Abstract
Glucose is the central molecule in many biochemical pathways, and numerous approaches have been developed for fabricating micro biosensors designed to measure glucose concentration in/near cells and/or tissues. An inherent problem for microsensors used in physiological studies is a low signal-to-noise ratio, which is further complicated by concentration drift due to the metabolic activity of cells. A microsensor technique designed to filter extraneous electrical noise and provide direct quantification of active membrane transport is known as self-referencing. Self-referencing involves oscillation of a single microsensor via computer-controlled stepper motors within a stable gradient formed near cells/tissues (i.e., within the concentration boundary layer). The non-invasive technique provides direct measurement of trans-membrane (or trans-tissue) analyte flux. A glucose micro biosensor was fabricated using deposition of nanomaterials (platinum black, multiwalled carbon nanotubes, Nafion) and glucose oxidase on a platinum/iridium microelectrode. The highly sensitive/selective biosensor was used in the self-referencing modality for cell/tissue physiological transport studies. Detailed analysis of signal drift/noise filtering via phase sensitive detection (including a post-measurement analytical technique) are provided. Using this highly sensitive technique, physiological glucose uptake is demonstrated in a wide range of metabolic and pharmacological studies. Use of this technique is demonstrated for cancer cell physiology, bioenergetics, diabetes, and microbial biofilm physiology. This robust and versatile biosensor technique will provide much insight into biological transport in biomedical, environmental, and agricultural research applications.
Keywords: glucose flux, self referencing, physiology, biosensor, carbon nanotubes
1. Introduction
Glucose plays a central role in metabolism, and glucose/glycolytic transport is commonly studied in cancer research (Busk et al., 2008), diabetes (Guillam et al. 2000), bioenergetics (Lagord et al., 1998), and other cell/tissue culture applications (Morten et al. 2008). Techniques for measuring glucose include wet chemistry approaches (Lanevschi and Kramer, 2009), hollow fiber hybridoma cell bioreactors (Nayak and Herman, 1997), radiolabeling (Guillam et al. 2000; Hellman et al. 1974; Sweet et al. 1996; Zawalich and Matschinsky 1977), nuclear magnetic resonance spectroscopy (Weiss et al. 1989), microfluorometry (Moley et al. 1998; Passonneau and Lowry 1993), fourier transform infrared spectrometry (Zhang and Fang, 2005), and electrochemical biosensors (reviewed in Wang, 2005). Due to high spatial resolution, excellent selectivity, rapid prototyping, low cost, and simple operating scheme, electrochemical biosensors have emerged as a vital tool for measuring glucose in a wide range of applications, including point of care diagnostics (Lowry et al., 1998) and preventative disease research (Choleau et al. 2002).
Most biosensors are based on enzymatic recognition of glucose by glucose oxidase (GOx), where oxidation to gluconic acid produces H2O2, which is detected using oxidative amperometry at a potential of +0.5–0.8V (Hrapovic et al. 2004; Wang 2005). In most biosensor designs, GOx is immobilized on a metal electrode via chemical linkage (Makino et al. 1988) or physical entrapment within a polymeric matrix (Rickus et al. 2002). Much research is focused on enhancement of biosensor performance by incorporating materials, such as carbon nanotubes (CNTs) (Wang et al. 2003; Gooding 2005; Wang 2005; Claussen et al., 2009; Claussen et al., 2010), Nafion (Ni et al. 1999), and amorphous platinum clusters (Jaffe and R. Nuccitelli, 1974).
Use of glucose biosensors in cell/tissue research and implantation often requires miniaturization (e.g., tip diameter less than approximately 10μm). Increased resistance due to miniaturization causes a significant decrease in maximum attainable signal. Thus, microsensors are highly susceptible to electromagnetic interferences/noise, and often have signal-to-noise ratios significantly lower than macroelectrodes (Bard and Faulkner, 2000). In addition to electronic noise, electrode drift in biological applications is an important consideration; as signal drift can be a continuous source of error due to active changes in the concentration of analyte(s) (due to metabolism, homeostasis, etc.). However, the resistance per area for microelectrodes is lower than that of macroelectrodes, and much current research is devoted to the use of nanomaterials for improving signal-to-noise ratio of microsensors.
Another approach for improving drift and noise inherent to microsensors is the use of a phase sensitive, noise-filtering microsensor technique known as self-referencing (SR) (Jaffe and Nuccitelli, 1974; Khutreiber and Jaffe, 1990). The SR microsensor technique was originally developed for measuring bioelectric current under physiological conditions (Jaffe and R. Nuccitelli, 1974), and has been adapted for studying active physiological transport of ions (Kochian et al., 1992; Krause et al., 1994; Shabala et al., 1997), oxygen (Porterfield et al., 2000; Chatni and Porterfield, 2009), electroactive molecules such as NO (Porterfield et al., 2001), and recently non-electroactive biomolecules such as glutamate (McLamore et al., 2010a). For a review on the SR microsensor technique, see Porterfield (2007).
The SR technique is designed to measure active flux within the concentration boundary layer formed near cells/tissues/organs, and involves computer-controlled translation of a single microsensor between two positions separated by a known distance. Output from a calibrated microsensor (C1) is recorded at the cell/tissue surface (known as the near pole position), the microsensor is translated a short distance (20–50μm) in thevector of transport (ΔX), and the concentration at the second position (known as the far pole position) is recorded (C2). Flux is then calculated using Fick’s first law according to equation 1:
| (equation 1) |
Due to filtering of background drift and noise (via phase-sensitive detection; Porterfield, 2007), SR converts a static concentration (magnitude) sensor into a dynamic biophysical flux sensor capable of directly measuring the magnitude and direction of transport. Under physiological conditions, this technique allows researchers to directly study transport under a wide range of physiological/pathophysiological conditions; based on the biophysical principle that the majority of membrane transport occurs in the vector perpendicular to the tangent of the cell/tissue surface.
This work introduces for the first time the use of a highly selective/sensitive GOx micro biosensor for directly measuring glucose flux. The bio-nanocomposite design combined the use of platinum black, Nafion, multiwalled carbon nanotubes, and GOx in a micro-biosensor format. Physiological glucose uptake is demonstrated in a wide range of metabolic and pharmacological studies, including: cancer cell physiology, diabetes, bioenergetics, muscle physiology, and microbial biofilm physiology. The use of this micro-biosensor technique allows direct quantification of physiological glucose transport under physiological conditions, which is a major technological improvement.
2. Materials and methods
2.1 Chemicals and reagents
Deionized water (DI) of resistivity 18.2 MΩ cm (Milli Q) was used to prepare solutions. Glucose oxidase (E.C.1.1.3.4, 2,000 10,000 units/g, from Aspergillus niger), potassium chloride (KCl, 99%), potassium ferricyanide (K3Fe(CN)6), Nafion (5% wt/wt), chloroplatinic acid solution (8% wt/wt), lead acetate (reagent grade, 95%), potassium cyanide (KCN), phloretin, β mercaptoethanol, HEPES buffer, sodium pyruvate, glutamine, penicillin, and streptomycin were purchased from Sigma Aldrich (St. Louis, MO). D-Glucose and sodium chloride (NaCl) were purchased from Mallinckrodt Baker, Inc (Phillipsburg, NJ). Sodium phosphate (Na2HPO4·7H2O), potassium phosphate (KH2PO4, monobasic) and potassium cyanide (KCN) were purchased from Fisher Scientific (Pittsburg, PA). PBS solutions (0.01M) were prepared by dissolving 8.0g NaCl, 1.2g Na2HPO4, 0.2g KCl and 0.2g KH2PO4 in 1.0L DI. MWNTs (-COOH Functionalized MWNTs 95wt% 8 15nm OD) were purchased from Cheap Tubes, Inc. (Brattleboro, VT). RPMI 1640 medium was purchased from GIBCO (Carlsbad, CA), and fetal calf serum was purchased from Invitrogen (Carlsbad, CA). Modified eagle’s media (MEM) was purchased from Fisher Scientific.
2.2 Biosensor fabrication
A Pt/Ir microelectrode (PI20033.0A10, 51mm length, 0.256mm shaft diameter, 1–2μm tip diameter, 3μm parylene C coated metal shaft) was used to fabricate the micro biosensor (Fig. 1). Pt black was electro deposited using a potentiostat (Applicable Electronics) in a solution of 0.72% chloroplatinic acid and 0.001% lead acetate. The microelectrode was connected to the cathode on the potentiostat, and a bare Pt wire (0.5mm in diameter; Alfa Aesar, Ward Hill, MA) was connected to the anode. A constant voltage (10V) was applied between the cathode and anode of the potentiostat for 1 minute.
Figure 1.
Schematic of glucose oxidase biosensor showing Pt microelectrode and parylene insulation. Inset (a) Amorphous Pt black nanoclusters electrodeposited on the surface of the Pt microelectrode. (b) MWNT/Nafion/GOx matrix deposited on the Pt black microelectrode tip.
The MWNT solution was prepared by mixing 2mg MWNT in 1 mL Nafion, and ultrasonicating for 120 minutes. The MWNT solution (2μL) was cast on the tip of the microelectrode using a pipette, and the microelectrode was subsequently air dried for 5 minutes. For enzyme immobilization, the microelectrode was then dipped in 100 μL of a solution containing 50 mg GOx/mL PBS for 30 min. When not used, the biosensor was stored in 50 mg GOx/mL PBS at 4 °C.
Cyclic voltammetry (CV) was carried out with a 3 electrode electrochemical (C-3) cell stand (BASi, West Lafayette, IN) with a 10 second quiet time. Ag/AgCl reference electrodes and auxiliary electrodes were purchased from BASi. Based on the peak obtained during CV analysis, all DC potential amperometry was conducted at a working potential of +500 mV versus a Ag/AgCl reference electrode with a sampling rate of 1kHz.
Amperometric sensitivity towards glucose was determined by measuring current at a constant working potential (+500 mV) while sequentially adding glucose to mixed solutions (all solutions stirred at 400 rpm). Following each glucose addition, measured current signal was allowed to reach steady state (defined as less than a 3% fluctuation for 10 sec). Average current values represent the arithmetic mean of observed current, and error bars represent the standard error of the mean for a minimum of three replicate experiments. Average current versus glucose concentration was used to characterize sensitivity, and the slope of each linear calibration curve used to estimate sensitivity (nA/mM). Following calibration, sensor efficiency was determined by calculating the sensitivity (μA mM−1) per geometric surface area.
Sensitivity was determined via DC potential amperometry at a working potential of +500 mV versus a Ag/AgCl reference electrode at a sampling rate of 1kHz. Lower detection limit was determined using the σ3 (i.e., 99% confidence method) (MacDougall and Crummett, 1980), and working range was defined as the linear sensing range where the R2 value for linear concentration-current plots was > 0.98. For determination of surface area, CV was performed in 4 mM Fe(CN)63−/1 M KNO3 solution in the potential range of 0 to +650 mV.
2.3 Self Referencing
The SR system (Porterfield 2007) included a vibration isolation table with Faraday cage (Technical Manufacturing Co., Peabody. MA), camera/zoomscope, micro biosensor, and reference electrode (Ag/AgCl) mounted on a head stage controlled by a motion control system (MCS) (Fig. S1). Automated Scanning Electrode Technique (ASET) software was used for data acquisition (A/D) and control functions (D/A) (Science Wares, Falmouth, MA). The A/D board with DC coupled differential amplifier, low/high pass filters, and video/data acquisition system were obtained through Applicable Electronics, Inc. (Sandwich, MA). As described previously (Porterfield 2007), computer controlled movement of sensors induced a phase dependant waveform on the measured output signal, where the amplitude was proportional to differential analyte concentration (ΔC). Preamplifier signals were capacitively coupled through a lock in amplifier to discretely subtract baseline signals (DC coupling), and the differential waveform was amplified to digitally analyze differential signals.
2.4 Abiotic validation of glucose flux
For abiotic validation of glucose flux measurements, a glass micropipette (1.5 mm diameter, World Precision Instruments, Sarasota, FL) was pulled on a horizontal puller (Sutter instrument Co., Novato, CA) to produce a tip diameter of 50 μm. A solution containing 5 mM glucose and 0.5% agar was heated to gel, mixed, injected into the pulled micropipette and allowed to cool. The micropipette containing 5 mM glucose and agar was mounted and submersed in PBS solution, creating a constant abiotic glucose concentration gradient near the pipette tip. The gradient was allowed to stabilize for 30 minutes, during which time a glucose micro biosensor and reference electrode were placed in the solution and polarized at +500mV. After a stable gradient formed near the micropipette tip, the micro biosensor was positioned at the surface of the pipette, and flux measured by oscillating in the direction perpendicular to the tangent of the micropipette tip surface. The sensor was oscillated within the excursion distance with oscillation frequency range suggested in the literature (Kuhtreiber and Jaffe 1990) to prevent mixing and ensure optimal performance. Following a minimum of nine measurements, the sensor was positioned 5 μm from the micropipette tip surface, and flux again recorded for a minimum of nine flux measurements. This step back process was repeated until no change (<1%) in glucose efflux was observed. An empirical model was developed based on concentration measurements for describing the flux, and the correlation coefficient between the measured and predicted flux was calculated to describe the performance of the oscillating biosensor (Porterfield 2007; McLamore et al. 2009). All experiments utilized the drift algorithm in McLamore et al. (2009).
2.5 Human breast endothelial cell culture
Human epithelial breast cells and MCF10A cells were stably transfected with Harvey ras oncogene (MCF10A-ras cells) (Zantek et al., 2001). Non-transfected MCF10A and MCF10A-ras were cultured in DMEM/F12 (1:1, v:v) containing 5% house serum and supplemented with 10 mg/L insulin, 20 μg/L epidermal growth factor, 50 μg/L cholera toxin, 50 mg/L hydrocortisone, 100 units/mL penicillin, and 0.1 mg/mL streptomycin in a humidified environment at 37°C with 5% CO2. Cells were used for experiments four days after achieving confluence.
2.6 Mouse muscle and intestine tissue extraction
All animal care procedures were approved by the Purdue Animal Care and Use Committee. Mice were maintained in a specific pathogen-free barrier facility with a 12-h light/dark cycle (6PM/6AM) with free access to food and water. Four month old, male, C57BL/6 mice fed high fat, semipurified diet (D12492, Research Diets, Inc., New Brunswick NJ) for two weeks were used for analysis of glucose transport into muscle and intestine tissue. Mice were anesthetized with Nembutal and euthanized by decapitation and exanguination. For analysis of muscle, soleus and gastrocnemius muscles were isolated, pinned down and incubated in low glucose Dulbecco’s Modified Eagle’s Medium (Gibco, Carlsbad, CA). The small intestine was divided into six, equal length parts and labeled S1 through S6 (proximal to distal) in relation to the stomach. S2 (upper jejunum) and S4 (lower jejunum), were cleaned thoroughly with PBS, cut longitudinally, pinned down luminal side up in low glucose Dulbecco’s Modified Eagle’s Medium. All tissues were maintained at 37°C throughout the experiment.
2.7 Pancreatic β cells and glucose transport inhibition
INS-1 cells (donated by Dr. Raghu Mirmira) isolated from an X-ray induced transplantable rat insulinoma (Hohmeier et al. 2000) were cultured on 10cm BD Falcon polystyrene tissue culture dishes (VWR, West Chester, PA). Cells were detached from culture dishes using a 0.05% trypsin/EDTA solution (Invitrogen, Carlsbad, CA) when 90% confluency was reached. After centrifugation the cells were suspended in 4mL of culture media. Clusters of adherent cells were formed on the culture dish by deposting 200μl droplets of suspended cells onto the plate using a pipette followed by incubation for 2 hours. Culture media (10mL) was then added to each dish and cells were incubated for an additional 12 hours at 37°C. Immediately prior to analysis the media was removed and exchanged with fresh warm media. Culture media was composed of RPMI 1640 mixed with 10% fetal calf serum, 50 μmol/L β mercaptoethanol, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 100 U/mL of penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA).
2.8 Glucose flux in Pseudomonas aeruginosa biofilms
P. aeruginosa is an opportunistic pathogen found in water distribution systems, and P. aeruginosa biofilms are the major cause of morbidity/mortality in cystic fibrosis patients (Costerson et al., 1999; Oliver et al., 2000). Pseudomonas aeruginosa (strain PA01) biofilms were grown on hollow fiber silicone membranes according to McLamore et al (2009) and McLamore et al (2010b). All P. aeruginosa biofilms were grown for at least 30 days at 37°C in a modified glucose media containing 10mM glucose, 50mM HEPES, 3mM NH4Cl, 43mM NaCl, 3.7mM KH2PO4, 1mM MgSO4, and 3.5μM FeSO4. Silver has been used for centuries as a biocide (Kim et al., 2000; Wu et al., 2007; Hwang et al., 2007), and ionic silver was used as an antimicrobial. Glucose flux was continuously measured at the biofilm surface for at least 30 minutes, and then 1.0 μM AgNO3 was added to the flowcell. The concentration of silver was significantly higher than the concentration used for disinfection of potable water on the International Space Station (Wing et al., 2010).
2.9 Experimental methods
Prior to each experiment described above, a background (reference) measurement was taken 3 mm from the cell/tissue surface for a minimum of 5 min as a null internal control. Glucose flux was measured at the surface of human breast endothelial cells, mouse muscle/intestinal tissue, glioblastoma neural clusters, and P. aeruginosa biofilms for at least 30 minutes. All experiments were conducted at 37°C, and repeated at least three times.
For determination of EC50 values, a SR glucose biosensor was positioned at the surface of cell clusters using the video zoomscope as previously described (McLamore et al., 2010a), phloretin was added (concentrations noted), and briefly mixed using a transfer pipette. Glucose flux was continuously recorded for at least 15 minutes at 37°C in all experiments. Relative activity-inhibitor plots were prepared by normalizing the glucose flux to the average pre-exposure flux measured in 18 replicate tissues.
2.10 Imaging
All field emission scanning electron microscopy (FESEM) graphs of the biosensor were obtained from a Hitachi S-4800 microscope with a power setting of 5.0kV and magnification settings of 3.5k and 25k. The biosensor was fixed in a vertical position for imaging (no additional preprocessing).
Cell viability before and after AgNO3 exposure was confirmed using a membrane integrity stain (BacLight Live/Dead viability kit; Invitrogen Molecular Probes, Carlsbad, CA). SYTO9 (green) was used to stain intact cells, and propidium iodide (red) was used to stain cells with compromised membranes (1:1 ratio). For all experiments, 3μL of stain was added to 1.5mL of PBS (all biofilms were stained for 20 minutes, and rinsed in PBS). A Zeiss LSM 710 (Thornwood, NY) confocal microscope with multi-wavelength lasers (488 and 514nm) was used for excitation, and Zen software (Zeiss, Thornwood, NY) was used for image capture and orthogonal image processing (Version 1.43f, NIH Image).
3. Results and discussion
3.1 Sensitivity, selectivity, and surface area, and validation of glucose flux
To characterize electrochemical behavior of the nanomaterials, CV was carried out for a bare Pt/Ir microelectrode and a micro biosensor made from the same electrode in Fe(CN)63− from 0 to +650 mV at a scan rate 20 mV/sec (Fig. 2). CV for the bare electrode exhibited a sigmoid curve and steady state diffusion-limited current, which is characteristic of microelectrodes (Heinze 1993). In addition to enhanced current, the biosensor also exhibited non-steady state diffusion-limited characteristics, likely due to the high film resistance of the MWNT/Nafion layer (Hrapovic et al. 2004). Diffusion limited current is defined as ilim = KnFDCr (Heinze 1993; Schulte and Chow 1996), where ilim is the diffusion-limited current, K is a geometric constant (K=2π for hemispherical diffusion model), n is the number of electrons transferred during the redox of Fe(CN)63−, F is the faradic constant, D is the diffusion coefficient for potassium ferricyanide ((6.70±0.02)×10−6 cm2/s), C is the concentration of potassium ferricyanide (4 mM) and r is the microelectrode tip radius. Based on this equation, the ratio of electrode tip radius between the nanomaterial modified biosensor and the bare electrode was estimated to be 11.5. This increase in effective surface area (i.e., tip radius measured via CV) is attributed to the deposition of catalytic nanomaterials (Hrapovic et al. 2004). Based on FESEM (Fig. 1b&c), low magnification images (inset; approximately 60 × 40μm) demonstrated the deposition of homogeneous Pt black and MWNT layers. High magnification images indicated that Pt black nanostructures were composed of a continuous film made up of amorphous nanoclusters of Pt particles. The highly porous MWNT provides a matrix for enzyme immobilization via absorption (Wang et al. 2003).
Figure 2.
(a) CV in 4 mM Fe(CN)63-/1M KNO3 for a bare micro-electrode and bionanocomposite (Pt black/MWNT/Nafion/GOx) micro-biosensor at a scan rate +100mV/s (b) Representative and (c) average sensitivity of glucose microbiosensor (n=5) in PBS at a constant working potential (+500 mV). Average current values (●) represent the arithmetic mean of observed current, and error bars represent the standard error of the mean for three replicate experiments. Average sensitivity was 531±149 pA mM−1 (n=3 biosensors).
As expected, calibration at 37°C via DC potential amperometry (+500mV) indicated significantly higher H2O2 sensitivity for the bionanocomposite sensor compared with bare Pt electrode (Fig. 2a). Bionanocomposite sensors exhibited a linear response towards glucose with a sensitivity of 531±149 pA/mM (n=3 replicate sensors) and an average response time (t95) of 0.88 sec (Fig. 2b&c). Average sensitivity per geometric tip area was 15.0±2.4 mA mM−1 cm−2.
A summary for the performance parameters of the bionanocomposite sensor and a comparison to designs in current literature is presented in the supplemental information (Table 1). The sensitivity per geometric surface area (15.0±2.4 mA mM−1 cm−2) was significantly larger than previously reported glucose micro biosensors (Hiratsuka et al. 2005; Jung et al. 2000; Jung et al. 2001; Kohma et al. 2007; Kurita et al. 2002; Miyashita et al. 2009). The lower limit of detection was 10 μM, and the linear range was 0.01 to 17.5 mM glucose with an R2 value of 0.99. Although the lower limit of detection was slightly higher than another microsensor (Kurita et al. 2002), the linear range covered glucose concentrations in common mammalian cell growth media (0.5–11.1 mM). This range is an improvement over previously reported micro biosensors (Hiratsuka et al. 2005; Jung et al. 2000; Jung et al. 2001; Kohma et al. 2007; Kurita et al. 2002; Tsai et al. 2005). Bionanocomposite sensors were stable for up to 7 days (11% change in sensitivity after 7 days, and less than 1% change after one day) when stored in 50 mg GOx/mL PBS at 4°C with enzyme solution replaced daily. Although this stability is slightly lower than other GOx biosensors (e.g., Claussen et al. 2009), the design herein was used continuously in physiological conditions, where the probability of surface fouling and enzyme denaturing are much higher than in “disposable” applications. The design used here is simple, robust, and can be used under physiological conditions.
To validate the use of the bionanocomposite sensor in the SR modality, glucose flux was measured in a known glucose concentration gradient near the surface of a tapered glass micropipette filled with 5 mM glucose and 0.5% agar in PBS (Fig. 3a). The sensor was oscillated at the surface of the pipette at a frequency of 0.3 Hz with an excursion distance of 30 μm for five minutes, and then moved to a position 5 μm from the surface. Based on the measured concentration values (near pole) throughout this “step back” experiment (SBE), an empirical Fickian diffusion model was developed (McLamore et al. 2009). The correlation between the predicted and measured glucose efflux was 0.99. The Fickian diffusion model did not consider convection, thus this correlation of predicted and measured values validated that the solution was not stirred by oscillating the sensor.
Figure 3.
(a) Average measured and predicted glucose flux microprofile near the surface of a tapered glass micropipette filled with 5mM glucose and 0.5% agar immersed in PBS (n=5 replicate experiments). Correlation between measured and predicted values (ε) was >0.98. (b) Continuous recording (raw A/D mode) of biosensor output during manual oscillation of a SR glucose biosensor at the surface of cultured pancreatic β cells. “Near pole” represents location of the sensor at the tissue surface, and far pole represents a position 30μm away from the surface. Current was converted to concentration via the average calibration plot in Fig. 2. (c) Glucose near pole (surface) and far pole (+30μm) concentration at the surface of cultured pancreatic β cells in DMEM at 37°C. Although the concentration output of the biosensor is full of drift and noise, the flux (i.e., concentration differential) was relatively constant due to phase sensitive detection and DC coupling (d) Glucose differential concentration data in panel C analyzed using the raw ΔC data (noted as “raw flux”), and the differential drift algorithm in McLamore et al. (2009).
To demonstrate the phase sensitive detection, glucose influx at the surface of cultured pancreatic β cells in DMEM at 37°C was continuously measured for 15 minutes. A trace recording of biosensor output during oscillation of the biosensor is shown in Fig. 3b (current was converted to concentration via the calibration in Fig. 2). The near pole position is representative of the biosensor when located at the tissue surface (positioned as described in McLamore et al., 2010a), and the far pole position represents the biosensor at a position 30μm in the direction of flux (perpendicular to the tangent of the tissue surface). The response time of the biosensor (0.88 sec) falls well within the move-wait-measure protocol used during oscillation at 0.33 Hz (total time of 1.5 seconds at each measurement position). Additionally, the DC coupled system utilized a signal filtering function to discretely remove background potential (Porterfield, 2007).
To demonstrate use of the differential drift algorithm originally presented in McLamore et al (2009), the glucose influx at the surface of cultured pancreatic β cells in DMEM at 37°C was continuously measured for thirty minutes. Fig 3c presents the concentration at the near pole and far pole during measurement of glucose flux. Due to the metabolic activity of β cells, the glucose concentration within the unstirred boundary layer continuously decreases. Although microsensors can be used in concentration mode to estimate uptake rates (for example, the rate in Fig. 3c is 1.67μM/min), application of such a technique is limited due to the requirement of long sample times, high cell densities, and/or small sample volumes. All of these approaches are not representative of in vivo physiological conditions, and techniques for analyzing data sets where signals are temporally dynamic are often very complex. On the other hand, oscillation of a single microsensor allows direct quantification of glucose transport with a temporal resolution of 2–5 sec under physiological conditions. For many cell/tissue applications, relatively high metabolic flux induces a differential drift artifact (noted as “raw flux” in Fig. 3d), which can be accounted for using the drift algorithm in McLamore et al. (2009) (noted as “differential drift algorithm” in Fig. 3d). This phase-sensitive detection strategy is invaluable for measuring biological analyte transport under physiological conditions, where background drift and noise (often in the nA to μA range) can be larger than sensor output at the cell/tissue surface (commonly in the pA to μA range).
3.2 Physiological glucose flux in cell/tissue applications
Average glucose influx (n=3) in tumorogenic breast endothelial cells (229.0±3.8 pmol cm−2 sec−1) was significantly higher than non-tumorogenic cells (126.0±2.1 pmol cm−2 sec−1) (p<0.01, α=0.05). Trace plots of glucose flux at the surface of confluent cultured cells are shown in Fig 4a (15 minutes of continuous data). This trend was expected, as cancer cells are known to utilize aerobic glycolysis (consumption of glucose and oxygen during simultaneous production of lactate), where non tumorogenic cells utilize a relatively low rate of glycolysis followed by subsequent ATP production via mitochondrial oxidative phosphorylation (Gatenby and Gillies, 2004; Zu and Guppy, 2004; Heiden et al., 2009). This upregulation of glycolysis (known as the Warburg effect, or aerobic glycolysis) is widely recognized as a survival advantage used by cancerous cells (Lu et al., 2002; Xu et al., 2005; Matoba et al., 2006; Pelicano et al., 2006). The Warburg effect has been measured in vivo using various techniques, including positron-emission tomography (Mochiki et al., 2004) and oxygen microelectrodes combined with p53 assays (Matoba et al., 2006). However, very little real time (~sec) data exists regarding aerobic glycolysis under physiological conditions, and the ability to measure glycolytic transport using simple biosensor approaches will be a very valuable tool in cancer research.
Figure 4.
(a) Average glucose flux at the surface of tumorogenic and non-tumorogeinc human breast endothelial cells in αMEM (n=3). Glucose flux in cancerous cells was significantly higher than non-tumorogenic cells (b) Average glucose flux at the surface of soleus and gastrocnemius muscle tissue from a mouse model (n=3). Glucose uptake in soleus tissue was significantly higher than gastrocnemius tissue for all preps. (c) Average glucose flux at the surface of upper jejunum and lower jejunum of excised mouse small intestinal tissue in RPMI media (n=3). Glucose flux in upper intestinal tissue was significantly higher than the lower intestine tissue.
The ability to measure physiological glucose flux via SR biosensors also provides useful data concerning general cell/tissue physiology. Average glucose flux in soleus (Type I) mouse muscle tissue (174.1±1.9 μmol cm−2 sec−1) was significantly higher than Type II (gastrocnemius) muscle (86.6±0.7 μmol cm−2 sec−1; p<0.01, α=0.05, n=3; Fig 4b). This is consistent with previous studies, where glucose flux was significantly higher in soleus than in gastrocnemius (Lagord et al., 1998; Holloszy and Hansen, 1996; Balon and Nadler, 1997). Soleus contains 75–85% slow-contracting type fibers, significantly more mitochondria, and typically more transmembrane glucose transport proteins, while gastrocnemius muscle contains a lower percentage of slow contracting fibers (approximately 57%; Gollnick et al., 1974), fewer mitochonodria, and significantly fewer glucose transporters (Slentz et al., 1992). Physiological glucose uptake in upper jejunum tissue (17.8±0.2 pmol cm−2 sec−1) was significantly higher than the average flux in lower jejunum tissue (5.5±0.2 pmol cm−2 sec−1) (p<0.01, α=0.05, n=3; Fig 4c). This metabolic trend is consistent with sucrase assays in rats (Goda and Koldovsky, 1985), and glucose studies in pigs, dogs, and humans (Baker et al., 1961; Holtug and Skadhauge, 1991).
For all experiments using excised tissue, flux was measured at a minimum of seven positions along the tissue separated by at least 0.1 cm, and no statistical difference was measured at any of the positions which were at least 1mm from the point of excision (p=0.012, α=0.05). While measurements of glucose transport/uptake via techniques such as perfusion and 2-deoxyglucose assays (Holloszy and Hansen, 1996; Balon and Nadler, 1997) continue to be useful techniques for studies of glucose transport, the ability to measure real time glucose uptake under physiological conditions without the addition of radiotracers will greatly contribute to our understanding of glucose membrane transport, exercise/diet physiology, and hypoxia.
3.3 Pharmacological glucose transport inhibition in pancreatic β cells
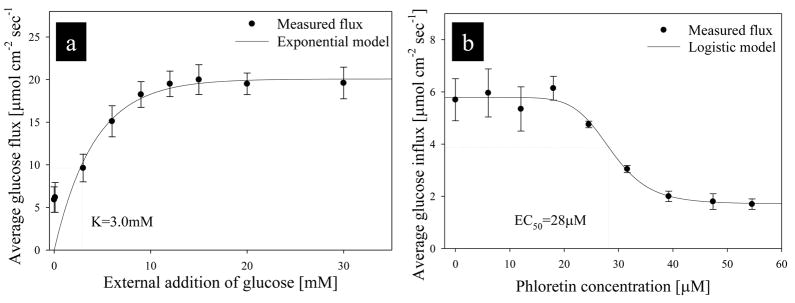
Glucose flux was measured at the surface of pancreatic INS-1 β cells in RPMI media at 37°C, and the median influx at the cell surface was 5.9±1.4 μmol cm−2 sec−1. This flux was significantly larger than a background measurement taken 3mm from the tissue surface (0.28±0.1 pmol cm−2 sec−1), which was used as an internal control (p>0.01, α=0.05). Glucose flux was continuously measured during external addition of glucose (Fig 5a) and the glucose transport inhibitor phloretin (Fig 5b). Glucose flux followed Michaelis-Menten type kinetics, with a maximum induced glucose uptake of 19.6±3.7 μmol cm−2 sec−1. An exponential curve was fit to the data, and the correlation between the exponential model and the average measured flux was 0.97 (n=12 replicate confluent cell layers). Using this data, the average calculated half saturation constant (K) was 3.1±1.3mM. Following addition of phloretin above concentrations of 20 μM, average glucose flux significantly decreased. Phloretin is an inhibitor of many sodium-independent transporters (e.g., GLUT1, GLUT2, GLUT3) (Guillam et al. 2000; Kellett and Helliwell 2000; Dominguez et al. 2002; Lam et al. 2004). This reduction in glucose transport is likely due to inhibition of GLUT 2 by phloretin (Kellett and Helliwell 2000; Dominguez et al. 2002). Based on current knowledge concerning GLUT transport proteins in pancreatic β cells, the residual glucose flux is likely due to GLUT9 (Evans et al. 2009). A four parameter logistic model was fit to the average glucose flux data during phloretin addition (calculated hillslope was 8.01, and correlation was 0.98). This logistic plot was used to calculate an inhibition constant (EC50), which had an average value of 28±1.6μM. This value is significantly lower than the concentration used to inhibit GLUT2 in HepG2 cells (200μM phloretin) measured using flow cytometry and DNA fragmentation studies; although no detailed inhibition kinetics were provided (Wu et al., 2009). Glucose assays based on concentration lack the temporal resolution demonstrated herein using the SR micro biosensor technique.
Figure 5.
(a) Average glucose dose-response curve for INS 1 β cells during addition of excess glucose. The calculated half saturation constant (K) for glucose uptake was 3.1±1.3mM, and the correlation between the exponential model and the measured data was 0.97 (n=12 replicate confluent cell layers) (b) Average phloretin dose-response curve for INS 1 cells. The calculated inhibition constant (EC50) was 28±1.6μM, the calculated hillslope was 8.01, and the correlation was 0.98 (n=12).
3.4 Glucose flux in Pseudomonas aeruginosa biofilms
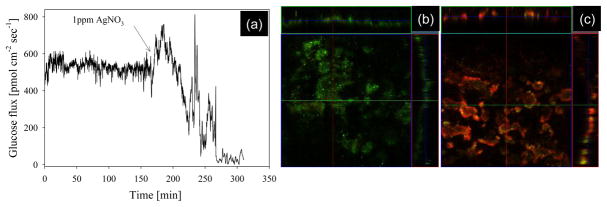
Monoculture P. aeruginosa mature (30 day old) biofilms were grown on hollow fiber silicon membranes using glucose as the primary substrate. Glucose flux into biofilms was relatively homogenous (525±81 pmol cm−2 sec−1) in three replicate biofilms (flux was measured for a minimum of 60 minutes at seven measurement positions along the biofilm surface). Figure 6 shows a representative trace of glucose influx for five hours, followed by addition of 0.8μM AgNO3. After an average of 185±24 minutes, glucose flux decreased by 96±5%, indicating a sharp reduction in metabolism (and likely cell lysis). This was confirmed by confocal microscopy using a membrane integrity stain, where green fluorescence is indicative of intact cell membranes, and red fluorescence is indicative of compromised cell membranes. Silver has been used as an antimicrobial for centuries (Kim et al., 2000; Wu et al., 2007; Hwang et al., 2007), and the mode of toxicity involves interaction with protein sulfhydryl groups, causing metabolic depression, inhibition of cell division, uncoupling of oxidative phosphorylation, and DNA damage (Donlan and Costerton, 2002; Rusin et al., 2003). Although microbial resistance to silver is rare, gram negative bacteria have indicated resistance to silver via upregulation of ATPase efflux pumps and metal oxidoreductases (Wu et al., 2007). In addition to genetic resistance, it is widely accepted that sessile bacteria associated with biofilms are much more resistant to biocide exposure than their planktonic counterpart (Sauer et al., 2002). Thus, the concentration used in this study was significantly higher than the concentration used for disinfection of potable water on the International Space Station (0.37 μM; Wing et al., 2010). The ability to monitor metabolic transport in microbial biofilms will greatly improve our understanding of biofilms in human infectious disease, and engineered systems such as potable water distribution systems.
Figure 6.
(a) Glucose flux into a mature (30 day old) Pseudomonas aeruginosa biofilm grown on a hollow fiber silicone membrane. Confocal microscopy images (orthogonal images at 10× magnification) showing (b) cells with intact membranes before exposure to 0.8 μM AgNO3, and (b) cells with damaged membranes after three hours of exposure to AgNO3.
We have demonstrated use of the SR micro-biosensor technique under a wide range of cell culture conditions. As opposed to sensors used in the concentration domain, the SR biosensor technique facilitates direct measurement of trans-membrane (or trans-tissue) flux, which will greatly improve our understanding of the dynamic, real time transport phenomena required to sustain the metabolic activity of cells. For detailed discussions on quantification of transmembrane flux compared to time-resolved concentration measurements, see Porterfield (2007), McLamore et al. (2010a), and McLamore et al. (2010b). This is a significant technological advancement, as microsensors used in concentration mode can be susceptible to a low signal-to-noise ratio due to drift/noise, limiting application. Due to use under physiological conditions, the possibility of contamination by sensor nanomaterials must be considered. A detailed discussion of the potential toxic effects of nanomaterials on cells/tissues is outside the scope of this manuscript; the reader is referred to the literature for more information (Oberdorster et al., 2005; Gratton et al., 2008).
4. Conclusions
The ability to measure glucose transport under physiological conditions is a vital tool to physiologists investigating a wide range of scientific hypotheses. Enzyme-based biosensors have proven to be one of the leading candidates for accurately measuring glucose concentration. However, in cell/tissue culture applications, miniaturization of biosensors limits application caused by low signal-to-noise ratios (due to electromagnetic interference/noise, and drift). To overcome these problems, a highly sensitive/selective glucose oxidase micro biosensor was used in the self-referencing modality. Sensitivity was enhanced via deposition of nanomaterials (platinum black, multiwalled carbon nanotubes), and physiological flux was facilitated via a phase sensitive, DC-coupled electronics scheme. Using this biosensor technique, trans-membrane and trans-tissue physiological glucose flux was measured in a wide range of biomedical and environmental applications.
Supplementary Material
Acknowledgments
The authors would like to thank the National Science Foundation (DMP), National Institutes of Health (DT, DMP, JLR), National Cancer Institute R25CA128770 (DT), and Cancer Prevention Internship Program (Y. Jiang) for funding the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad F, Yusof APM, Bainbridge M, Ghania SA. Biosens Bioelectron. 2008;23:1862–1868. doi: 10.1016/j.bios.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. J Appl Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- Baker RD, Searle GW, Nunn AS. Amer J Physiol. 1961;200(2):301–305. doi: 10.1152/ajplegacy.1961.200.2.301. [DOI] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2. Wiley; New York: 2000. [Google Scholar]
- Busk M, Horsman MR, Kristjansen PE, van der Kogel AJ, Bussink J, Overgaard J. Int J Cancer. 2008;122(12):2726–2734. doi: 10.1002/ijc.23449. [DOI] [PubMed] [Google Scholar]
- Chatni MR, Porterfield DM. Analyst. 2009;134:2224–2232. doi: 10.1039/b903092a. [DOI] [PubMed] [Google Scholar]
- Choleau C, Klein JC, Reach G, Aussedat B, Demaria-Pesce V, Wilson GS, Gifford R, Ward WK. Biosens Bioelectron. 2002;17(8):647–654. doi: 10.1016/s0956-5663(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Claussen JC, Franklin AD, ul Haque A, Porterfield DM, Fisher TS. ACS Nano. 2009;3:37–44. doi: 10.1021/nn800682m. [DOI] [PubMed] [Google Scholar]
- Claussen JC, Sungwon KS, ul Haque A, Artiles MS, Porterfield DM, Fisher TS. J Diabetes Sci and Technol. 2010;4:312–319. doi: 10.1177/193229681000400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Dominguez JH, Soleimani M, Batiuk T. Kidney Int. 2002;62(1):127–136. doi: 10.1046/j.1523-1755.2002.00429.x. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Nature Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Goda T, Koldovsky O. Biochem J. 1985;229:751–758. doi: 10.1042/bj2290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Sjödin B, Karlsson J, Jansson E, Saltin B. Pflügers Archiv European J Physiol. 1974;348(3):247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Gong K, Yan Y, Zhang M, Su L, Xiong S, Mao L. Anal Sci. 2005;21:1383–1394. doi: 10.2116/analsci.21.1383. [DOI] [PubMed] [Google Scholar]
- Gooding JJ. Electrochim Acta. 2005;50(15):3049–3060. [Google Scholar]
- Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. Proc Nat Acad Sci (USA) 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillam MT, Dupraz P, Thorens B. Diabetes. 2000;49:1485–1491. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- Heiden MG, Cantley LC, Thompson CB. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B, Idahl LA, Lernmark A, Taljedal IB. Proc Natl Acad Sci U S A. 1974;71(9):3405–3409. doi: 10.1073/pnas.71.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka A, Kojima K, Muguruma H, Lee K, Suzuki H, Karube I. Biosens Bioelectron. 2005;21(6):957–964. doi: 10.1016/j.bios.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen GX, Henkel-Rieger R, Prentki M, Newgard CB. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- Holtug K, Skadhauge E. Am J Physiol. 1991;260(2):G220–31. doi: 10.1152/ajpgi.1991.260.2.G220. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Hansen RA. Rev Physiol Biochem Pharmacol. 1996;128:99–193. doi: 10.1007/3-540-61343-9_8. [DOI] [PubMed] [Google Scholar]
- Hrapovic S, Liu Y, Male KB, Luong JH. Anal Chem. 2004;76(4):1083–1088. doi: 10.1021/ac035143t. [DOI] [PubMed] [Google Scholar]
- Hwang MG, Katayama H, Ohgaki S. Water Res. 2007;41:4097–4104. doi: 10.1016/j.watres.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Nuccitelli R. J Cell Biol. 1974;80(63):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SK, Kauri LM, Qian W, Kennedy RT. J Bio Chem. 2000;275:6642–6650. doi: 10.1074/jbc.275.9.6642. [DOI] [PubMed] [Google Scholar]
- Jung SK, Trimarchi JR, Sanger RH, Smith PJS. Anal Chem. 2001;73(15):3759–3767. doi: 10.1021/ac010247u. [DOI] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. Biochem J. 2000;15(350):155–162. [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Thomas S, Fogler HS. Appl Environ Microbiol. 2000;66(3):976–981. doi: 10.1128/aem.66.3.976-981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Shaff JE, Kühtreiber WM, Jaffe LF, Lucas WJ. Planta. 1992;188:601–610. doi: 10.1007/BF00197055. [DOI] [PubMed] [Google Scholar]
- Kohma T, Oyamatsu D, Kuwabata S. Electrochem Comm. 2007;9(5):1012–1016. [Google Scholar]
- Kühtreiber WM, Jaffe LF. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TL, Fishman HM, Ballinger ML, Bittner GD. J Neurosci. 1994;14(11):6636–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita R, Hayashi K, Fan X, Yamamoto K, Kato T, Niwa O. Sens Actuat B: Chem. 2002;87(2):296–303. [Google Scholar]
- Lanevschi A, Kramer JW. Veterinary Clinic Pathol. 2009;25(1):10–13. doi: 10.1111/j.1939-165x.1996.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Lowry JP, O’Neill RD, Boutelle MG, Fillenz M. J Neurochem. 1998;70(1):391–396. doi: 10.1046/j.1471-4159.1998.70010391.x. [DOI] [PubMed] [Google Scholar]
- Lu HS, Forbes RA, Verma A. J Bio Chem. 2002;277(26):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- Makino K, Maruo S, Morita Y, Takeuchi T. Biotech Bioengin. 1988;31(6):617–619. doi: 10.1002/bit.260310615. [DOI] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. Science. 2006;312(5780):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Mohanty S, Shi J, Rickus JL, Porterfield DM. J Neurosci Methods. 2010;189:14–22. doi: 10.1016/j.jneumeth.2010.03.001. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Zhang W, Porterfield DM, Banks MK. Environ Sci Technol. 2010 doi: 10.1021/es1012356. in press. [DOI] [PubMed] [Google Scholar]
- McLamore ES, Porterfield DM, Banks MK. Biotech Bioengin. 2009;102(3):791–799. doi: 10.1002/bit.22128. [DOI] [PubMed] [Google Scholar]
- Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. World J Surgery. 2004;28(3):247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- Morten B, Horsman MR, Kristjansen PEG, van der Kogel AJ, Bussink J, Overgaard J. Int J Cancer. 2008;122(12):2726–34. doi: 10.1002/ijc.23449. [DOI] [PubMed] [Google Scholar]
- Nayak RC, Herman IM. J Immunol Methods. 1997;205(2):109–114. doi: 10.1016/s0022-1759(97)00068-9. [DOI] [PubMed] [Google Scholar]
- Ni JA, Ju HX, Chen HY, Leech D. Analytica Chimica Acta. 1999;378(1–3):151–157. [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Environ Health Perspect. 2005;133:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Cantón R, Campo P, Baquero F, Blázquez J. Science. 2000;288:1251–1253. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Oncogene. 2006;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Corkey RF, Sanger RH, Tornheim K, Smith PJ, Corkey BE. Diabetes. 2000;49(9):1511–1516. doi: 10.2337/diabetes.49.9.1511. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Laskin JD, Jung SK, Malchow RP, Billack B, Smith PJS, Heck DE. Am J Physiol Lung Cell Mol Physiol. 2001;281:L904–L912. doi: 10.1152/ajplung.2001.281.4.L904. [DOI] [PubMed] [Google Scholar]
- Porterfield DM. Biosens Bioelectron. 2007;22(7):1186–1196. doi: 10.1016/j.bios.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Rickus JL, Dunn B, Zink JI, Frances SL, Chris ART. Optically Based Sol-Gel Biosensor Materials. In: Ligler FS, Rowe-Taitt CA, editors. Optical Biosensors. Elsevier Science; Amsterdam: 2002. pp. 427–456. [Google Scholar]
- Shabala SN, Newman IA, Morris J. Plant Phys. 1997;113(1):111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slentz CA, Gulve EA, Rodnick KJ, Henriksen EJ, Youn JH, Holloszy JO. J Appl Physiol. 1992;73(2):486–492. doi: 10.1152/jappl.1992.73.2.486. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Li G, Najafi H, Berner D, Matschinsky FM. Am J Physiol Endocrinol Metab. 1996;271:E606–E625. doi: 10.1152/ajpendo.1996.271.3.E606. [DOI] [PubMed] [Google Scholar]
- Wang J, Musameh M, Lin Y. J Amer Chem Soc. 2003;125(9):2408–2409. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- Wang J. Electroanalysis. 2005;17(1):7–14. [Google Scholar]
- Weiss RG, Chacko VP, Glickson JD, Gerstenblith G. Proc Natl Acad Sci USA. 1989;(16):6426–6430. doi: 10.1073/pnas.86.16.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MY, Suryanarayanan K, van Ooij WJ, Oerther DB. Water Sci Techn. 2007;55(8–9):413–419. doi: 10.2166/wst.2007.285. [DOI] [PubMed] [Google Scholar]
- Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. Inter J Cancer. 2009;124(9):2210–2219. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Cancer Research. 2005;65(2):613–621. [PubMed] [Google Scholar]
- Zantek ND, Walker-Daniels J, Stewart J, Hansen RK, Robinson D, Miao H, Wang B, Kung HJ, Bissell MJ, Kinch MS. Clin Cancer Res. 2001;7:3640–3648. [PubMed] [Google Scholar]
- Zawalich WS, Matschinsky FM. Endocrinol. 1977;100(1):1–8. doi: 10.1210/endo-100-1-1. [DOI] [PubMed] [Google Scholar]
- Zhang T, Fang HHP. Environ Technol. 2005;26(2):155–160. doi: 10.1080/09593332608618574. [DOI] [PubMed] [Google Scholar]
- Zu XL, Guppy M. Biochem Biophys Res Comm. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.