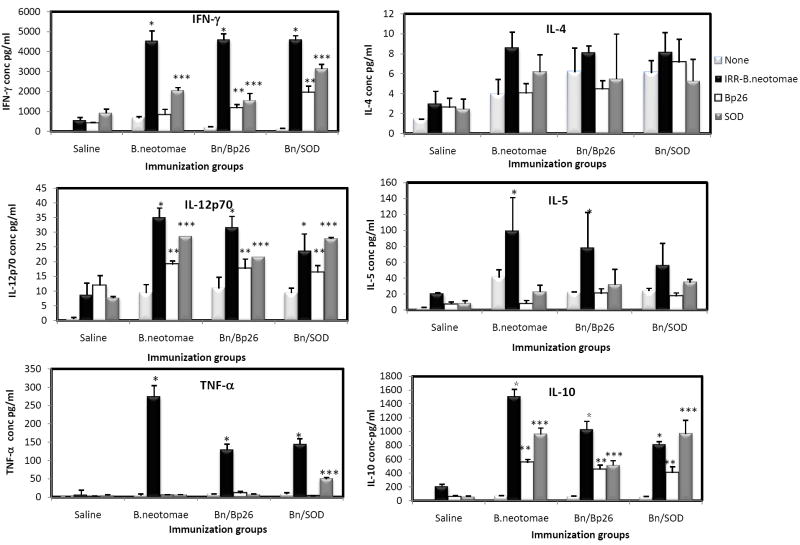
When stimulated with gamma-irradiated B. neotomae, splenocytes from all vaccinated mice, but not the saline inoculated ones, secreted significantly higher levels of IFN-γ, IL-12p70, IL-5 and IL-10 compared to the unstimulated controls (Fig 5). The concentrations of IL-4 in all culture supernatants were low (< 10 pg/ml) and were not significantly different from the corresponding unstimulated controls (Fig. 5).
Figure 5.
Production of specific cytokines by splenocytes of BALB/c mice vaccinated with gamma irradiated B. neotomae and its recombinants after in vitro stimulation with irradiated B. neotomae (IRR-B.neotomae), MBP-Bp26 (Bp26), or SOD. Values are means ± standard deviation (n=4).
* Significantly different from the corresponding unstimulated control with B. neotomae stimulation (P<0.05)
** Significantly different from the corresponding unstimulated control with MBP-Bp26 stimulation (P<0.05)
*** Significantly different from the corresponding unstimulated control with SOD stimulation (P<0.05)
Stimulation with MBP-Bp26 and SOD resulted in the secretion of significantly higher amounts of IL-12p70 and IL-10 by splenocytes from all vaccinated mice compared to the unstimulated controls; the concentrations of IFN-γ and IL-5 were low and variable among the different vaccine groups (Fig. 5).
Splenocytes from all vaccinated, but not saline inoculated, mice secreted significantly higher amounts of TNF-α upon stimulation with irradiated B. neotomae (Fig. 5).
Stimulation with irradiated B. neotomae, MBP-Bp26 and SOD induced secretion of similar high levels of GM-CSF from splenocytes of all vaccinated, but not saline inoculated, mice (data not shown). In contrast, splenocytes from all groups secreted similar low levels of IL-2 upon in vitro stimulation with all the antigens (data not shown). Mitogen stimulation with conA resulted in the secretion of similarly high levels of all the cytokines from splenocytes of all groups (data not shown).
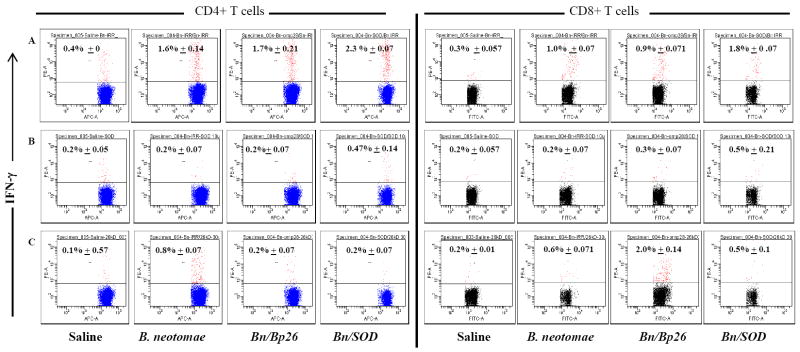
Using flow cytometry analysis of splenocytes stimulated in vitro with irradiated B. neotomae, significantly higher proportions of IFN-γ-secreting CD4+ (1.5-2.3%) and CD8+ (0.9-1.8%) T cells were detected in all vaccinated mice compared to the saline inoculated ones (Fig. 6). In vitro stimulation with MBP-Bp26 and SOD resulted in detection of low proportion of IFN-γ-secreting CD4+ and CD8+ T cells in splenocytes from vaccinated mice.
Figure 6.
Flow cytometric analysis showing the percentage of interferon-γ secreting CD4+ and CD8+ T cells in the spleens of BALB/c mice immunized with gamma irradiated B. neotomae and its recombinants following in vitro stimulation with: (A) gamma irradiated B. neotomae, (B) SOD,(C) MBP-Bp26.
3.6. Protection against challenge with virulent Brucella spp
Compared to the saline-inoculated controls, vaccination of mice with 2 doses of irradiated B. neotomae, B. neotomae/Bp26, or B. neotomae/SOD prior to challenge with virulent B. abortus 2308, B. suis 1330 or B. melitensis 16M significantly reduced the number of virulent brucellae in the spleens 2 weeks after challenge (Table 2). The vaccinated mice contained approximately 2-3 logs, 3.5 logs, and 1.3-2.3 logs lower B. abortus 2308, B. suis 1330, and B. melitensis 16M, respectively (Table 2). For each challenge strain, there were no statistically significant differences in the spleen bacterial loads among the different vaccine groups.
Table 2.
Protection against challenge with 3 virulent Brucella species in mice vaccinated with 2 doses of gamma-irradiated B. neotomae and its recombinant strains.
| Challenge strain | Vaccine | Bacterial load in spleen log10 CFU (mean ± stdev) | Units of protectiona | P value vs corresponding control group |
|---|---|---|---|---|
| B.abortus 2308 | None (saline) | 5.27 ± 0.09 | - | - |
| B. neotomae | 3.32 ± 0.84 | 2.95 | 0.0015 | |
| B. neotomae/Bp26 | 2.29 ± 0.32 | 2.98 | 0.00006 | |
| B. neotomae/SOD | 3.24 ± 0.19 | 2.03 | 0.00005 | |
| B. suis 1330 | None (saline) | 5.71 ± 0.29 | - | - |
| B. neotomae | 2.13 ± 1.16 | 3.58 | 0.003 | |
| B. neotomae/Bp26 | 2.15 ± 0.81 | 3.56 | 0.0028 | |
| B. neotomae/SOD | 2.31 ± 0.99 | 3.4 | 0.0005 | |
| B. melitensis 16M | None (saline) | 5.66 ± 0.11 | - | - |
| B. neotomae | 3.62 ± 1.4 | 2.04 | 0.025 | |
| B. neotomae/Bp26 | 4.32 ± 0.43 | 1.34 | 0.003 | |
| B. neotomae/SOD | 3.33 ± 1.73 | 2.33 | 0.045 |
Units of protection were calculated by subtracting the mean log10 CFU for a vaccinated group from the mean log10 CFU of the corresponding saline control group.
4. Discussion
In this study, we demonstrated that vaccination of mice with gamma-irradiated B. neotomae results in the development of protective immunity against virulent B. abortus, B. suis, and B. melitensis. The vaccine potential of B. neotomae was first suggested in 1963 by Stoenner for controlling swine brucellosis [24]. However, to the best of authors’ knowledge, there are no published reports of examining the usefulness of B. neotomae as a brucellosis vaccine. Though B. neotomae is not known to be a pathogen, safety concerns may preclude its use as a live vaccine, especially in humans. Therefore, we used exposure to a minimum dose of gamma radiation as a means to abolish the ability of B. neotomae to replicate. The use of ionizing radiation has been used in the development of vaccines for preventing infectious diseases of animals and humans that are caused by different viruses, bacteria, and parasites [13, 25-29]. However, a recently developed strategy specifically uses exposure to a minimum dose of radiation, which is sufficient to abolish replication of the organism, for developing safer vaccines for diseases caused by intracellular pathogens [13, 28-29]. Similar to the previously reported findings for B. abortus RB51 and B. melitensis Rev1 [13, 29], B. neotomae exposed to a minimum of 300-350 krads of radiation lost its ability to replicate on nutrient rich TSA medium. The irradiated B. neotomae, however, remained metabolically active as demonstrated by the bacteria’s ability to reduce Alamar blue dye and prevent the penetration of propidium iodide through the cell membranes (Fig. 2). By retaining the metabolic activity, gamma irradiated bacteria can mimic the actual host cell infection of the live bacteria [28-29]. In addition, the bacteria exposed to a minimum dose of radiation retain their de novo protein synthesis capabilities [13, 29]. This feature in case of intracellular bacterial pathogens can lead to elicitation of CMI responses to antigens that are expressed when the bacteria are inside the host cells. Our PCR analysis suggests that the irradiated bacteria persisted at higher numbers and for longer time than the heat-killed bacteria in the spleens of the inoculated mice. Though we have not compared the immune responses induced by the irradiated and heat-killed B. neotomae, similar previous studies with B. abortus RB51 and B. melitensis showed that the irradiated bacteria are better at inducing antigen-specific and protective immune responses [13, 29].
All three irradiated B. neotomae vaccines used in this study conferred similar levels of protection against each of the virulent Brucella spp (Table 1). Overexpression of a protective antigen in the live vaccine strain was previously used as a strategy to induce enhanced protective immunity against brucellosis and tuberculosis in mouse models [18, 30-31]. The selection of SOD and Bp26 for overexpression in B. neotomae was based on the previous findings that these are protective antigens of B. abortus and B. melitensis, respectively [20, 32]. Moreover, when used as a live vaccine, overexpression of SOD in B. abortus RB51 (strain RB51SOD) led to induction of increased SOD-specific immune responses and enhancement of its protective efficacy against B. abortus challenge in BALB/c mice [18]. Unexpectedly, in this study, we did not detect significantly enhanced SOD-specific antibody and T-cell immune responses in mice vaccinated with the irradiated B. neotomae/SOD. Though the irradiated B. neotomae/SOD vaccine, unlike the live RB51SOD vaccine, cannot replicate, we expected that there would be a significantly enhanced SOD-specific immune responses following the booster immunization. This lack of enhanced SOD-specific immune responses suggests that the amount of SOD present in the irradiated vaccine dose was not sufficiently high enough or that the other immunodominant antigens of B. neotomae affected the immunogenic potential of SOD. It is also possible that the overexpression of SOD did not occur under in vivo conditions. In case of Bp26, mice vaccinated with the irradiated B. neotomae/Bp26 developed increased levels of antibodies and more numbers of IFN-γ-secreting CD8+ T cells specific to Bp26. Nevertheless, these increased Bp26-specific immune responses did not translate to enhanced protection against the challenge with any of the 3 virulent Brucella spp. (Table 1). If overexpression of some other protective proteins can lead to enhancement of the vaccine efficacy remains to be tested.
In general, a Th1 type of immune responses is considered desirable for protection against intracellular bacterial infections, such as brucellosis. However, some published reports document that the acquired resistance against brucellosis can occur even in presence of a mix of Th1 and Th2 immunity [33-34]. The presence of antigen-specific IgG1 and IgG2a antibodies in serum of the vaccinated mice suggest that the irradiated B. neotomae vaccines induced a mix of Th1 and Th2 immune responses. However, based on cytokine secretion by the antigen-specific splenocytes, it appears that the induced Th1 response was more prominent, because of the significantly higher concentration of IFN-γ in supernatants of cultures stimulated with irradiated B. neotomae; the concentrations of Th2 cytokines IL-4 and IL-5 were marginally higher, compared to the spontaneous release cell controls (Fig. 5). The presence of increased concentration of IL-12p70 and TNF-α in the antigen-stimulated culture supernatants also suggests the development of Th1 type effector cells. Although T cells are not a major source for IL-12, a key facilitator of Th1differentiation during an immune response, antigen-specific Th1 cells can positively affect IL-12 secretion by antigen-presenting cells through contact-dependent manner [35].
All three B. neotomae vaccines induced the development of antigen-specific IL-10-secreting lymphocytes (Fig. 5). IL-10 is usually considered to be an anti-inflammatory cytokine which participates in reducing the adverse effects resulting from the excessive production of proinflammatory cytokines such as IFN-γ [36]. Previous research demonstrated that inhibition of IL-10 activity leads to increased clearance of B. abortus from infected mice [37]. However, there appears to be no correlation between IL-10 secretion by the vaccine-induced antigen-specific T cells and resistance to virulent Brucella challenge. For example, studies with some experimental Brucella vaccines, such as certain deletion mutants of B. abortus and B. melitensis, and plasmid DNA and recombinant protein of Omp31, induction of IL-10 secreting antigen-specific lymphocytes had no negative effect on protection to challenge infection [38-39]. Recently, it has been shown that IL-10 can also promote inflammation during active inflammatory responses [36]. Therefore, it is possible that vaccine-induced IL-10 secretion may play a beneficial role in mediating protection against virulent Brucella challenge.
Both T cells and anti-smooth LPS antibodies play a role in mediating protection against brucellosis [40-41]. Antigen-specific T cells that secrete IFN-γ are primarily responsible for the acquired CMI against virulent Brucella infection [41]. Passive transfer experiments showed that antibodies to the O-antigen can mediate protection [42-47]. Anti-O antibodies of isotypes IgM, IgG2a, IgGb and IgG3 are effective at affording protection against brucellosis in mice [45-47]. All three irradiated B. neotomae vaccines induced LPS-specific antibodies of these isotypes, which increased upon booster immunization (Fig. 4). Usually, IgM is mainly produced during the primary immune response by short-lived plasma cells, thereafter, following the isotype switching in germinal centers, long-lived plasma cells develop which produce antibodies of other isotypes and the production of IgM decreases. Therefore, it is unclear why the levels of IgM remained elevated even after 4 weeks after the booster immunization. Whether this feature is specific to B. neotomae remains to be determined.
Immunization of mice with any of the three gamma-irradiated B. neotomae vaccines conferred a significant level of protection against virulent B. abortus, B. suis, and B. melitensis challenges. Based on the units of protection afforded by the three vaccines, the level of resistance against B. abortus and B. suis appeared to be better than that against B. melitensis challenge (Table 2). One contributing factor for this difference could be the resistance of B. melitensis to complement-mediated lysis. Both smooth and rough B. melitensis are more resistant to complement-mediated killing than B. abortus with similar phenotypes [48-49]. Another factor could be the variation in epitope dominance in the O antigen of Brucella smooth LPS between B. melitensis and B. abortus or B. suis. Two epitopes, designated C (for common among all Brucella smooth LPS) and C/Y (for common between smooth LPS of Brucella and Yersinia enterocolitica O:9), are present in smooth LPS from all Brucella species [50]. Two additional epitopes, designated A (for abortus) and M (for melitensis), are identified in the O-antigen portion of the smooth LPS [51-52]. Different smooth Brucella strains possess varying proportions of A and M epitopes. The O-antigen of Brucella smooth LPS is a homopolymer of 4.6-dideoxy-4-formamido-α-D-mannopyranosyl units. These units are linked in α-1,2 in A-dominant smooth Brucella strains, but every fifth residue is linked in α-1,3 in M-dominant strains. Antibodies to the dominant epitope of the O-antigen are more effective in mediating protection [50]. B. neotomae, B. abortus 2308, and B. suis 1330 are A-dominant strains, while B. melitensis 16M is a M-dominant strain [50]. Although we did not identify the epitope specificity of the anti-smooth LPS antibodies produced by the vaccinated mice, it is highly likely that the irradiated B. neotomae vaccines induced more antibodies to specific to A than M epitope. In addition, antigen specificity of the T cell responses could also have affected the level of protection conferred by the vaccines against B. melitensis challenge. Enhancement of T cell responses through strategies such as microencapsulation might increase the protective efficacy of the irradiated B. neotomae vaccines [53].
In the previous studies with the irradiated B. abortus RB51 and B. melitensis Rev1, a single dose of 109 CFU equivalent per mouse was used for immunization [13, 29]. However, in our preliminary experiments, we observed that the mice inoculated with 109 CFU equivalent of irradiated B. neotomae exhibited clinical signs consistent with the vaccine-induced sickness during the first 3-5 days after immunization. On the contrary, mice inoculated with 108 CFU equivalent of irradiated B. neotomae did not show any signs of distress. Therefore, an empirical regimen of 2 doses of 108 CFU equivalent of the irradiated bacteria each at 2-weeks apart was used for the studies. Further studies are warranted to determine the minimum effective dose of the vaccine and the duration of the protective immune status in the vaccinated animals. In addition, examining the potential of the irradiated B. neotomae as an oral vaccine would be very pertinent for human application.
In conclusion, the results suggest that gamma-irradiated B. neotomae can be used for developing an effective vaccine against brucellosis caused by B. abortus, B. suis and B. melitensis. The non-pathogenic feature of B. neotomae along with the inability of the irradiated bacteria to replicate in the host makes it a safer alternative to the other live attenuated vaccine candidates for human brucellosis.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grant AI065667-01A2 from the National Institute of Allergy and Infectious Diseases. We thank Lynn Guptill and Harm HogenEsch for critical review of the manuscript and Kay Carlson for help with the mouse protection studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213–21. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–86. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 3.Cutler SJ, Whatmore AM, Commander NJ. Brucellosis - new aspects of an old disease. J Appl Microbiol. 2005;98(6):1270–81. doi: 10.1111/j.1365-2672.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 4.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–9. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 5.Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM, Rice-Ficht AC. Brucellosis: The case for live, attenuated vaccines. Vaccine. 2009;27:D40–D3. doi: 10.1016/j.vaccine.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. 2002;90(1-4):479–96. doi: 10.1016/s0378-1135(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 7.Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. 2010;34(3):379–394. doi: 10.1111/j.1574-6976.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 8.Blasco JM, Díaz R. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. The Lancet. 1993;342(8874):805. doi: 10.1016/0140-6736(93)91571-3. [DOI] [PubMed] [Google Scholar]
- 9.Stoenner HG, Lackman DB. A new species of Brucella isolated from the desert wood rat, Neotoma lepida Thomas. Am J Vet Res. 1957;18(69):947–51. [PubMed] [Google Scholar]
- 10.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, 2nd, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 166(1):175–6. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 11.Vemulapalli R, He Y, Boyle SM, Sriranganathan N, Schurig GG. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1 type immune responses. Infect Immun. 2000;68(6):3290–6. doi: 10.1128/iai.68.6.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuiston JR, Schurig GG, Sriranganathan N, Boyle SM. Transformation of Brucella species with suicide and broad host-range plasmids. Methods Mol Biol. 1995;47:143–8. doi: 10.1385/0-89603-310-4:143. [DOI] [PubMed] [Google Scholar]
- 13.Sanakkayala N, Sokolovska A, Gulani J, HogenEsch H, Sriranganathan N, Boyle SM, et al. Induction of antigen-specific Th1-type immune responses by gamma-irradiated recombinant Brucella abortus RB51. Clin Diagn Lab Immunol. 2005;12(12):1429–36. doi: 10.1128/CDLI.12.12.1429-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bounaadja L, Albert D, Chenais B, Henault S, Zygmunt MS, Poliak S, et al. Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31 and per target genes. Vet Microbiol. 2009;137(1-2):156–64. doi: 10.1016/j.vetmic.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Morrison DC, Leive L. Fractions of Lipopolysaccharide from Escherichia coli O111-B4 prepared by two extraction procedures. J Biol Chem. 1975;250(8):2911–9. [PubMed] [Google Scholar]
- 17.Phillips M, Pugh GW, Deyoe BL. Chemical and protective properties of Brucella lipopolysaccharide obtained by butanol extraction. Am J Vet Res. 1989;50(3):311–7. [PubMed] [Google Scholar]
- 18.Vemulapalli R, He Y, Cravero S, Sriranganathan N, Boyle SM, Schurig GG. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect Immun. 2000;68(6):3286–9. doi: 10.1128/iai.68.6.3286-3289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vemulapalli R, Duncan AJ, Boyle SM, Sriranganathan N, Toth TE, Schurig GG. Cloning and sequencing of yajC and secD homologs of Brucella abortus and demonstration of immune responses to YajC in mice vaccinated with B. abortus RB51. Infect Immun. 1998;66(12):5684–91. doi: 10.1128/iai.66.12.5684-5691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onate AA, Vemulapalli R, Andrews E, Schurig GG, Boyle S, Folch H. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect Immun. 1999;67(2):986–8. doi: 10.1128/iai.67.2.986-988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 22.Schurig GG, Roop RM, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 199;28(2):171–88. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 23.Bricker BJ, Tabatabai LB, Judge BA, Deyoe BL, Mayfield JE. Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect Immun. 1990;58(9):2935–9. doi: 10.1128/iai.58.9.2935-2939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoenner HG. The behavior of Brucella neotomae and Brucella suis in reciprocal superinfection experiments in mice and guinea pigs. Am J Vet Res. 1963;24:376–80. [PubMed] [Google Scholar]
- 25.Hubbert WT, Miller JN. Studies on immunity in experimental leptospirosis: the immunogenicity of Leptospira icterohemorrhagiae attenuated by gamma-irradiation. J Immunol. 1965;95(4):759–64. [PubMed] [Google Scholar]
- 26.Jarrett WF, Jennings FW, Mc IW, Mulligan W, Urquhart GM. Irradiated helminth larvae in vaccination. Proc R Soc Med. 1958;51(9):743–4. doi: 10.1177/003591575805100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SS, Bakken RR, Lind CM, Garcia P, Jenkins E, Glass PJ, et al. Comparison of the immunological responses and efficacy of gamma-irradiated V3526 vaccine formulations against subcutaneous and aerosol challenge with Venezuelan equine encephalitis virus subtype IAB. Vaccine. 2010;28(4):1031–40. doi: 10.1016/j.vaccine.2009.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta SK, Okamoto S, Hayashi T, Shin SS, Mihajlov I, Fermin A, et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity. 2006;25(1):143–52. doi: 10.1016/j.immuni.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Magnani DM, Harms JS, Durward MA, Splitter GA. Nondividing but metabolically active gamma-irradiated Brucella melitensis is protective against virulent B. melitensis challenge in mice. Infect Immun. 2009;77(11):5181–9. doi: 10.1128/IAI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhar N, Rao V, Tyagi AK. Recombinant BCG approach for development of vaccines: cloning and expression of immunodominant antigens of M. tuberculosis. Fems Microbiol Lett. 2000;190(2):309–16. doi: 10.1111/j.1574-6968.2000.tb09304.x. [DOI] [PubMed] [Google Scholar]
- 31.Rao V, Dhar N, Tyagi AK. Modulation of host immune responses by overexpression of immunodominant antigens of Mycobacterium tuberculosis in bacille Calmette-Guérin. Scand J Immunol. 2003;58(4):449–61. doi: 10.1046/j.1365-3083.2003.01321.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW. Selection of protective epitopes of Brucella melitensis by DNA vaccination. Infect Immun. 2005;73(11):7297–303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velikovsky CA, Goldbaum FA, Cassataro J, Estein S, Bowden RA, Bruno L, et al. Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect Immun. 2003;71(10):5750–5. doi: 10.1128/IAI.71.10.5750-5755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delpino MV, Estein SM, Fossati CA, Baldi PC, Cassataro Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine. 2007;25(37-38):6721–9. doi: 10.1016/j.vaccine.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Ria F, Penna G, Adorini L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur J Immunol. 1998;28(6):2003–16. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Sharif MN, Tassiulas I, Hu Y, Mecklenbrauker I, Tarakhovsky A, Ivashkiv LB. IFN-alpha priming results in a gain of proinflammatory function by IL-10: implications for systemic lupus erythematosus pathogenesis. J Immunol. 2004;172(10):6476–81. doi: 10.4049/jimmunol.172.10.6476. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes DM, Baldwin CL. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995;63(3):1130–3. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassataro J, Velikovsky CA, Bruno L, Estein SM, de la Barrera S, Bowden R, et al. Improved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boosting. Clin Vaccine Immunol. 2007;14(7):869–74. doi: 10.1128/CVI.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahl-McDonagh MM, Ficht TA. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect Immun. 2006;74(7):4048–57. doi: 10.1128/IAI.01787-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez D, Grillo MJ, De Miguel MJ, Ali T, Arce-Gorvel V, Delrue RM, et al. Brucellosis vaccines: assessment of Brucella melitensis lipopolysacharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS One. 2008;3(7):e2760. doi: 10.1371/journal.pone.0002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yingst S, Hoover DL. T cell immunity to brucellosis. Crit Rev Microbiol. 2003;29(4):313–31. doi: 10.1080/713608012. [DOI] [PubMed] [Google Scholar]
- 42.Limet J, Plommet AM, Dubray G, Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987;138(3):417–24. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- 43.Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143(10):3330–3337. [PubMed] [Google Scholar]
- 44.Montaraz JA, Winter AJ, Hunter DM, Sowa BA, Wu AM, Adams LG. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986;51(3):961–3. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elzer PH, Jacobson RH, Jones SM, Nielsen KH, Douglas JT, Winter AJ. Antibody-mediated protection against Brucella abortus in BALB/c mice at successive periods after infection: variation between virulent strain 2308 and attenuated vaccine strain 19. Immunology. 1994;82(4):651–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Elzer PH, Jacobson RH, Nielsen KH, Douglas JT, Winter AJ. BALB/c mice infected with Brucella abortus express protracted polyclonal responses of both IgG2a and IgG3 isotypes. Immunol Lett. 1994;42(3):145–50. doi: 10.1016/0165-2478(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 47.Cloeckaert A, Jacques I, de Wergifosse P, Dubray G, Limet JN. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect Immun. 1992;60(1):312–5. doi: 10.1128/iai.60.1.312-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Prada CM, Nikolich M, Vemulapalli R, Sriranganathan N, Boyle SM, Schurig GG, et al. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect Immun. 2001;69(7):4407–16. doi: 10.1128/IAI.69.7.4407-4416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Prada CM, Zelazowska EB, Nikolich M, Hadfield TL, Roop RM, 2, Robertson GL, et al. Interaction between Brucella melitensis and human phagocytes: bacterial surface O-polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect Immun. 2003;71(4):2110–9. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cloeckaert A, Weynants V, Godfroid J, Verger JM, Grayon M, Zygmunt MS. O-Polysaccharide epitopic heterogeneity at the surface of Brucella spp. studied by enzyme-linked immunosorbent assay and flow cytometry. Clin Diagn Lab Immunol. 1998;5(6):862–70. doi: 10.1128/cdli.5.6.862-870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caroff M, Bundle DR, Perry MB, Cherwonogrodzky JW, Duncan JR. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984;46(2):384–8. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bundle DR, Cherwonogrodzky JW, Perry MB. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry. 1987;26(26):8717–26. doi: 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- 53.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect Immun. 2008;76(6):2448–55. doi: 10.1128/IAI.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.