Abstract
Amebiasis in the murine model can be prevented by vaccination with the Gal/GalNAc lectin through a T cell-dependent mechanism. In this work we further decipher the mechanism of this protection. Mice vaccinated with the recombinant “LecA” fragment of the Gal/GalNAc lectin with alum were capable of transferring protection to naïve recipients by both CD4+ T cells and surprisingly CD8+ T cells. We then examined the cytokine profile of these cells. CD4+ T cells from PBMC of LecA-alum vaccinated mice were observed to be a major source of IFN-γ, known to be a protective cytokine with this vaccine. In contrast, CD8+ T cells produced relatively little IFN-γ but more IL-17 than the CD4 compartment. We thus examined the role of IL-17 in vaccine mediated protection and found through neutralization experiments that this cytokine contributed to LecA-alum vaccine protection. In addition we examined whether these cells exhibited direct amebicidal activity in vitro and found that both populations had amebicidal activity at high concentrations (1000:1) but CD8+ T cells appeared more potent, capable of cytotoxicity at a 100:1 ratio. In conclusion, both CD4 and CD8 T cells exert protection with this amebiasis vaccine. The mechanism of CD8 T cell-mediated protection may include direct amebicidal activity and/or IL-17 production. Both IL-17 and IFN- γ are useful surrogates for immune protection.
Keywords: Vaccine, Entamoeba histolytica, CD8+ T cell, IL-17, Amebicidal activity
1. Introduction
The protozoan parasite Entamoeba histolytica is the causative agent of amebiasis, a parasitic illness that manifests most commonly as amebic colitis and liver abscess. E. histolytica infection remains a worldwide health problem that particularly affects people in areas of poor sanitation and nutrition, and it persists as one of the major causes of diarrhea in pre-school children in developing countries [1]. Despite of the efficacy of metronidazole treatment and improvements in diagnosis, an effective amebiasis vaccine remains a desirable and feasible goal to prevent Entamoeba histolytica infection globally.
We have previously demonstrated that vaccines derived from the amebic Gal/GalNAc lectin (native lectin or its recombinant subunit, LecA) provided effective protection in mice against intestinal infection by E. histolytica [2] [3]. Interestingly, disparate adjuvants provided comparable protection regardless of their different immunogenicity, suggesting the presence of multiple redundant protective mechanisms [3]. Our previous studies have indicated that T cell but not immune serum is sufficient to transfer Gal/GalNAc lectin-provided protection, and IFN-γ appears to be required for lectin or LecA-elicited protective immunity across three different formulations [3]. Interestingly, although the LecA/alum formulation elicited a Th2-type response characterized by substantial production of IL-4 and predominant levels of IgG1 isotype, IFN-γ remained a protective factor and immune correlate to the LecA/alum-based vaccine [3]. In addition to IFN-γ, LecA also elicited antigen-specific IL-17 production by splenocytes and mesenteric lymph node cells [3], but how this cytokine might contribute to the immunity to amebiasis remains unclear. In this study, to determine the effector mechanisms of LecA/alum-elicited protection, we investigated the contribution of CD4+ and CD8+ T cells for vaccine protection, and examined the amebicidal activity of vaccinated T cells. We also found that IL-17, predominantly produced by CD8+ T cells, contributed to LecA/alum-mediated protection, which might serve as another effector cytokine elicited by this regimen.
2. Materials and Methods
2.1. Animals
Male CBA/J mice (3–4 week old) were obtained from The Jackson Laboratory for vaccination and adoptive transfer experiments. Mice were maintained under specific pathogen-free conditions at University of Virginia.
2.2. Immunization regimen
The production of the antigen: recombinant subunit “LecA” (amino acids 578–1154 of the Gal/GalNAc lectin heavy chain), was described previously [2;3]. Briefly, LecA-expressing bacterial cells were lysed and treated with 0.7% NP-40 and 0.7% Sodium deoxycholate (Sigma-Aldrich). The isolated inclusion bodies were washed with 0.05% Triton X-100 and denatured in 8 M urea containing 50 mM DTT. To renature the proteins, the solution was diluted 10 fold and the pH gradually titrated from 11 to 7.5. Immobilized Metal ion Affinity Chromatography (IMAC) purification was performed with a HisPrep 16/10 Fast Flow column (GE Healthcare) eluting LecA with 300 mM Imidazole (Sigma-Aldrich).
Mice were immunized subcutaneously with 100 µL of LecA/alum emulsion containing 20µg of recombinant LecA formulated in 1mg/ml of alum (Rehydragel LV, Reheis, Inc.). Animals were boosted twice at 4-week intervals by the same regimen as the priming dose. Endotoxin levels of purified proteins were monitored with the Endosafe PTS™ instrument (Charles River Laboratory) and ranged from 0.021 EU/µg to 0.079 EU/µg. In all sham-immunized mice the amount of LPS (Sigma-Aldrich) contained in 20 µg LecA was included as a control.
2.3. Animal model for intestinal amebic infection
Trophozoites for inoculation were originally derived from laboratory strain HM1: IMSS (ATCC) that were sequentially passed in vivo through the mouse cecum. For all intracecal inoculations, trophozoites were grown to the log phase and 2 × 106 trophozoites in 150 µl were injected intracecally after laparotomy as described previously [2;3]. Mice were sacrificed at day 7 or day 14 post-infection. The cecum was harvested and longitudinally bisected, cecal content was rinsed with PBS and cultured in complete TYI-S-33 medium with supplemental antibiotics for 5 days at 37°C. Infection status was determined by cultural positivity.
2.4. Adoptive transfer experiments
Mice were immunized with recombinant LecA formulated with alum. Two weeks after the final immunization, vaccine-treated and PBS sham-immunized mice were bled for serum and sacrificed to obtain splenocytes. Pooled single cell suspensions from either vaccine or PBS-immunized donors were enriched for CD3+ T cells or CD4+ and CD8+ T cells by magnetic separation (EasySep Mouse T Cell Enrichment Kit, StemCell Technologies). To determine the efficiency of T cell enrichment, the donor lymphocytes were stained with anti-B220, anti-CD3, anti-CD4 and anti-CD8 antibodies (all from BD Biosciences) by flow cytometry before and after T cell enrichment. For T cell transfer, 4 × 107 enriched donor CD3+ T cells or 2 × 107 CD4+ or CD8+ T cells were injected intravenously to naive CBA recipients. For serum transfer, recipients received 400µl of serum intraperitoneally. Both T cell and serum recipients were challenged 3 days after transfer and sacrificed 10 days post challenge.
2.5. Intracellular cytokine staining of PBMC
5 × 105 PBMCs were cultured in complete DMEM (Invitrogen) and stimulated with 10 µg/ml of LecA for 22 h. Anti-CD28 and Golgiplug (BD Biosciences) were added in the last 12 h of stimulation. Phorbol myristoyl acetate (PMA) (50ng/mL) and ionomycin (800ng/mL) (Sigma-Aldrich) were used as positive controls to assess cytokine production capacity for each sample.
For intracellular staining, PBMC were washed and incubated with CD16/CD32 mAb (BD Biosciences) to block Fc binding, followed by surface staining with CD4-PE and CD8-PerCP.Cy5.5 (BD Biosciences). Cells were permeabilized and stained with IFN-γ-FITC (BD Biosciences), IL-17-APC (eBioscience) and run on a BD FACSCalibur (BD Biosciences). The data were analyzed with FlowJo software.
2.6. In vitro amebicidal acitivity
Splenocyte single cell suspensions from either vaccine (LecA/alum) or PBS/alum-immunized donors were enriched for CD4+ and CD8+ T cells by magnetic separation (EasySep Mouse T Cell Enrichment Kit, StemCell Technologies) as described in 2.4. Purified T cells (2.0 × 106 cells per well) were incubated at 37°C in 5% CO2 with HM1 trophozoites (at T cell: HM1 ratios of 10:1, 100:1 and 1000:1) for 6 h in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. At the end of the incubation period, the pellet was suspended by gentle pipetting and the numbers of viable amebae were determined by using trypan blue (0.4%) exclusion criteria.
2.7. Cytokine depletion in vivo
For IL-17 neutralization, mice were treated with 125 µg of anti-IL-17 mAb (LEAF™ Purified Anti-mouse IL-17A, clone TC11-18H10.1, BioLegend) or control rat IgG intraperitoneally at day -1, day 1 and day 4 post-infection (3 doses total with 125µg per dose). The mice were sacrificed at day 8 post-infection.
2.8. Statistical analysis
All statistical analyses were performed using GraphPad Prism for Windows, version 5.0 (GraphPad Software). Student t tests were used to compare values of vaccinated versus control groups, or protected versus unprotected individuals. Fisher’s exact test was used to compare the infection rates. Two tailed p values < 0.05 were considered significant.
3. Results
3.1. LecA-mediated protection is transferable by CD3+, CD4+ or CD8+ T cells but not by serum
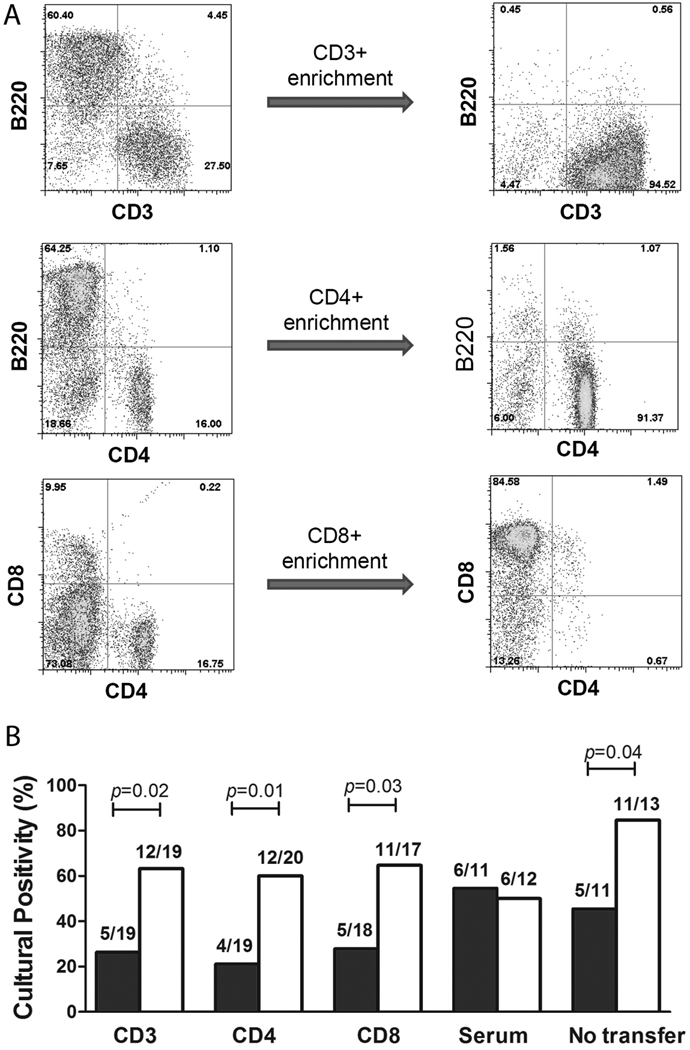
To determine the contributions of T cell subsets and immune serum in LecA/alum-mediated protection, adoptive transfer experiments were performed using CBA donor and recipient mice. Splenocytes and serum were obtained from LecA/alum-vaccinated or PBS sham-immunized mice. The donor splenocytes were purified for CD3+, CD4+, and CD8+ T cells. After the immunomagnetic selection, CD3+ T cells were enriched from 28% to 95%, CD4+ T cells from 16% to 91% and CD8+ T cells from 10% to 85% (Fig. 1A). Three days after transfer of these purified T cells, naïve recipients of T cells or serum were challenged with E. histolytica and sacrificed at day 12 post-infection. As determined by culture positivity, recipients for immune CD3+ or CD4+ or CD8 + T cells showed significantly lower infection rates compared with the control recipients (58%, 65%, and 57% efficacies, respectively, Fig. 1 B). As seen previously [3], immune serum was unable to transfer protection to recipients (Fig. 1 B).
Figure 1. LecA/alum-mediated protection is adoptively transferred by CD3+, CD4+ or CD8+ T cells, not serum antibodies.
LecA/alum-immunized and PBS/alum sham-immunized CBA donor mice were sacrificed 4 weeks after the final boost. Splenocytes were isolated and enriched for CD3+, CD4+ and CD8+ T cells. (A) The purity of enriched cells was determined by FACS staining for CD3, B220, CD4 and CD8 prior and after enrichment. (B) T cells or serum from vaccinated vs. control donor mice were transferred to naïve recipients, which were challenged with E. histolytica trophozoites 3 days after transfer. The recipients were sacrificed 12 days post-challenge to assess protection as determined by culture of cecal contents. Close bars: recipients that were transferred with cells or serum from LecA-immunized donors; Open bars: recipients that were transferred with cells or serum from PBS control donors. “No transfer” refers to LecA/alum immunized (close bar) or PBS control mice (open bar) directly challenged with E. histolytica without performing adoptive transfer.
3.2. CD8+ T cells preferentially make IL-17 over IFN-γ
Next we examined the cytokine profile of these cells. CD4+ T cells from PBMC of LecA-alum vaccinated mice were observed to be a major source of IFN-γ (in IFN-γ+ population, 44% of CD4, versus 23% of CD8 and 33% of non-CD4 non-CD8 cells), known to be a protective cytokine with this vaccine (Fig. 2A). In contrast, CD8+ T cells produced relatively little IFN-γ but more IL-17 compared with the CD4 compartment (in IL-17+ population, 55% of CD8, 23% of CD4 and 13% of non-CD4 non-CD8 cells) (Fig. 2B).
Figure 2. IFN-γ- and IL-17-producing CD4 and CD8 T cells in peripheral blood.
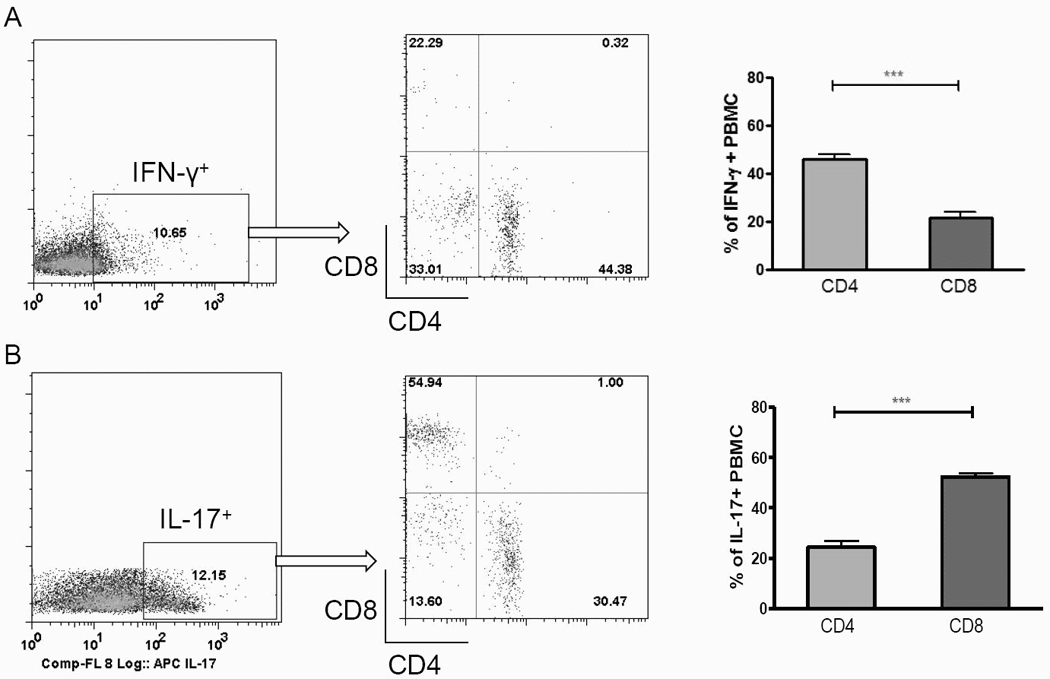
PBMC were isolated from LecA/alum-immunized and PBS/alum control CBA mice 3 weeks after the final boost. Cells were in vitro stimulated with LecA for 20 hrs and stained for CD4, CD8 and intracellular IFN-γ and IL-17. Cells were gated on IFN-γ+ (A, left) or IL-17+ (B, left) populations for their CD4 or CD8 phenotype (A, B, middle and right, n=16). *** p <0.001.
3.3. IL-17 contributes to LecA/Alum-elicited protection
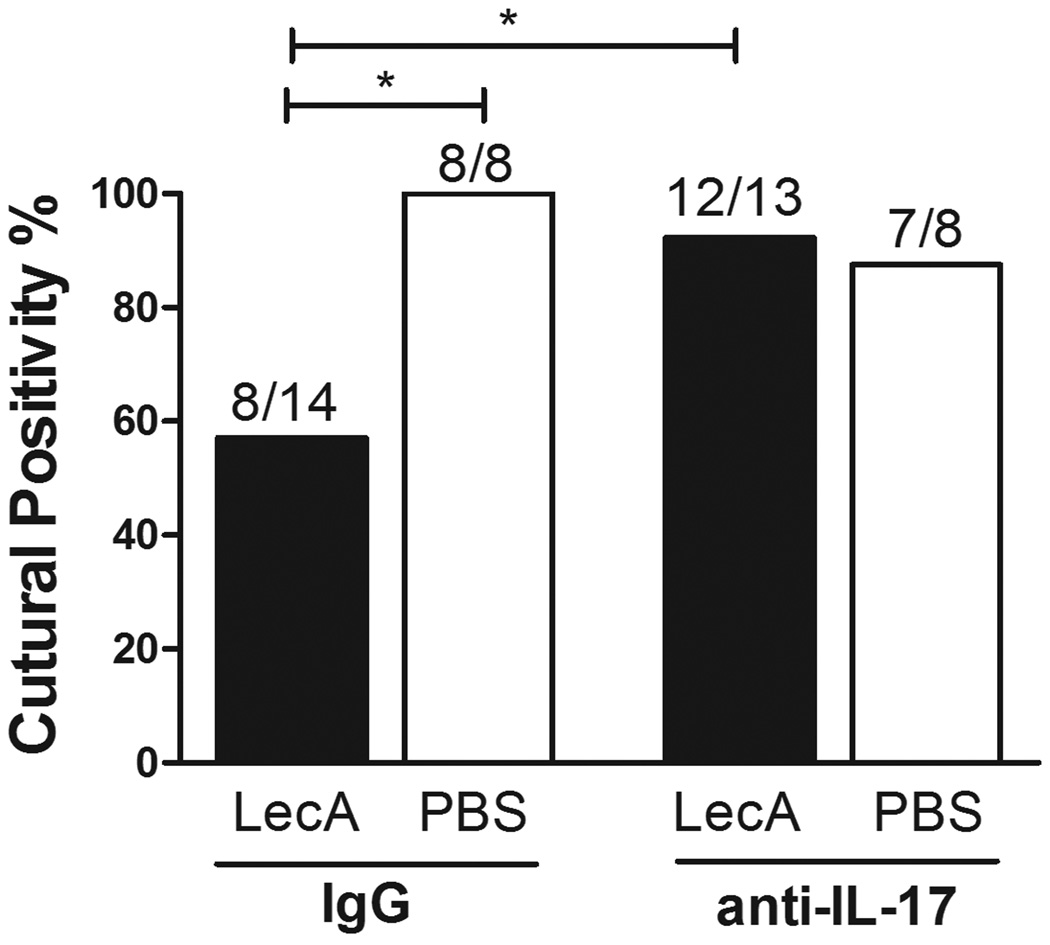
To test the role of IL-17 in LecA/alum-elicited protection, vaccinated mice were treated with anti-IL-17 mAb or control IgG and challenged with E. histolytica. As shown in Fig. 3, IL-17 neutralization abrogated protection conferred by LecA/alum, as immunized mice treated with anti-IL-17 lost protection that the immunized/control IgG treated mice exhibited. Notably, IL-17 neutralization had no observed effect on infection in wild-type, PBS/sham immunized mice, suggesting IL-17 exhibits little impact during natural infection.
Figure 3. IL-17 contributes to LecA/alum-mediated protection.
Mice immunized with LecA (closed bars) or PBS (open bars) in the alum regimen were administered anti-IL-17 or control IgG from 1 day prior to 4 days after challenge (3 doses total). Mice were sacrificed seven days post-infection. *p <0.05.
3.4. In vitro amebicidal activity of CD8+ and CD4+ T cells from LecA-vaccinated mice
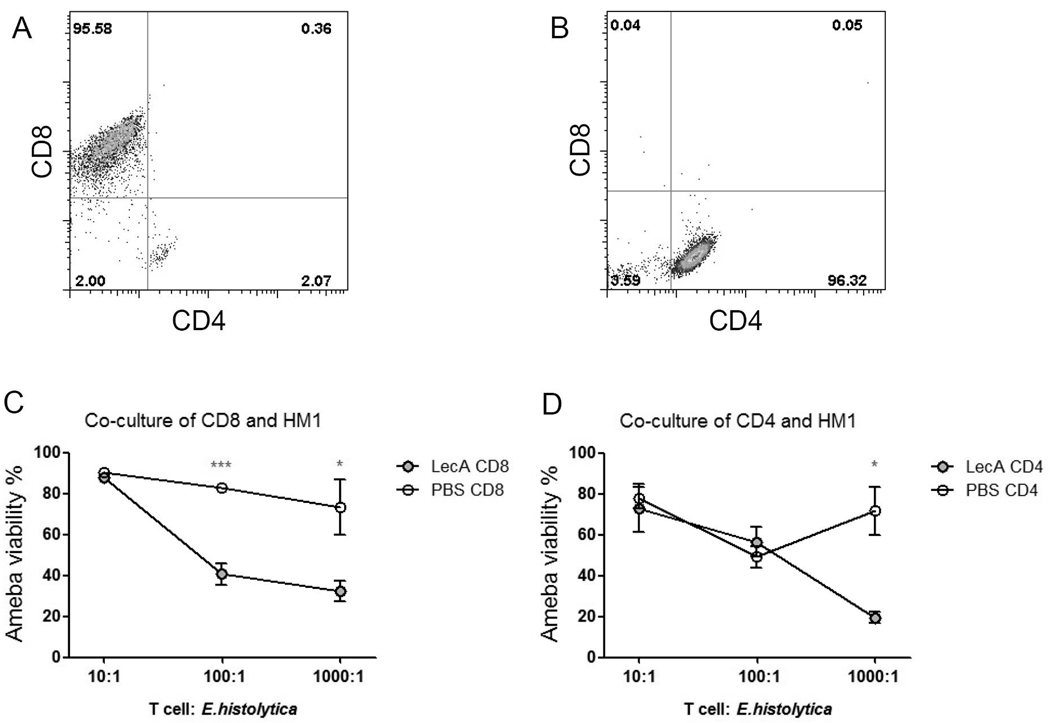
To examine another mechanism whereby CD4 and CD8 T cells may be protective, an in vitro amebicidal assay was performed by co-culturing E. histolytica trophozoites (HM1) with CD4 or CD8 T cells (purified from spleen) from LecA or PBS-immunized mice. The purity of isolated CD8 and CD4 T cells were 95% (Fig. 4A) and 96% (Fig. 4B) respectively. Compared to the PBS CD8 T cells, CD8+ cells from LecA-immunized mice elicited significantly greater amebicidal acitivity at a ratio of 100:1 (T cell: ameba), and the killing capability persisted at a higher ratio 1000:1 (Fig. 4C). The immune CD4+ T cells however, only killed amebae at the high concentration (1000:1) (Fig. 2D).
Figure 4. In vitro amebicidal activity of immune CD8 and CD4 T cells.
LecA/alum-immunized and PBS/alum sham-immunized CBA mice were sacrificed 4 weeks after the final boost. Splenocytes were isolated from vaccinated mice (close circles) or PBS controls (open circles) and enriched for CD8+ (A) and CD4+ (B) T cells. T cells were co-cultured with HM1 E. histolytica trophozoites at various ratios indicated in the×axis. Viability of HM1 co-cultured with (C) CD8+ T cells and (D) CD4+ T cells was determined by trypan blue exclusion. * p <0.05, *** p <0.001.
4. Discussion
Studies in animal models [3–5] and during human infection [6] have established that ameba-specific IFN- γ production is critically involved in the clearance of infection and host protection. Therefore it is commonly assumed that amebiasis vaccines require Th1 responses, as indicated by our earlier findings that the frequency of IFN- γ -producing CD4+ cells in blood predicts protection [3]. In this study we showed that CD4+ T cells appeared to be the major source of IFN- γ at least in the spleen, therefore it is not surprising that CD4+ T cells are sufficient to transfer protection, which presumably is dependent on the protective effects of IFN- γ [7–9].
However, it is somehow unexpected that vaccinated CD8+ T cells could also protect. CD8+ cells are mainly perceived as mediators of immune responses against intracellular pathogens. However, the phenomenon of cross-priming [10–12] and previous work describing an antigen-non-specific stimulation of CD8 T cells by cytokines such as IL-2, IL-15 or IL-21 [13] provided tentative explanations for how CD8 T cells might be activated by E. histolytica antigens. In fact a previous study showed that PHA-stimulated CD8 T cells had direct amebicidal activity in vitro [14], therefore it would be of interest to discern whether the CD8+ cells killed amebae via direct cytotoxicity or through activating accessory cells (eg. neutrophils and macrophages). In this study we showed that purified immune CD8+ and CD4+ T cells both had amebicidal activity at high concentrations (1000:1), but CD8+ T cells appeared more potent, capable of killing amebae at a 100:1 ratio. Additional studies are required to reveal whether accessory cells are required for amebicidal activity of CD8+ and CD4+ T cells as the small portion of contaminated CD4− CD8− cells might be a confounder. However we think the latter is unlikely, since other cells such as macrophages or neutrophils, which can be cytotoxic for ameba require significant 100:1 excess which would not be present at our assay conditions [15;16].
While the Th1 response has been well-known for mediating resistance to amebiasis [4;6;17], limited information is available for the role of IL-17 during amebic infection. We have observed increased IL-17 response in both naturally infected CBA mice [4] and lectin-based vaccine immunized mice [3]. In the present study, we found that neutralization of IL-17 abrogated LecA/alum-elicited protection, indicating a protective role of this cytokine in vaccine immunity to amebiasis. Th17 cells are known to play important roles in mucosal defense against infection with bacterial pathogens like Mycobacterium tuberculosis [18], Listeria monocytogenes [19], Helicobacter pylori [20] and Salmonella enterica [21] etc. The mechanism of IL-17-mediated protection involves neutrophil recruiting to infected sites and regulation of DC function and Th1 responses through cytokines and chemokines induced by IL-17 [22;23]. In a pneumococcal vaccine study IL-17 was protective, potentially through a neutrophil-dependent mechanism [24]. In a Mycobacterium tuberculosis vaccine mycobacterial antigen-specific Th17 responses enhance protective Th1 type immunity through induction of chemokines that recruit mycobacterial antigen-specific Th1 T cells into the infected lung and inhibit bacterial growth [18]. Therefore, vaccination that induces IL-17 could represent a new strategy to prevent E. histolytica infection through inducing protective Th1 response. Interestingly we also observed a marked decrease in IL-17 production upon IFN-γ depletion in vaccinated mice (data not shown), suggesting an interplay between Th1 and Th17 cells, which has been reported by several other groups [22;25;26]. These results suggest that IL-17 may play cooperative roles with IFN- γ in vaccine-primed protective immunity against E. histolytica, which provides a tentative explanation for why neutralization of either IFN-γ or IL-17 is sufficient to abrogate LecA-conferred protection.
In LecA/alum-immunized mice both CD4+ and CD8+ cells were able to produce IFN- γ and IL-17, but CD8+ T cells produced a higher level of IL-17 than CD4+ T cells, whereas CD4+ cells mainly made IFN- γ. It has been demonstrated that CD8+ T cells are able to produce IL-17 that contributes to tissue inflammation [27;28]. We speculate that IL-17-producing cells are primed elsewhere but produce IL-17 only when located in an active inflammation site containing relevant inducer cytokines.
In conclusion, we demonstrated that protection by Gal/GalNAc lectin-derived vaccines was transferable to naïve animals by transfer of immune T cells (CD4, CD8), and that IFN-γ and IL-17 were both essential for protection. IL-17 may play cooperative roles with IFN- γ in vaccine-primed protective immunity. Therefore, selection of protective regimens that stimulate CD4+ Th1 cells or IL-17-producing CD8+ cells for a subunit vaccine might be central to the current vaccine design effort.
Acknowledgements
This research was supported by U.S. National Institutes of Health grants U01 AI070384 (to W.A.P.) and R01 AI071373 (to E.R.H.).
We thank members of the Advisory Committee for grant U01 (Alan Shaw, Brian Kelsall, Myron Levine, Steven Reed, and Annie Mo) for valuable input. We thank Stephen Becker, Suzanne Stroup and Bradley Podd for assistance in mouse surgery and ameba trophozoite culture and Caitlin Foley (TechLab) for IMAC purification and evaluation of LecA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Haque R, Mondal D, Kirkpatrick BD, et al. Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003 Oct;69(4):398–405. [PubMed] [Google Scholar]
- 2.Houpt E, Barroso L, Lockhart L, et al. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004 Jan 26;22(5–6):611–617. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Barroso L, Becker SM, et al. Protection against intestinal amebiasis by a recombinant vaccine is transferable by T cells and mediated by gamma interferon. Infect Immun. 2009 Sep;77(9):3909–3918. doi: 10.1128/IAI.00487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Stroup SE, Houpt ER. Persistence of Entamoeba histolytica infection in CBA mice owes to intestinal IL-4 production and inhibition of protective IFN-gamma. Mucosal Immunol. 2008 Mar;1(2):139–146. doi: 10.1038/mi.2007.18. [DOI] [PubMed] [Google Scholar]
- 5.Seydel KB, Smith SJ, Stanley SL., Jr. Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect Immun. 2000 Jan;68(1):400–402. doi: 10.1128/iai.68.1.400-402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haque R, Mondal D, Shu J, et al. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007 Feb;76(2):340–344. [PubMed] [Google Scholar]
- 7.Ghadirian E, Denis M. Entamoeba histolytica extract and interferon-gamma activation of macrophage-mediated amoebicidal function. Immunobiology. 1992 Jun;185(1):1–10. doi: 10.1016/S0171-2985(11)80312-8. [DOI] [PubMed] [Google Scholar]
- 8.Ghadirian E, Denis M. In vivo activation of macrophages by IFN-gamma to kill Entamoeba histolytica trophozoites in vitro. Parasite Immunol. 1992 Jul;14(4):397–404. doi: 10.1111/j.1365-3024.1992.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 9.Denis M, Ghadirian E. Activated mouse macrophages kill Entamoeba histolytica trophozoites by releasing reactive nitrogen intermediates. Microb Pathog. 1992 Mar;12(3):193–198. doi: 10.1016/0882-4010(92)90053-q. [DOI] [PubMed] [Google Scholar]
- 10.Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995 Sep 1;182(3):639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995 Jan 13;267(5195):243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 12.Pedras-Vasconcelos JA, Pearce EJ. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J Immunol. 1996 Oct 1;157(7):3046–3053. [PubMed] [Google Scholar]
- 13.Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp (Warsz) 2008 Sep;56(5):311–323. doi: 10.1007/s00005-008-0033-2. [DOI] [PubMed] [Google Scholar]
- 14.Salata RA, Cox JG, Ravdin JI. The interaction of human T-lymphocytes and Entamoeba histolytica: killing of virulent amoebae by lectin-dependent lymphocytes. Parasite Immunol. 1987 Mar;9(2):249–261. doi: 10.1111/j.1365-3024.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Seguin R, Mann BJ, Keller K, Chadee K. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect Immun. 1997 Jul;65(7):2522–2527. doi: 10.1128/iai.65.7.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis M, Chadee K. Human neutrophils activated by interferon-gamma and tumour necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989 Sep;46(3):270–274. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- 17.Lin JY, Seguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994 May;62(5):1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007 Apr;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto M, Emoto M, Emoto Y, et al. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J Immunol. 2003 May 15;170(10):5228–5234. doi: 10.4049/jimmunol.170.10.5228. [DOI] [PubMed] [Google Scholar]
- 20.Vorobjova T, Watanabe T, Chiba T. Helicobacter pylori immunology and vaccines. Helicobacter. 2008 Oct;13 Suppl 1:18–22. doi: 10.1111/j.1523-5378.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008 Sep;20(9):1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 22.Bai H, Cheng J, Gao X, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009 Nov 1;183(9):5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Gao L, Lei L, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009 Jul 15;183(2):1291–300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Ritchea S, Logar A, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009 Nov 20;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Liu XF, Zhuang Y, et al. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol. 2010 May 1;184(9):5121–5129. doi: 10.4049/jimmunol.0901115. [DOI] [PubMed] [Google Scholar]
- 27.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006 Nov 15;177(10):6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega C, Fernandez A, Carrillo JM, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009 Aug;86(2):435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]