Abstract
Tobacco addiction is a chronic disorder that is characterized by craving for tobacco products, withdrawal upon smoking cessation, and relapse after periods of abstinence. Previous studies demonstrated that systemic administration of α2-adrenergic receptor agonists attenuates stress-induced reinstatement of drug seeking in rats. The aim of the present experiments was to investigate the role of noradrenergic transmission in the central nucleus of amygdala (CeA) in stress-induced reinstatement of nicotine seeking. Rats self-administered nicotine for 14–16 days and then nicotine seeking was extinguished by substituting saline for nicotine. The effect of the intra-CeA infusion of the α2-adrenergic receptor agonists clonidine and dexmedetomidine, the nonselective β1/β2-adrenergic receptor antagonist propranolol, and the α1-adrenergic receptor antagonist prazosin on stress-induced reinstatement of nicotine seeking was investigated. In all the experiments, exposure to footshocks reinstated extinguished nicotine seeking. The administration of clonidine or dexmedetomidine into the CeA attenuated stress-induced reinstatement of nicotine seeking. The administration of propranolol or prazosin into the CeA did not affect stress-induced reinstatement of nicotine seeking. Furthermore, intra-CeA administration of clonidine or dexmedetomidine did not affect operant responding for food pellets. This suggests that the effects of clonidine and dexmedetomidine on stress-induced reinstatement of nicotine seeking were not mediated by motor impairments or sedation. Taken together, these findings indicate that stimulation of α2-adrenergic receptors, but not blockade of α1 or β-adrenergic receptors, in the CeA attenuates stress-induced reinstatement of nicotine seeking. These findings suggest that α2-adrenergic receptor agonists may at least partly attenuate stress-induced reinstatement of nicotine seeking by stimulating α2-adrenergic receptors in the CeA.
Keywords: Nicotine, footshocks, clonidine, dexmedetomidine, propranolol, prazosin
1. Introduction
Tobacco addiction is a chronic disorder that is characterized by a loss of control over smoking, affective withdrawal symptoms upon smoking cessation, and relapse after periods of abstinence (American Psychiatric Association, 2000; McLellan et al., 2000; O’Brien, 2003). Nicotine is one of the main components of tobacco smoke that leads to and maintains smoking (Bardo et al., 1999; Crooks and Dwoskin, 1997; Stolerman and Jarvis, 1995). This is supported by studies that show that rodents, dogs, and nonhuman primates readily learn to self-administer nicotine (Corrigall and Coen, 1989; Goldberg et al., 1981; Risner and Goldberg, 1983). Furthermore, nicotine induces conditioned place preference and facilitates intracranial self-stimulation (ICSS) in rats (Harrison et al., 2002; Le Foll and Goldberg, 2005; Pradhan and Bowling, 1971). Conditioned place preference and facilitation of ICSS are indicative of a potentiation of brain reward function (Bardo and Bevins, 2000; Schaefer and Michael, 1992). Nicotine mediates its positive reinforcing effects (e.g., mild euphoria) at least partly via the activation of central nicotinic acetylcholine receptors (nAChRs). Pharmacological blockade of nAChRs or genetic deletion of the β2-subunit of the nAChR decreases nicotine self-administration in rodents (Corrigall et al., 1994; Corrigall and Coen, 1989; Donny et al., 1999; Picciotto et al., 1998; Watkins et al., 1999). Cessation of smoking in humans leads to an acute withdrawal syndrome that is characterized by affective symptoms such as depressed mood, anxiety, and difficulty concentrating (American Psychiatric Association, 2000; Bruijnzeel and Gold, 2005). It has been hypothesized that the acute negative affective aspects of nicotine withdrawal provide a powerful motivational force for the continuation of smoking (Koob et al., 1997; Markou et al., 1998).
Epidemiological studies indicate that exposure to stressors increases the risk for relapse to smoking in humans (Cohen and Lichtenstein, 1990; Doherty et al., 1995; Kassel et al., 2003; McKee et al., 2003; Niaura et al., 2002; Shiffman and Waters, 2004; Swan et al., 1988). Stressors also increase the risk for relapse to other drugs of abuse such as alcohol and opioids (Brown et al., 1995; Kosten et al., 1986). Animal models have been developed to delineate the neuronal mechanisms that underlie stress-induced relapse. In one of these models, rats are allowed to self-administer a drug for a specific amount of time and then drug seeking is extinguished by substituting saline for the drug solution. Exposure to footshocks leads to a resumption of extinguished drug seeking (Shaham et al., 2003). Footshocks have been shown to reinstate extinguished heroin, cocaine, alcohol, and nicotine seeking in rats (Buczek et al., 1999; Erb et al., 2001; Liu and Weiss, 2002; Shaham and Stewart, 1995). In a previous study we reported that exposure to the contextual stimuli associated with prior footshock-stress does not reinstate extinguished nicotine seeking (Zislis et al., 2007). Several studies have reported that footshocks do not reinstate operant responding that was previously maintained by food pellets, sucrose pellets, or sucrose solutions (Ahmed and Koob, 1997; Buczek et al., 1999; Le et al., 1998; Mantsch and Goeders, 1999). This suggests that stressors reinstate nonreinforced responding for addictive substances, but not for natural reinforcers, such as food pellets or sucrose.
Although exposure to stressors plays an important role in relapse to smoking in humans, only a few preclinical studies have investigated the neuronal mechanisms that mediate stress-induced relapse to smoking. Previous studies in our laboratory investigated the role of CRF and noradrenergic transmission in stress-induced reinstatement of nicotine seeking in rats. These studies demonstrated that central administration of the non-specific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) and the specific CRF1 receptor antagonist R278995/CRA0450, but not the CRF2 receptor antagonist astressin-2B, attenuates stress-induced reinstatement of nicotine seeking (Bruijnzeel et al., 2009; Zislis et al., 2007). Furthermore, it was shown that systemic administration of the α2-adrenergic receptor agonist clonidine attenuates footshock-induced reinstatement of nicotine seeking (Zislis et al., 2007). These studies suggest that CRF and noradrenergic transmission plays an important role in stress-induced reinstatement of nicotine seeking. This pattern of results is in line with the findings of studies that investigated the neurobiological substrates underlying footshock-induced reinstatement of cocaine and alcohol seeking (Erb et al., 2000; Le et al., 2000). The α2-adrenergic receptor agonists clonidine, lofexidine, and guanabenz attenuate stress, but not cue, induced reinstatement of cocaine seeking in rats (Erb et al., 2000). Furthermore, the intracerebroventricular (icv) administration of the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) or systemic administration of the specific CRF1 receptor antagonist CP-154,526 attenuates stress-induced reinstatement of alcohol seeking (Le et al., 2000). Recent studies indicate that the activation of α1-adrenergic receptor plays a role in cue and drug-induced reinstatement of drug seeking. The α1-adrenergic receptor antagonist prazosin attenuates nicotine and cue-induced reinstatement of extinguished nicotine seeking in rats (Forget et al., 2010). Furthermore, the norepinephrine transport inhibitor nisoxetine induces the reinstatement of cocaine seeking in squirrel monkeys and this effect is blocked by prazosin (Fuller et al., 1975; Platt et al., 2007). In contrast, prazosin does not prevent forced swim stress-induced reinstatement of cocaine-induced conditioned place preference in mice (Mantsch et al., 2010). These studies suggest that α1-adrenergic receptor antagonists attenuate drug and cue-induced reinstatement of drug seeking but not stress-induced reinstatement of drug seeking.
Experimental evidence suggests that noradrenergic transmission in the central nucleus of the amygdala (CeA) plays a role in stress-induced reinstatement of drug seeking. This is supported by the observation that footshocks induce the release of norepinephrine in the amygdala (Galvez et al., 1996; Iimori et al., 1982). In addition, the administration of a mixture of the β1-adrenergic receptor antagonist betaxolol and the β2-adrenergic receptor antagonist ICI-118,551 into the CeA attenuates footshock-induced reinstatement of cocaine seeking (Leri et al., 2002). The aim of the present experiments was to investigate the role of noradrenergic transmission in the CeA in stress-induced reinstatement of nicotine seeking. The first experiment investigated the effects of clonidine in the CeA and the second experiment investigated the effects of dexmedetomidine in the CeA on stress-induced reinstatement of nicotine seeking. Clonidine (Ki α2: 3.2 nM; Ki α1: 713 nM) and dexmedetomidine (Ki α2: 1.1 nM; Ki α1: 1750 nM) are potent α2-adrenergic receptor agonists and they also bind with a relatively low affinity to α1-adrenergic receptors and imidazoline 1 (I1) and I2 receptors (Smith et al., 2009; Virtanen et al., 1988). Dexmedetomidine is a somewhat more selective α2-adrenergic receptor agonist than clonidine. Dexmedetomidine has a 7 fold greater selectivity for α2 over α1-adrenergic receptors than clonidine (Dexmedetomidine, α2/α1 ratio: 1620; Clonidine, α2/α1 ratio: 220) (Savola and Virtanen, 1991; Virtanen et al., 1988). Both the I1 and I2 receptor have been detected in the central nervous system (Dardonville and Rozas, 2004; Smith et al., 2009). Dexmedetomidine has a lower affinity for the I1 receptor than clonidine (Dexmedetomidine Ki: 637 nM; Clonidine Ki: 55 nM)(Piletz and Sletten, 1993). Both dexmedetomidine and clonidine have a very low affinity for the I2 receptor (Dexmedetomidine Ki: 38,700 nM; Clonidine Ki: 300,000 nM)(Piletz and Sletten, 1993). The third experiment investigated the effects of the nonselective β1/β2-adrenergic receptor antagonist propranolol in the CeA on footshock-induced reinstatement of nicotine seeking. The fourth experiment investigated the effects of the specific α1-adrenergic receptor antagonist prazosin in the CeA on footshock-induced reinstatement of nicotine seeking. These studies may improve the understanding of the role of noradrenergic transmission in the CeA in stress-induced reinstatement of nicotine seeking.
2. Materials and Methods
2.1. Subjects
Male Wistar rats (Charles River, Raleigh, NC) weighing 250–300 g at the beginning of the experiments were used. Animals were single-housed in a temperature and humidity-controlled vivarium and maintained on a 12 hr reversed light-dark cycle (lights off at 9 AM). All testing occurred during the first 3 hours of the dark cycle. Food and water were available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine hydrogen tartrate salt, prazosin hydrochloride, clonidine hydrochloride, propranolol hydrochloride, and pentobarbital sodium salt were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Dexmedetomidine hydrochloride was purchased from Tocris (Tocris Bioscience, Ellisville, MO, USA). Nicotine and pentobarbital were dissolved in saline (0.9% sodium chloride). Clonidine, dexmedetomidine, prazosin, and propranolol were dissolved in distilled water. Drug doses are expressed as salt with the exception of the nicotine dose which is expressed as free base.
2.3. Surgical Procedures
2.3.1. Catherization surgery
Rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3% isoflurane) and prepared with a chronic catheter in the right jugular vein as described previously (Bruijnzeel et al., 2009; Zislis et al., 2007). Catheters consisted of silastic tubing (length 13.5 cm, 0.51 mm inside diameter × 0.94 mm outside diameter, Dow Corning, Midland, MI) that was connected to a 22 gauge stainless steel guide cannula, which was molded onto a durable polyester fiber mesh (Plastics One, Roanoke, VA). The tubing was passed subcutaneously from the mid scapular region to the ventral thorax/lower part of the neck, inserted into the jugular vein (4.0 cm), and secured with silk suture thread. After the implantation of the catheters, the animals were allowed to recover for 7 days. During the recovery period, the rats received daily iv infusions with the antibiotic Timentin (7.0 mg/kg day, 0.2 ml, ticarcillin disodium and clavulanate potassium, Beecham Laboratories, Piscataway, NJ) to prevent postoperative infections. To ensure long-term patency of the catheters, 0.1 ml lock solution (heparinized glycerol, 10000 unites/ml; Amresco Inc., Solon, Ohio) was infused into the catheter after the administration of Timentin. When the catheters were not being used, they were closed with a short length of Tygon tubing (Saint-Gobain Performance Plastics, Valley Forge, PA) plugged with monofilament and covered with a stainless steel cap. Catheter patency was tested with 0.1 ml Brevital (1% methohexital sodium, King Pharmaceuticals, Bristol, TE) at the end of the recovery period or when an animal displayed a sudden decrease in responding for nicotine. Animals with patent catheters exhibit pronounced loss of muscle tone within 2 seconds of intravenous injection of Brevital.
2.3.2. Intracranial cannulae implantations
For all experiments, the rats were prepared with cannulae above the CeA. The rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3% isoflurane) and placed in a Kopf stereotaxic frame (Model 940, David Kopf Instruments, Tujunga, CA) with the incisor bar set 3.3 mm below the interaural line (flat skull). Then the rats were prepared with 11 mm stainless steel 23 gauge cannulae above the CeA according to Paxinos and Watson, 1998, and a previous study by Koob and colleagues (Funk et al., 2006). Bilateral cannulae were implanted 2.5 mm above the CeA (anterior-posterior [AP] −2.3, medial lateral [ML] ±4.0 mm, dorsal-ventral [DV] −4.7 from dura), At the end of the surgery, 11 mm removable 30 gauge wire stylets were inserted in the cannulae to maintain patency. The cannulae were permanently secured to the skull with four stainless steel skull screws (shaft length, 1.60 mm; shaft diameter 1.57 mm; Plastics One, Roanoke, VA) and dental cement (CO-Oral-Ite Dental, Diamond Springs, CA).
2.4. Apparatus
Food training and drug self-administration sessions were conducted in sixteen operant conditioning chambers that were located inside sound attenuating boxes (Med Associates, St. Albans, VT). One side of the operant conditioning chambers was equipped with an active and an inactive lever, and above each lever was a cue light. Data collection and test sessions were controlled by a desktop Windows-based computer. Delivery of the nicotine solution was controlled by a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). Tygon tubing (0.508 mm inside diameter × 1.524 mm outside diameter, Saint-Gobain Performance Plastics, Valley Forge, PA) connected a 10 ml syringe, which was placed in the pump, to the backmount of the rat. A protective metal spring covered the tubing. One side of the spring was connected to a stainless steel swivel (Instech Laboratories, Plymouth Meeting, PA) and the other side of the spring was connected to the backmount.
2.5. Food training
The rats were food deprived for 48 hours (5 gram of lab chow/day) prior to the onset of food training. After the onset of food training, the rats were fed 17–20 gram (80–95% of baseline ad libitum calories) of lab chow per day. The rats were fed about one hour after the end of the food training sessions. Standard 45-mg food pellets were used in all the operant conditioning sessions (Bio-Serv, Frenchtown, NJ). During the first training session, the rats received 3.4 gram of food pellets at fixed intervals (one pellet every 12 seconds for 5 minutes, then a 5 minute break; this sequence was repeated during the initial 30 minute session; total number of pellets delivered was 75) with no requirement to respond on the active lever. After this session, the rats had to respond on the active lever to receive food pellets. Instrumental training started on a fixed-ratio 1, time-out 1 second (FR1 TO1-s) schedule of reinforcement and the training sessions lasted 1 hour. The training schedule was progressively changed according to the following sequence: FR1 TO1, FR1 TO10, FR1 TO20, FR2 TO20, FR5 TO20-s. The rats had to reach the criterion of 100 pellets earned during a 1-hour session before training at the next level started. Food training continued until the subjects earned 100 food pellets in a 1-hour session on an FR5 TO20-s schedule of reinforcement. Food training typically required 7–9 days. After the completion of the food training sessions, all the rats were fed 20 gram of lab chow per day (95% of baseline ad libitum calories) 1 hour after the end of testing.
2.6. Intracranial microinjections
Drugs were administered bilaterally into the CeA by using 30 gauge stainless steel injectors that extended 2.5 mm (length of injector tip was 13.5 mm) beyond the guide cannulae. The injection volume was 0.5 μl/side and the drug was infused over a 66 second period as described previously (Bruijnzeel and Markou, 2004). The rats were gently retrained by hand during the infusions. The infusion speed was regulated by a Harvard Apparatus syringe pump (model 975) and the pump was equipped with 10 μl syringes (Hamilton, Rena, NE). The syringes were connected to the injectors with Tygon microbore PVC tubing (0.25 mm ID × 0.76 mm OD). The injectors were left in place for 30 seconds post-injection to allow diffusion from the injector tip. The dummy stylets, 11 mm, were re-inserted immediately after the injectors were removed.
2.7. Nicotine self-administration, extinction, and stress-induced reinstatement
After the successful completion of the food training and catherization surgeries, the rats were allowed to self-administer nicotine at the 0.03 mg/kg/infusion (free base) dose by switching the delivery of a food pellet for the delivery of a nicotine infusion as described previously (Bruijnzeel and Markou, 2003). The operant conditioning chambers were equipped with two retractable levers. Responding on the active lever resulted in the delivery of a nicotine infusion and responding on the inactive lever was recorded but had no scheduled consequences. The delivery of an infusion (0.1 ml/infusion over a 5.6 second time-period) was earned by responding five times on the active lever (FR5 TO20-s). The initiation of the delivery of an infusion was paired with a cue light, which remained illuminated throughout the 20-second time-out period (initiated simultaneously with the initiation of delivery of a nicotine infusion). The active lever was retracted during the time-out period. Responding for nicotine was extinguished by replacing nicotine with saline. Extinction training was considered completed when the average number of infusions was less then 2. Reinstatement sessions started one day after extinction training was completed. Nicotine seeking behavior was reinstated by the administration of footshocks (8 shocks in a 10-minute time-period, 0.8 mA, 1 second shocks, mean off period 37 seconds) immediately prior to the self-administration session. Shock parameters were based on reinstatement studies conducted by Stewart and Shaham (Buczek et al., 1999). The nicotine self-administration and extinction sessions were conducted 7 days per week.
2.8. Histology
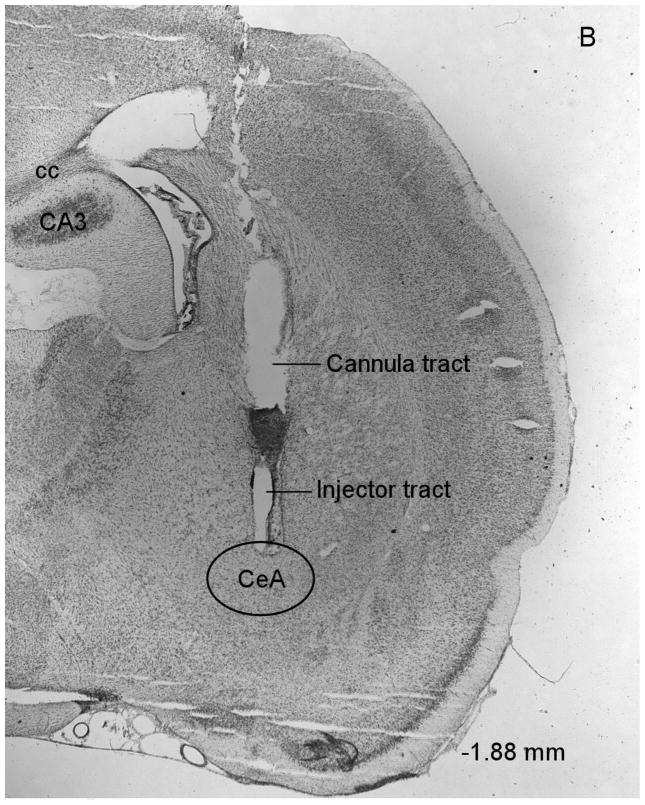
At the end of the experiments the rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, ip). The rats were then perfused via the ascending aorta with 100 ml of physiological saline followed by 150 ml of a 10% phosphate buffered formalin (4% formalin, w/v) solution. The rats were perfused with the intracranial drug injectors in place in order to enhance the visibility of the injection tracts in the brain sections. Brains were postfixed for 24 hours in phosphate buffered formalin and then stored in 0.1 M phosphate buffered saline until further processing. Sections were cut on a Leica CM3050 S cryostat (coronal sections of 40 μm at −15 °C) and mounted on Superfrost Plus microscope slides. The sections were stained with cresyl violet. The locations of the guide cannulae and injections sites were verified with a Leica DM2500 light microscope and with reference to a stereotaxic atlas of the rat brain (Fig. 1, Paxinos and Watson, 1998).
Figure 1.
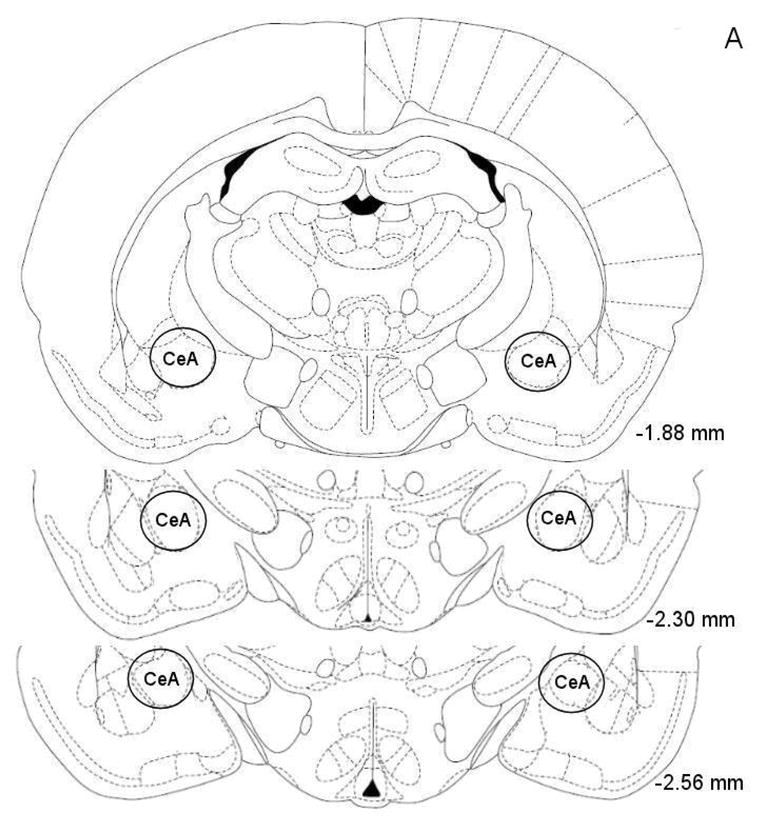
Histological overview of bilateral injection sites in the CeA (A). Drug injections were administered within or at the boundaries of the CeA (−1.88 to −2.56 anterior-posterior). The injections sites were evenly distributed over the 3 levels depicted in this figure. The figures are copies from the Paxinos and Watson brain atlas (Paxinos and Watson, 1998). Figure 1B depicts a photomicrograph of a representative cresyl violet-stained brain section at the level of the CeA. The photomicrograph shows the cannula tract and the injector tract that ends within the CeA. Abbreviations: cc, corpus callosum, CA3, hippocampal CA3 region.
2.9. Experimental Design
2.9.1. Experiment 1–4: Effects of the intra-CeA administration of clonidine, dexmedetomidine, propranolol, and prazosin on stress-induced reinstatement of nicotine seeking
At the beginning of the experiments the rats were trained to respond for food pellets and prepared with intravenous catheters and bilateral cannulae above the CeA. After at least one week of recovery from the surgery, the rats were allowed to self-administer nicotine (FR5 TO20-s, 0.03 mg/kg of nicotine per infusion; free base) for 14–16 consecutive days. Footshocks reliably reinstate extinguished nicotine seeking in rats that have self-administered nicotine for 14–16 days (Buczek et al., 1999; Zislis et al., 2007). Nicotine seeking was extinguished by substituting saline for nicotine. In the second experiment (dexmedetomidine), the number of saline infusions stabilized at about 3 infusions per session. After three weeks of extinction training the number of saline infusions was 2.47 and thus approached the extinction criterion of 2.0. At this point the extinction training was discontinued in order to prevent large differences in the duration of extinction training between experiments. Reinstatement sessions started one day after extinction training was completed. Clonidine (0, 0.5, 1 μg, n = 15, expt. 1), dexmedetomidine (0, 0.5, 1 μg, n = 17, expt. 2), propranolol (0, 1, 5, 10 μg, n = 12, expt. 3), and prazosin (0, 0.3, 1, 3 μg, n = 17, expt. 4) were administered bilaterally into the CeA 15 minutes before the footshock sessions. The aforementioned doses refer to the unilateral doses and all drugs were administered according to a Latin-square design. Drug naive rats were used in all the drug self-administration studies. There were at least 3 days (72 hours) between the intracranial injections. The half-lives of clonidine, dexmedetomidine, propranolol and prazosin are between 1 and 2 hours and therefore all the drugs will be metabolized after a 72-hour period (Conway and Jarrott, 1980; Elghozi et al., 1979; Hamilton et al., 1985; Salonen, 1989). The rats were left undisturbed in the vivarium on days that they were not tested. The design of this study was based on previous studies that showed that repeated footshock sessions or repeated exposure to cues associated with nicotine delivery reliably reinstates extinguished nicotine-seeking behavior (Bruijnzeel et al., 2009; Buczek et al., 1999; Paterson et al., 2005). At the end of the experiment the rats were perfused and the brains were removed to verify the anatomical localization of the injection sites. Histological verification indicated that all the injector tracts ended within the CeA and therefore all the rats were included in the statistical analyses. See figure 1 for the boundaries of the CeA.
2.9.2. Experiment 5: Effects of clonidine and dexmedetomidine on responding for food pellets
Rats (n = 10) were prepared with bilateral cannulae above the CeA and after at least one week of recovery they were allowed to respond for food pellets under a FR5 TO20-s schedule of reinforcement. This experiment was conducted in two parts. First the effect of clonidine (0, 0.5, 1 μg, unilateral dose) on responding for food pellets was investigated and then the effect of dexmedetomidine (0.5 and 1 μg, unilateral dose) on responding for food pellets was investigated in the same group of animals. The rats received one injection with vehicle and this treatment condition served as the control condition for the clonidine part and the dexmedetomidine part of the experiment. Clonidine, including vehicle, was administered according to a Latin-square design 15 minutes before the rats were placed in the operant conditioning chambers. When the clonidine part was completed the injections with dexmedetomidine were initiated. One half of the rats received the 0.5 μg dose of dexmedetomidine first and the other half of the rats received the 1 μg dose of dexmedetomidine first. There were at least 72 hours between subsequent drug injections and there were 5 days between the last clonidine injection and the first dexmedetomidine injection. The operant conditioning sessions were conducted 7 days per week and the duration of each session was 20 minutes. At the end of the experiment the rats were deeply anesthetized, perfused, and the brains were removed in order to verify the placements of the injections sites.
2.10. Data analyses
The dependent variables were the number of responses on the active lever and the number of responses on the inactive lever. The number of responses on the active lever and inactive lever during extinction training were analyzed using a one-way repeated-measures analyses of variance (ANOVA) with time as the within subjects factor. The effects of footshocks on responding on the active and inactive lever were analyzed using one-way repeated-measures ANOVA with footshocks as the within subjects factor. The effects of clonidine, dexmedetomidine, propranolol and prazosin on responding on the active and inactive lever after the administration of footshocks were analyzed using one-way repeated-measures ANOVAs with the dose of the drug as the within subjects factor. The effects of clonidine and dexmedetomidine on responding on the active and inactive lever during food sessions were analyzed using one-way repeated-measures ANOVA with the dose of clonidine or dexmedetomidine as the within subjects factor. The criterion for significance was set at 0.05. The statistical analyses were performed using PASW Statistics 18 for Windows software.
3. Results
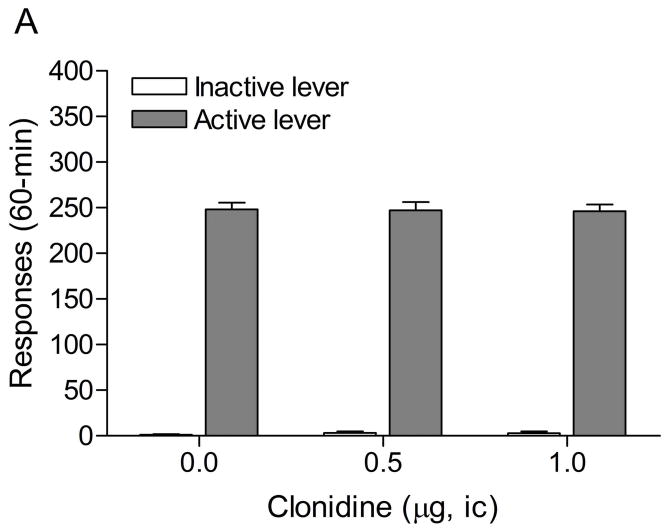
3.1. Experiment 1: Effects of intra-CeA administration of clonidine on stress-induced reinstatement of nicotine seeking
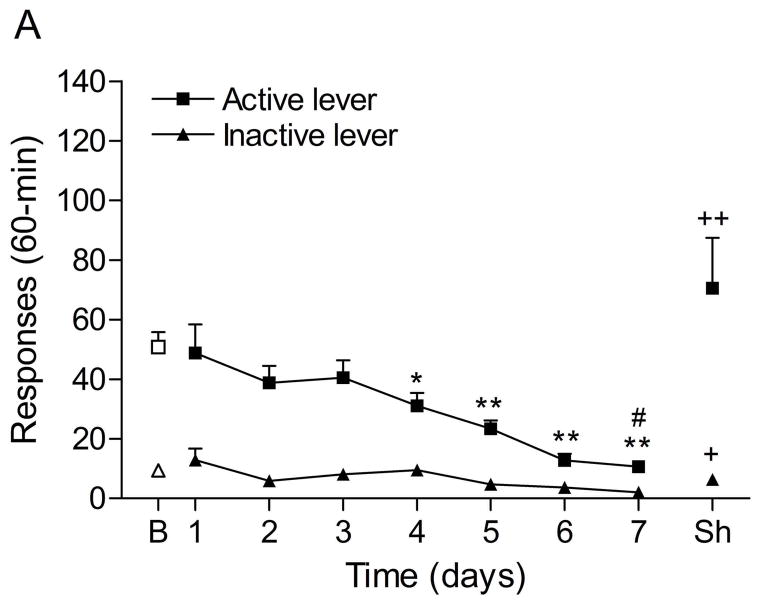
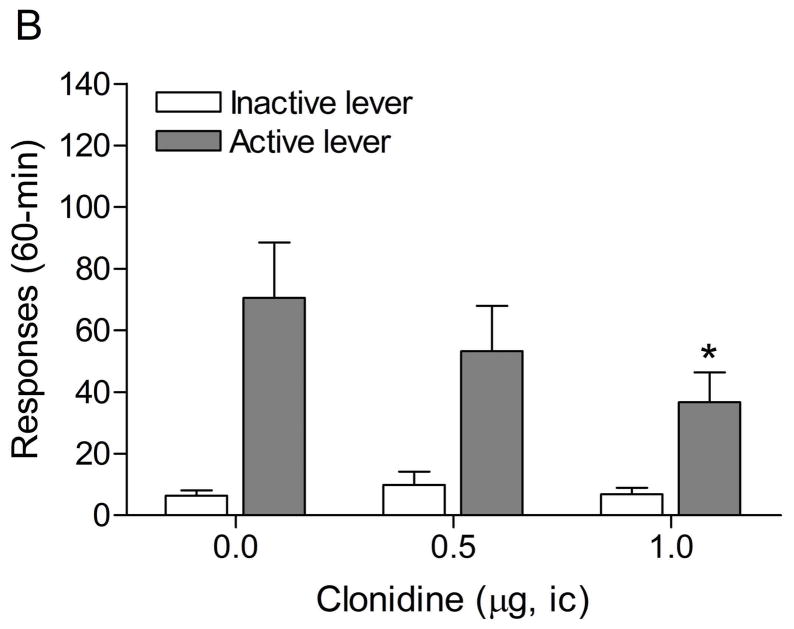
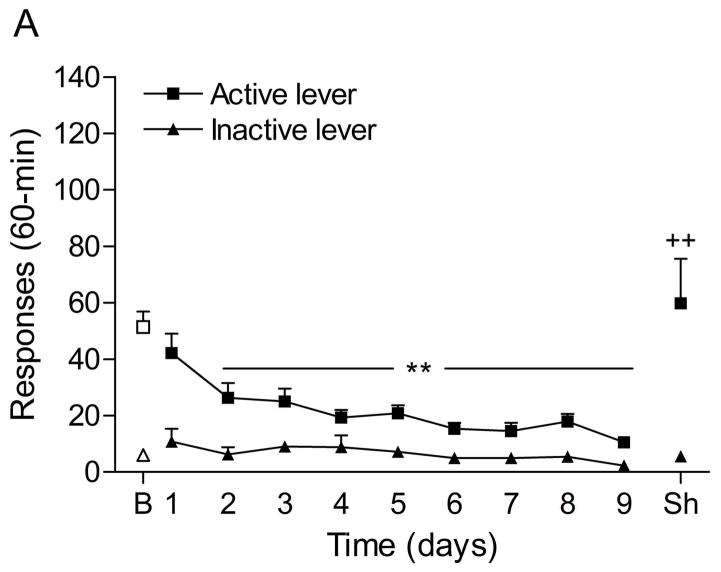
The mean (±S.E.M.) number of responses on the active lever and responses on the inactive lever during the last day of nicotine self-administration were 50.8 ± 5.3 and 9.4 ± 5.3, respectively. Substituting saline for nicotine lead to a decrease in the number of responses on the active lever (Fig. 2A; F7,98=10.70, P<0.0001) and the inactive lever (F7,98=4.11, P<0.0005). The extinction criterion (fewer than 2 infusions) was met on day 7. The administration of footshocks increased the number of responses on the active lever (last day of extinction vs. footshocks vehicle condition (F1,29=11.17, P<0.002) and the inactive lever (F1,29=5.95, P<0.05). Clonidine attenuated stress-induced responding on the active lever (Fig. 2B; F2,28=4.65, P<0.05) and did not affect responding on the inactive lever. Posthoc analyses revealed that the highest dose of clonidine, 1 μg per side, attenuated stress-induced nicotine seeking compared to the vehicle condition (P<0.05).
Figure 2.
Effects of clonidine on stress-induced reinstatement of nicotine seeking. Figure 2A depicts responding on the active and inactive lever during extinction of nicotine self-administration and stress-induced reinstatement of nicotine seeking (n = 15). In figure 2A, asterisks (* P<0.05, ** P<0.01) indicate a decrease in responding on the active lever compared to the last day of nicotine self-administration. The pound sign (# P<0.05) indicates a decrease in response on the inactive lever compared to the last day of nicotine self-administration. Plus signs (+ P<0.05, ++ P<0.01) indicate a footshock-induced increase in responding on the active lever or inactive lever compared to day 7 of extinction training. In figure 2B, Asterisks (P<0.05) indicate a decrease in responding on the active lever compared to rats that were exposed to footshocks and pretreated with vehicle. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as mean ± SEM.
3.2. Experiment 2: Effects of intra-CeA administration of dexmedetomidine on stress-induced reinstatement of nicotine seeking
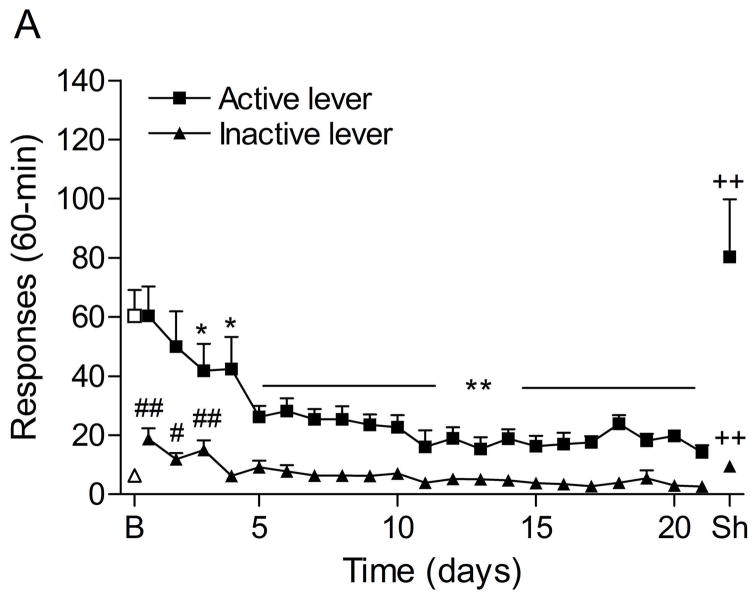
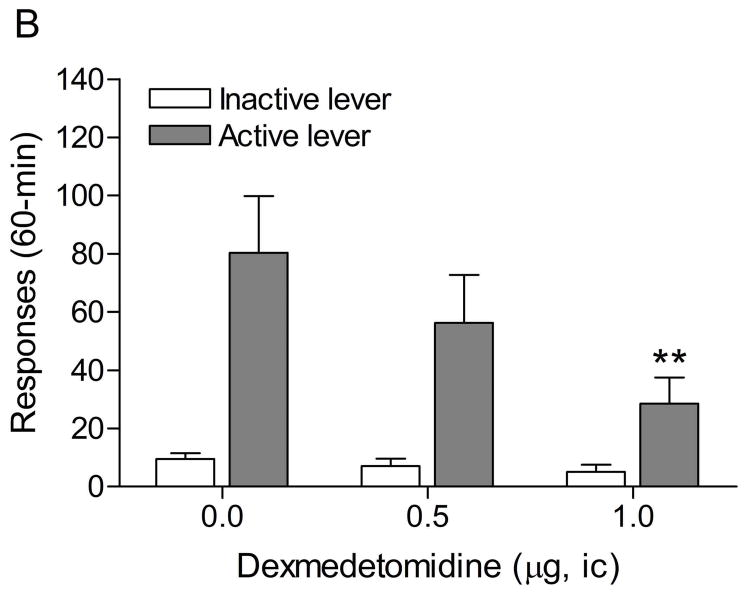
The mean (±S.E.M.) number of responses on the active lever and responses on the inactive lever during the last day of nicotine self-administration were 60.4 ± 8.8 and 6.4 ± 1.8, respectively. Responding on the active lever decreased after the nicotine solution was replaced with saline (Fig. 3A; F21,336=12.21, P<0.0001). There was initially a rapid decline in the number of saline infusions and then the number of infusions stabilized at about 3 per session. At day 21, the average number of saline infusions was 2.47 and the extinction training was discontinued. Extinction training also affected the number of responses on the inactive lever (F21,336=6.87, P<0.0001). Posthoc analyses indicated that the number of responses on the inactive lever was increased during the first, second, and third extinction training session. The administration of footshocks increased the number of responses on the active lever (last day of extinction vs. footshocks vehicle condition; Fig. 3B; F1,33=11.32, P<0.002) and the inactive lever (F1,33=10.22, P<0.003). Dexmedetomidine attenuated stress-induced responding on the active lever (F2,32=6.68, P<0.004) and did not affect responding on the inactive lever. Posthoc analyses indicated that 1 μg of dexmedetomidine (unilateral dose) significantly (P<0.01) decreased footshock-induced responding on the active lever.
Figure 3.
Effects of dexmedetomidine on stress-induced reinstatement of nicotine seeking. Figure 3A depicts responding on the active and inactive lever during extinction of nicotine self-administration and stress-induced reinstatement of nicotine seeking (n = 17). In figure 3A, asterisks (* P<0.05, ** P<0.01) indicate a decrease in responding on the active lever compared to the last day of nicotine self-administration. The pound signs (# P<0.05, ## P<0.01) indicate an increase in responding on the inactive lever compared to the last day of nicotine self-administration. Plus signs (++ P<0.01) indicate a footshock-induced increase in responding on the active lever or inactive lever compared to day 21 of extinction training. In figure 3B, Asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to rats that were exposed to footshocks and pretreated with vehicle. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as mean ± SEM.
3.3. Experiment 3: Effects of intra-CeA administration of propranolol on stress-induced reinstatement of nicotine seeking
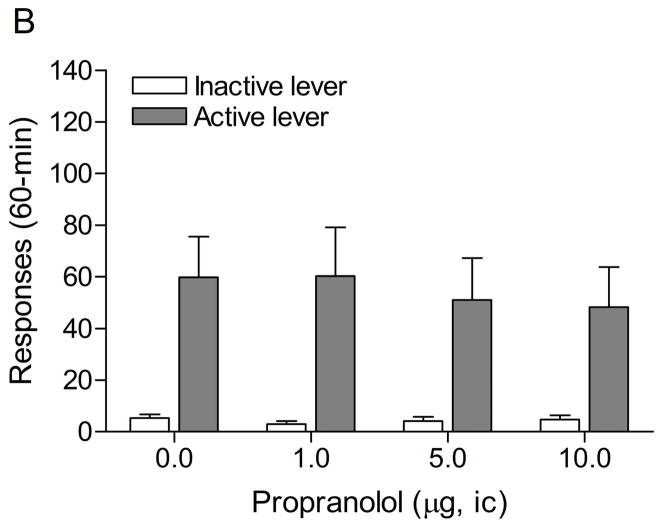
The mean (±S.E.M.) number of responses on the active lever and responses on the inactive lever during the last day of nicotine self-administration were 51.4 ± 5.4 and 6.0 ± 2.0, respectively. Substituting saline for nicotine lead to a decrease in the number of responses on the active lever (Fig. 4A; F9,99=13.60, P<0.0001) and did not affect the number of responses on the inactive lever. The extinction criterion was met on day 9. The administration of footshocks increased the number of responses on the active lever (last day of extinction vs. footshocks vehicle condition (Fig. 4B; F1,23=9.52, P<0.005) and there was a trend towards an increase in responding on the inactive lever (F1,23=3.45, P<0.08, not significant). The administration of propranolol into the CeA did not affect the stress-induced increase in responding on the active lever.
Figure 4.
Effects of propranolol on stress-induced reinstatement of nicotine seeking. Figure 4A shows responding on the active and inactive lever during extinction of nicotine self-administration and stress-induced reinstatement of nicotine seeking (n = 12). In figure 4A, asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to the last day of nicotine self-administration. Plus signs (++ P<0.01) indicate a footshock-induced increase in responding on the active lever compared to day 9 of extinction training. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as mean ± SEM.
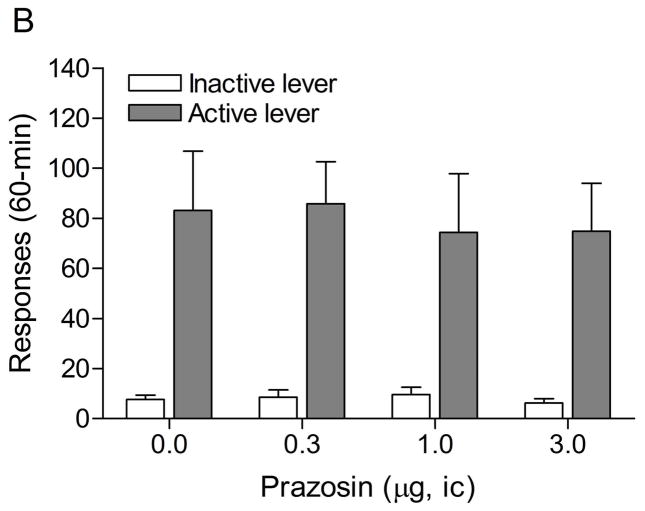
3.4. Experiment 4: Effects of intra-CeA administration of prazosin in the CeA on stress-induced reinstatement of nicotine seeking
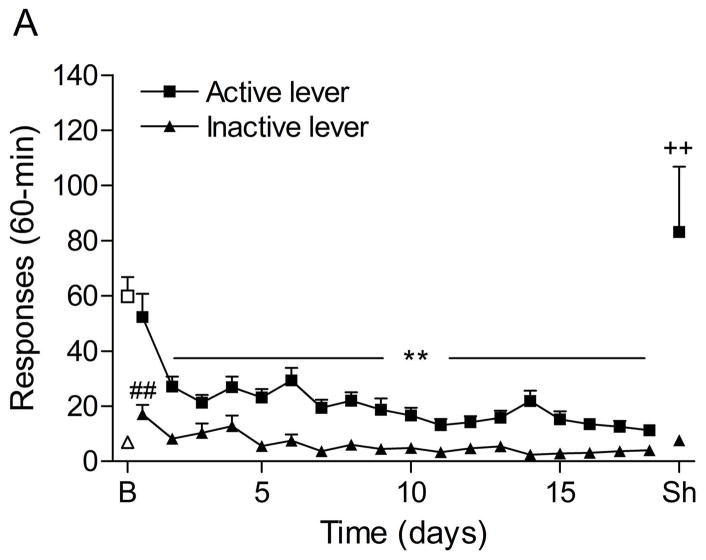
The mean (±S.E.M.) number of responses on the active lever and responses on the inactive lever during the last day of nicotine self-administration were 59.8 ± 7.3 and 6.9 ± 1.7, respectively. Substituting saline for nicotine caused a rapid decline in the number of responses on the active lever (Fig. 5A; F18,288=18.58, P<0.0001) and the inactive lever (F18,288=5.59, P<0.0001). The extinction criterion was met on day 18. The administration of footshocks increased the number of responses on the active lever (last day of extinction vs. footshocks vehicle condition (Fig. 5B; F1,33=9.19, P<0.005) and there was a trend towards an increase in responding on the inactive lever (F1,33=3.56, P<0.07, not significant). Prazosin did not affect the stress-induced increase in responding on the active lever.
Figure 5.
Effects of prazosin on stress-induced reinstatement of nicotine seeking. Figure 5A shows responding on the active and inactive lever during extinction of nicotine self-administration and stress-induced reinstatement of nicotine seeking (n = 17). In figure 5A, asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to the last day of nicotine self-administration. The pound sign (## P<0.01) indicate an increase in responding on the inactive lever compared to the last day of nicotine self-administration. Plus signs (++ P<0.01) indicate a footshock-induced increase in responding on the active lever compared to day 18 of extinction training. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as mean ± SEM.
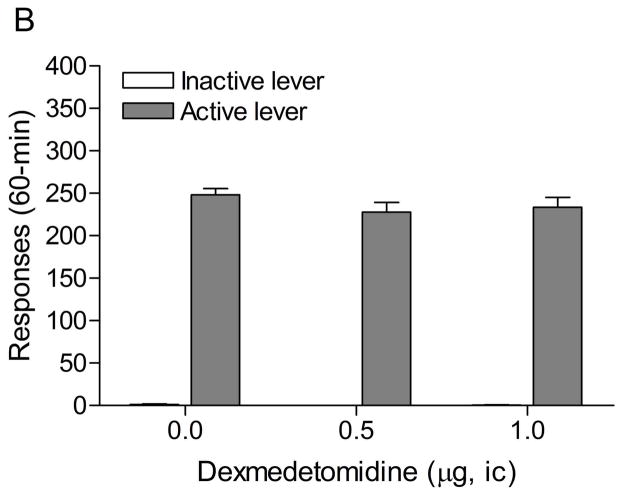
3.5. Experiment 5: Effects of intra-CeA administration of clonidine and dexmedetomidine on responding for food pellets
The mean (±S.E.M.) number of responses on the active lever and inactive lever on the test-day prior to the onset of the drug injections were 239.1 ± 12.9 and 6.5 ± 3.3, respectively. Figure 6A shows the effects of clonidine on responding for food pellets under a FR5 TO20-s schedule of reinforcement. Clonidine did not affect responding on the active (F2,18=0.13, n.s.) or the inactive lever (F2,18=0.64, n.s.). Figure 6B shows the effects of dexmedetomidine on responding for food pellets. Dexmedetomidine did not affect responding on the active (F2,18=2.76, n.s.) or the inactive lever (F2,18=3.07, n.s.). This indicates that the doses of clonidine and dexmedetomidine that attenuated stress-induced reinstatement of extinguished nicotine seeking did not cause motor impairments or have sedative effects.
Figure 6.
Effects of clonidine (A) and dexmedetomidine (B) on responding for food pellets (n = 10). Data are expressed as mean ± SEM.
4. Discussion
These studies investigated the role of α2, α1, and β1/β2-adrenergic receptors in the CeA in stress-induced reinstatement of nicotine seeking in rats. It was shown that pretreatment with the α2-adrenergic receptor agonists clonidine and dexmedetomidine in the CeA attenuated stress-induced reinstatement of nicotine seeking. Doses of clonidine and dexmedetomidine that attenuated stress-induced reinstatement did not affect responding for food pellets. In contrast to the α2-adrenergic receptor agonists, the intra-CeA administration of the nonselective β1/β2-adrenergic receptor antagonist propranolol and the α1-adrenergic receptor antagonist prazosin did not attenuate stress-induced reinstatement of nicotine seeking.
The present studies indicated that the administration of α2-adrenergic receptor agonists into the CeA attenuates stress-induced reinstatement of nicotine seeking while the administration of α1 or β-adrenergic receptors antagonists into the CeA is ineffective. It is unlikely that the lack of an effect of the α1-adrenergic antagonist prazosin or the nonselective β-adrenergic receptor antagonist propranolol was due to an absence of α1 or β-adrenergic receptors in the CeA. Previous studies have shown moderate to high levels of β1, β2, and α1-adrenergic receptors in the CeA (Jones et al., 1985; Rainbow et al., 1984). It is more likely that the discrepancy in the effectiveness of α2-adrenergic receptor agonists and α1 or β-adrenergic receptor antagonists was due to the fact that they have different effects on neuronal activity in the CeA. Freedman and Aghajanian showed that the administration of clonidine into the CeA leads to a dramatic decrease in the firing rate of CeA neurons. The effect of clonidine on the firing rate of neurons in the CeA was completely blocked by pretreatment with the α2-adrenergic receptor antagonist idazoxan (Freedman and Aghajanian, 1985). In contrast, prazosin and propranolol did not affect the firing rate of neurons in the CeA (Freedman and Aghajanian, 1985). Stressors have been shown to increase the activity of neurons in the CeA (Campeau et al., 1991; Honkaniemi et al., 1992). Therefore, it might be possible that α2-adrenergic agonists block stress-induced reinstatement of nicotine seeking by inhibiting stress-induced activation of CeA neurons.
In the present study, the administration of propranolol into the CeA did not attenuate stress-induced reinstatement of nicotine seeking. It is interesting to note that one study reported that the administration of a mixture of the relatively selective β1-adrenergic receptor antagonist betaxolol and the somewhat selective β2-adrenergic receptor antagonist ICI-118,551 into the CeA attenuates stress-induced reinstatement of cocaine seeking (Leri et al., 2002). It is unlikely that the discrepancy between the study by Leri and colleagues and the present nicotine study is due to the fact that different antagonists were used. Propranolol has a higher affinity and potency for the β1-adrenergic receptor than the β1-adrenergic receptor antagonist betaxolol (Rimele et al., 1988; Satoh et al., 1993). Furthermore, propranolol has only a slightly lower affinity for the β2-adrenergic receptor than the β2-adrenergic receptor antagonist ICI-118,551 (Bilski et al., 1983; Satoh et al., 1993). Therefore, it might be expected that the overall effect of a mixture of betaxolol and ICI-118,551 on β1 and β2-adrenergic receptors is very similar as that of propranolol. It is also unlikely that in the present study the doses of propranolol were too low. Leri and colleagues reported that the coadministration of 1 nmol of betaxol and 1 nmol of ICI-118,551 (unilateral dose) in the CeA attenuates footshock-induced reinstatement of cocaine seeking (Leri et al., 2002). Therefore, the total unilateral dose was 2 nmol of betaxol and ICI-118,551. The lowest dose of propranolol in the present study was 1 μg (3.4 nmol, unilateral dose) which is somewhat higher than the highest dose in the study by Leri and colleagues. A possible explanation for the discrepancy between these two studies could be that β-adrenergic receptors in the CeA play a more important role in stress-induced reinstatement of cocaine seeking than in stress-induced reinstatement of nicotine seeking. However, before firm conclusions can be drawn, additional studies are needed that systematically compare the effects of various β-adrenergic receptor antagonists on stress-induced reinstatement of nicotine and cocaine seeking.
The CeA plays an important role in the regulation of behavioral and autonomic responses to stressors. The CeA regulates autonomic responses by modulating the activity of brain stem areas such as the parabrachial nucleus, the nucleus of the solitary tract, and the dorsal motor nucleus of the vagus nerve (Price and Amaral, 1981; Veening et al., 1984). Neurons in the CeA also project heavily to brain areas that have been implicated in orchestrating behavioral responses to stressors such as the bed nucleus of the stria terminals, the lateral hypothalamus, the substantia nigra, and the pericoerulear region (Krettek and Price, 1978; Price and Amaral, 1981; Van Bockstaele et al., 1996). The pericoerulear region contains a dense networks of locus coeruleus (LC) dendrites (Shipley et al., 1996). The projections from the CeA to pericoerulear region have been suggested to modulate the activity of LC neurons (Van Bockstaele et al., 1996). It is, however, unlikely that the LC plays a role in stress-induced reinstatement of nicotine seeking. Shaham and colleagues investigated the role of the LC in stress-induced reinstatement of heroin seeking (Shaham et al., 2000). They demonstrated that systemic administration, but not intra-LC administration, of clonidine attenuates stress-induced reinstatement of heroine seeking. Furthermore, it has also been shown that chemical lesioning of LC projections does not affect stress-induced reinstatement of morphine-induced conditioned place preference (Wang et al., 2001). Taken together, these studies suggest that the LC does not play a role in stress-induced reinstatement of drug seeking.
Erb and colleagues explored the role of the projection from the CeA to the BNST in stress-induced reinstatement of cocaine seeking in rats (Erb et al., 2001; Sakanaka et al., 1986). They demonstrated that the unilateral administration of the sodium channel blocker tetrodotoxin into the CeA in combination with the unilateral administration of D-Phe CRF(12–41) into the BNST in the opposite hemisphere attenuates footshock-induced reinstatement of cocaine seeking. Based on this finding it was suggested that a CRF-containing pathway from CeA to BNST at least partly mediates stress-induced reinstatement of cocaine seeking. The same pathway might play a role in stress-induced reinstatement of nicotine seeking. In our study, it was shown that the administration of clonidine or dexmedetomidine into the CeA, which decreases noradrenergic transmission, attenuated stress-induced reinstatement of nicotine seeking. Histological evidence indicates that noradrenergic terminals form synapses with dendrites of CRF neurons in the BNST (Phelix et al., 1994). We are not aware of any studies that investigated if there are synaptic connections between noradrenergic terminals and CRF neurons in the CeA. However, because there are strong neurochemical similarities between the amygdala and BNST (Alheid, 2003) there might be similar synaptic connections between noradrenergic terminals and CRF neurons in the CeA as in the BNST. Furthermore, there is evidence that indicates that norepinephrine stimulates the release of CRF in the brain (Dunn and Swiergiel, 2008). Thus, the administration of clonidine and dexmedetomidine into the CeA may have attenuated stress-induced reinstatement of nicotine seeking by diminishing the stress-induced activation of the CRF projection from the CeA to the BNST.
It might also be possible that in the present study the administration of clonidine or dexmedetomidine into the CeA attenuated stress-induced reinstatement of nicotine seeking by modulating glutamatergic transmission in this brain site. Several lines of evidence suggest that increased glutamate release in the CeA may contribute to relapse to drug seeking (Lu et al., 2007; Watanabe et al., 2002). Stressors increase glutamate release in the CeA which can lead to the activation of brain stress systems and negative mood states (Ebner et al., 2005; Reznikov et al., 2007; Watanabe et al., 2002). The activation of brain stress systems contributes to the reinstatement of drug seeking (Bruijnzeel and Gold, 2005; Koob and Le, 2008). Additional evidence for a role of glutamatergic transmission in the CeA in reinstatement of drug seeking is provided by a study with the group II metabotropic glutamate (mGlu2/3) receptor agonist LY379268 (Lu et al., 2007). The mGlu2/3 receptors are autoreceptors and stimulation of these receptors leads to a decrease in the release of glutamate (Cartmell and Schoepp, 2000). The administration of LY379268 into the CeA has been shown to decrease cue-induced reinstatement of cocaine seeking (Lu et al., 2007). Taken together, the aforementioned studies suggest that the release of glutamate in the CeA may contribute to relapse to drug seeking. Numerous studies have reported that clonidine and dexmedetomidine inhibit glutamate release in the brain (Bickler and Hansen, 1996; Dolphin, 1982; Li and Eisenach, 2001). Therefore, the administration of clonidine or dexmedetomidine into the CeA might have attenuated stress-induced reinstatement of nicotine seeking by inhibiting footshock-induced glutamate release in this brain site.
Taken together, the present studies demonstrate that the administration of the α2-adrenergic receptor agonists clonidine and dexmedetomidine into the CeA attenuates stress-induced reinstatement of nicotine seeking. Doses of clonidine and dexmedetomidine that attenuated stress-induced reinstatement of nicotine seeking did not affect responding for food pellets. The administration of the β1/β2-adrenergic receptor antagonist propranolol and the α1-adrenergic receptor antagonist prazosin into the CeA did not affect stress-induced reinstatement of nicotine seeking. Clonidine and dexmedetomidine may have attenuated stress-induced relapse by inhibiting the release of glutamate in the CeA and/or inhibiting excitatory CRF projections from the CeA to the BNST. However, additional studies are needed before firm conclusions about the neurochemical mechanisms by which clonidine and dexmedetomidine attenuated stress-induced reinstatement of nicotine seeking can be drawn. It is suggested that studies that investigate the role of noradrenergic transmission in the reinstatement of nicotine seeking may contribute to the development of novel, non-addictive, treatments for tobacco addiction in humans.
Acknowledgments
This work was funded by National Institute on Drug Abuse grant (DA023575) to Adrie Bruijnzeel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Hansen BM. Alpha 2-adrenergic agonists reduce glutamate release and glutamate receptor-mediated calcium changes in hippocampal slices during hypoxia. Neuropharmacology. 1996;35:679–687. doi: 10.1016/0028-3908(96)84639-9. [DOI] [PubMed] [Google Scholar]
- Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551) J Cardiovasc Pharmacol. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-Releasing Factor-1 Receptor Activation Mediates Nicotine Withdrawal-Induced Deficit in Brain Reward Function and Stress-Induced Relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Conway EL, Jarrott B. Clonidine distribution in the rat: temporal relationship between tissue levels and blood pressure response. Br J Pharmacol. 1980;71:473–478. doi: 10.1111/j.1476-5381.1980.tb10960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Dardonville C, Rozas I. Imidazoline binding sites and their ligands: an overview of the different chemical structures. Med Res Rev. 2004;24:639–661. doi: 10.1002/med.20007. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Noradrenergic modulation of glutamate release in the cerebellum. Brain Res. 1982;252:111–116. doi: 10.1016/0006-8993(82)90983-0. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Elghozi JL, Bianchetti G, Morselli PL, Meyer P. Brain distribution of propranolol in the rat. Eur J Pharmacol. 1979;55:319–322. doi: 10.1016/0014-2999(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le FB. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JE, Aghajanian GK. Opiate and alpha 2-adrenoceptor responses of rat amygdaloid neurons: co-localization and interactions during withdrawal. J Neurosci. 1985;5:3016–3024. doi: 10.1523/JNEUROSCI.05-11-03016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD, Molloy BB. Blockade of amine depletion by nisoxetine in comparison to other uptake inhibitors. Psychopharmacol Commun. 1975;1:455–464. [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Reid JL, Vincent J. Pharmacokinetic and pharmacodynamic studies with two alpha-adrenoceptor antagonists, doxazosin and prazosin in the rabbit. Br J Pharmacol. 1985;86:79–87. doi: 10.1111/j.1476-5381.1985.tb09437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hokfelt T, Pelto-Huikko M. Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. Neuroreport. 1992;3:849–852. doi: 10.1097/00001756-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Iimori K, Tanaka M, Kohno Y, Ida Y, Nakagawa R, Hoaki Y, Tsuda A, Nagasaki N. Psychological stress enhances noradrenaline turnover in specific brain regions in rats. Pharmacol Biochem Behav. 1982;16:637–640. doi: 10.1016/0091-3057(82)90429-4. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le MM. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry. 1986;43:733–738. doi: 10.1001/archpsyc.1986.01800080019003. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Eisenach JC. alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J Pharmacol Exp Ther. 2001;299:939–944. [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of Noradrenergic Neurotransmission in the Stress- but not Cocaine-Induced Reinstatement of Extinguished Cocaine-Induced Conditioned Place Preference in Mice: Role for beta-2 Adrenergic Receptors. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addict Behav. 2002;27:241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12(Suppl 2):S36–S47. [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. 1994;33:109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Merlo Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Sletten K. Nonadrenergic imidazoline binding sites on human platelets. J Pharmacol Exp Ther. 1993;267:1493–1502. [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Pradhan SN, Bowling C. Effects of nicotine on self-stimulation in rats. J Pharmacol Exp Ther. 1971;176:229–243. [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Rimele TJ, Henry DE, Giesa FR, Buckley SK, Geiger G, Heaslip RJ, Lee DK, Grimes D. Comparison of the beta-adrenoceptor affinity and selectivity of cetamolol, atenolol, betaxolol, and ICI-118,551. J Cardiovasc Pharmacol. 1988;12:208–217. doi: 10.1097/00005344-198808000-00011. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Salonen JS. Pharmacokinetics of medetomidine. Acta Vet Scand Suppl. 1989;85:49–54. [PubMed] [Google Scholar]
- Satoh E, Narimatsu A, Hosohata Y, Tsuchihashi H, Nagatomo T. The affinity of betaxolol, a beta 1-adrenoceptor-selective blocking agent, for beta-adrenoceptors in the bovine trachea and heart. Br J Pharmacol. 1993;108:484–489. doi: 10.1111/j.1476-5381.1993.tb12829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola JM, Virtanen R. Central alpha 2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol. 1991;195:193–199. doi: 10.1016/0014-2999(91)90535-x. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Schedule-controlled brain self-stimulation: has it utility for behavioral pharmacology? Neurosci Biobehav Rev. 1992;16:569–583. doi: 10.1016/s0149-7634(05)80197-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu WL, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J Comp Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Smith KL, Jessop DS, Finn DP. Modulation of stress by imidazoline binding sites: implications for psychiatric disorders. Stress. 2009;12:97–114. doi: 10.1080/10253890802302908. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity. J Neurosci Res. 1996;45:289–302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;58:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]