Abstract
Rapidly proliferating epithelial crypt cells of the small intestine are susceptible to radiation-induced oxidative stress, yet there is a dearth of data linking this stress to expression of antioxidant enzymes and to alterations of intestinal nutrient absorption. We previously showed that 5 – 14 d after acute γ-irradiation, intestinal sugar absorption decreased without change in antioxidant enzyme expression. In the present study, we measured antioxidant mRNA and protein expression in mouse intestines taken at early times postirradiation. Observed changes in antioxidant expression are characterized by a rapid decrease within 1 h postirradiation, followed by dramatic upregulation within 4 h, and then downregulation a few days later. The cell type and location expressing the greatest changes in levels of the oxidative stress marker 4HNE and in antioxidant enzymes are, respectively, epithelial cells responsible for nutrient absorption and the crypt region comprised mainly of undifferentiated cells. Consumption of a cocktail of antioxidant vitamins A, C and E, before irradiation, prevents reductions in transport of intestinal sugars, amino acids, bile acids and peptides. Ingestion of antioxidants may blunt radiation-induced decreases in nutrient transport, perhaps by reducing acute oxidative stress in crypt cells, thereby allowing the small intestine to retain its absorptive function when those cells migrate to the villus days after the insult.
Keywords: SOD, catalase, glutathione peroxidase, oxidation, enzyme, sugars
INTRODUCTION
The gastrointestinal (GI) tract is the second most radiosensitive critical organ system in the body, preceded only by the hematopoietic system. The continuously regenerating epithelial cells in the mucosal lining of the small intestinal lumen are readily perturbed by radiation [1, 2]. These cells are located mainly in the crypt and crypt/villus junction of the intestinal mucosa. It is well established that γ-rays generate free radicals and reactive oxygen species (e.g. H2O+, e+, H3O+, OH−) through the radiolysis of water, the major constituent of tissue, thereby indirectly damaging DNA and other biomolecules.
The small intestine has four tissue types: epithelial, muscle, nervous and connective tissue. Epithelial cells comprise the mucosal layer that lines the luminal surface. High levels of radiation exposure can cause intestinal epithelial denudation, and even higher doses can result in little or no subsequent regeneration, thereby affecting water, electrolyte and nutrient uptake. Because the epithelial cells in the mucosal layer are responsible for digestive, absorptive, and barrier functions, acute radiation injury in humans contributes significantly to sugar malabsorption and diarrhea [3]. Secondary changes include infection, ulceration and perforation. Early toxicity is mainly due to dysfunction of the mucosal epithelia [4]. Delayed or chronic radiation-induced toxicity typically involves all other tissue types and is a progressive condition with few therapeutic options and with substantial long-term morbidity and mortality. Severe delayed radiation enteritis is also serious, and about 10% die as a direct result of radiation enteropathy. It is characterized by malabsorption, dysmotility and transit abnormalities, and features mucosal atrophy, intestinal fibrosis and vascular sclerosis [4]. cDNA microarray analysis of the human ileum from patients with radiation enteritis revealed that genes involved in collagen synthesis, matrix metalloproteinase genes, and metalloproteinase inhibitor genes changed in expression, indicating morphological [5] changes being retained even several months after an acute 10 Gy insult. Early and delayed radiation enteritis were initially thought to be unrelated, but it is now known that the severity of early events may determine the subsequent incidence and severity of chronic enteritis [6].
We recently studied intestinal sugar transport prior to overt radiation-induced damage to the intestinal epithelium [7]. Here, the effect of ionizing radiation on nutrient absorption was delayed as demonstrated by reductions in mouse intestinal glucose and fructose transport about 5 – 14 d after acute whole-body γ-irradiation with 7 – 10 Gy. Transporter mRNA levels decreased in a dose-dependent manner, paralleling reductions in nutrient transport. While the morphological and functional effects of radiation are well known, the response of the endogenous antioxidant network in the small intestine is poorly characterized and has not been correlated to physiological responses. Our previous attempts could not detect radiation-induced changes in antioxidant enzyme expression at the same time that sugar absorption rates decreased [7].
In the present study, we tested the general hypothesis that changes in expression of antioxidant enzymes occurs prior to overt changes in mucosal morphology and function. This was achieved by testing a variety of interrelated hypotheses: i) radiation alters the expression of antioxidant enzymes within hours postirradiation, even though overt symptoms of malabsorption occur many days later. ii) changes in antioxidant expression should be greater in the crypt region where most differentiating intestinal cells are located. iii) radiation-induced changes in levels of 4-hydroxy-nonenal (4HNE, a biomarker of oxidative stress [8]) are greater in the intestinal epithelia than in other tissues of the small intestine, and they occur at similar crypt-villus locations as adaptive increases in antioxidant enzymes. iv) consumption of a cocktail of antioxidant vitamins A, C, and E should protect the small intestine from the harmful effects of irradiation. A cocktail of these vitamins was chosen based on their previously demonstrated radioprotective capacity. Studies in our laboratory showed that vitamin A substantially mitigates the deleterious effects of chronic exposure to both low- and high-LET radiations in mouse testes [9]. Vitamin C mitigates radiation-induced damage even when administered after irradiation [10] and patients have benefited from taking vitamin C after abdominal radiotherapy [11]. Vitamin E has been reported to maintain jejunal, ileal, and colonic fluid absorption in irradiated rats [12]. Small intestinal crypt cell numbers, mucosal height, and goblet cell numbers were significantly protected from radiation effects by dietary vitamin E [13]. Cocktails of radioprotectors offer a means to further improve radioprotection. In fact, a combination of vitamins A, C and E reduced bone marrow toxicity caused by radioimmunotherapy with 131I labeled antibodies [14]. Vitamin C and E supplements reduced bleeding and diarrhea in patients with chronic radiation proctitis caused by pelvic irradiation [15]. Hence, vitamins A, C and E have already been shown to protect the small intestine from radiation and therefore are excellent candidates for protecting against radiation-induced reductions in intestinal nutrient transport.
MATERIALS AND METHODS
Radiochemicals
D-glucose, 3-O-methyl-D-glucose, L-carnosine and taurocholic acid were purchased from Sigma (St Louis, MO), D-fructose from Mallinckrodt (St Louis, MO) and L-proline from Eastman (Kingsport, TN). D-[14C]glucose (31 MBq/mL) and L-[3H]glucose (37 MBq/mL) were purchased from Sigma, D-[14C]fructose (19 MBq/mL), [3H]proline (37 MBq/mL) and [3H]taurocholic acid (37 MBq/mL) from ARC (St Louis, MO), [3H]carnosine (37 MBq/mL) from Moravek Biochemicals (Brea, CA), and [14C]3-O-methyl-D-glucose (3.7 MBq/mL) and inulin [carboxyl-14C] (37 MBq/mL) from Perkin-Elmer (Waltham, MA).
Animals and irradiation
Swiss Webster adult male mice (average weight: 37 g), 7–8 weeks of age, were purchased from Taconic Farms, Germantown, NY. The experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee (IACUC), University of Medicine and Dentistry of New Jersey, New Jersey Medical School, (Newark, NJ). Mice were housed, one per cage, in a sterilized, filtered, positive air-flow cage with feed and water provided ad libitum. Pelleted AIN-76A rodent diet (Research Diets, New Brunswick, NJ) was used for the control diet (Table 1). Cages were maintained in a climate controlled room with 12 h light/dark cycles in our Cancer Center Comparative Medicine Resource Center. As detailed in our earlier study [7], whole-body radiation absorbed doses were delivered acutely with a Mark I 137Cs-irradiator (661 keV gamma rays) operating at a calibrated dose rate of 1.14 Gy/min (JL Shepherd, San Fernando, CA). Sham irradiated mice (0 Gy) were placed in the irradiator for the time equivalent to the maximum 137Cs source exposure period without activating the source. All irradiations were performed at room temperature.
Table 1.
Compositions of control diet and vitamin A, C and E supplemented diet.
| Control diet | A C E diet | |||
|---|---|---|---|---|
| Composition | % (g) | % (kcal) | % (g) | % (kcal) |
| Protein | 20 | 21 | 19 | 21 |
| Carbohydrates | 66 | 68 | 63 | 68 |
| Fat | 5 | 12 | 5 | 12 |
| AIN-76A Ingredients | g | kcal | g | kcal |
| Casein, 80 mesh | 200 | 800 | 200 | 800 |
| DL-methionine | 3 | 12 | 3 | 12 |
| Corn starch | 150 | 600 | 150 | 600 |
| Sucrose | 500 | 2000 | 500 | 2000 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Corn oil | 50 | 450 | 50 | 450 |
| Mineral mix, S10001 | 35 | 0 | 35 | 0 |
| Vitamin mix, V10001 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| Supplements | ||||
| Vitamin A acetate, 500,000 IU/g | 0 | 0 | 0.8 | 0 |
| Vitamin E acetate, 500 IU/g | 0 | 0 | 25 | 0 |
| Ascorbic acid phosphate (35 % active) | 0 | 0 | 28.6 | 0 |
| Total | 1000 | 3902 | 1054.4 | 3902 |
Oxidative stress in irradiated animals
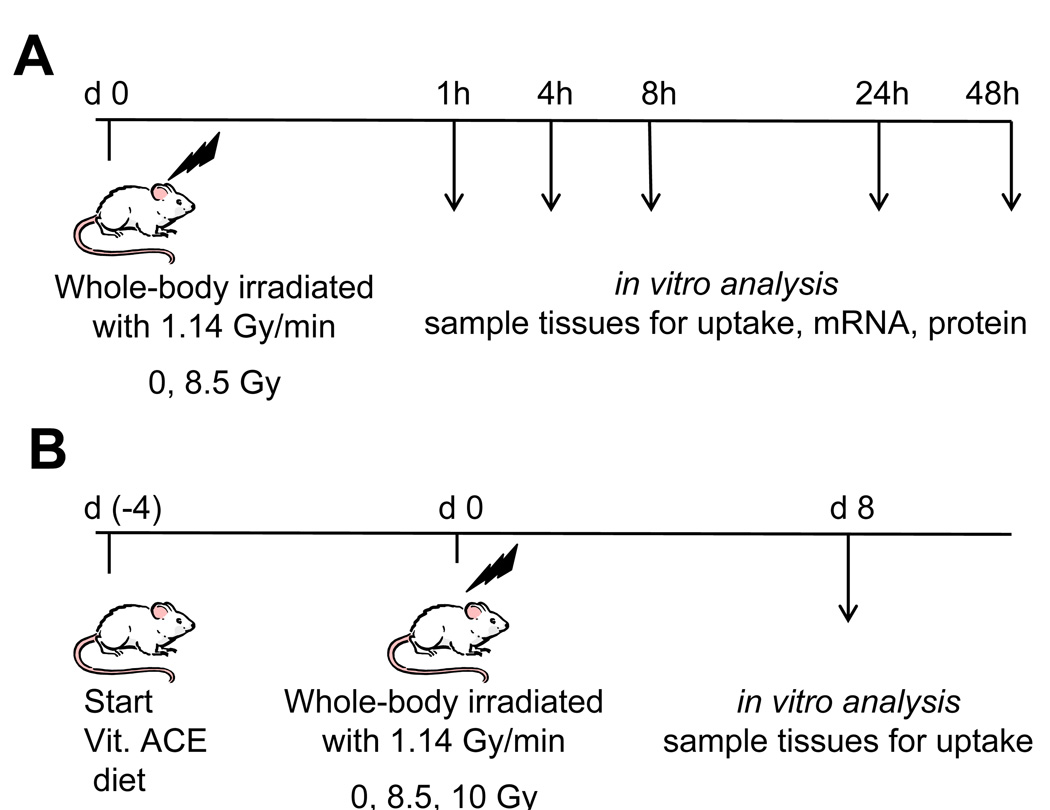
The study was divided into 4 trials done in series, with a total of 10–20 mice per trial (Fig. 1A). Mice were fed control diet throughout the oxidative stress study. Whole-body doses of 8.5 Gy were delivered acutely and mice were randomly assigned to one of 5 groups. Groups were sacrificed at 1, 4, 8, 24, and 48 h postirradiation. Data from all trials were combined.
Fig. 1.
Design of experiments wherein mice were acutely irradiated with low-LET γ-rays and sacrificed at different times postirradiation. (A) Mice were fed control diet, irradiated, and sacrificed 1 – 48 h postirradiation. (B) Mice were fed control diet or diet supplemented with vitamin A, C, and E. Arrows refer to times postirradiation when mice were sacrificed. Day 8 was chosen for B based on a previous experiment tracking the time course of radiation-induced reductions in nutrient transport.
Protection against radiation-induced reductions in nutrient transport with a cocktail of vitamins A, C, and E
The study was divided into 6 separate, sequential but identical trials with 6 mice per trial (Fig. 1B). Data from all trials were combined. Mice were maintained as described above. A 2×3 factorial experimental design was followed with two diets and three radiation absorbed doses (0, 8.5 and 10 Gy) for a total of six groups. Group mean body weights were 34.4 – 37.7 g (n = 6 per group). Mice were fed isocaloric diets, either AIN-76A or a modified AIN-76A rodent diet supplemented with 400 IU/g retinyl acetate (100× control), 28.6 mg/g ascorbic acid phosphate (35% active), and 12.5 IU/g alpha-tocopherol acetate (250× control) (Research Diets) (Table 1). See discussion for rationale of dietary vitamin concentrations. This diet is referred to as the vitamin ACE enriched diet.
All mice were acclimated for 10 d to the laboratory environment and the control rodent diet. Following the ten day acclimation period, the vitamin ACE enriched diet was substituted for the control diet for mice in the vitamin ACE group. This change provided a 4 d pre-irradiation ingestion of the vitamin-supplemented diet. Acute whole body irradiation was carried out at absorbed doses of 8.5 or 10 Gy. Sham-irradiated mice (0 Gy) were placed in the irradiator, without activating the radiation source, for a duration equivalent to the time required to deliver the highest dose. Mouse weight and diet consumption were monitored prior to and after irradiation. The vitamin ACE enriched diet was continued postirradiation until the animal was sacrificed.
Animal surgery
Mice were anesthetized with a cocktail containing 0.97% ketamine, 0.097% xylazine, 0.02% acepromazine in sterile water (5 mL/kg body weight). The small intestine was surgically removed 1, 4, 8, 24 or 48 h or 8 d postirradiation. The intestinal lumen was subsequently rinsed with an ice-cold Krebs Ringer bicarbonate solution (KRb1: 128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2·5H2O, 1.2 mM MgSO4, 19 mM NaHCO3, and 1.2 mM KH2PO4, pH 7.3–7.4), and immediately processed for determinations of nutrient uptake, and freezing for mRNA analyses, protein analyses, and sectioning for immunohistochemistry staining. After excision of the small intestine, the mouse was killed by intracardiac injection of 0.2 mL Euthasol® (Virbac, Fort Worth, TX).
Intestinal uptakes
Uptake rates were determined in excised intestine as described earlier [7, 16]. Briefly, several 1-cm segments of the duodenum and jejunum were individually mounted and everted on grooved steel rods (3-mm diameter). Since active transport of bile acids occurs only in the distal ileum, only this region was used for taurocholate uptake. Rods with the everted intestinal segment were preincubated at 37°C for 5 min in a KRb1 buffer, and bubbled with 95% O2 - 5% CO2.
For glucose uptake, the tissue was incubated at 37°C for 1 min in a freshly made oxygenated solution of KRb2 buffer (KRb1 buffer with 103 mM NaCl) containing 50 mM D-glucose, 380 µM D-[14C]glucose and 0.2 µM L-[3H]glucose. Similarly, fructose uptake was measured after 2 min incubation in 50 mM D-fructose, 1.6 µM D-[14C]fructose and 0.2 µM L-[3H]glucose. Uptake of the non-metabolizable glucose analog, 3-O-methyl-D-glucose, was measured after 1 min incubation in 25 mM 3-O-methyl-D-glucose, 1.2 µM D-[14C]-3-O-methyl-glucose, and 0.2 µM L-[3H]glucose. L-[3H]glucose was used to correct for adherent fluid and passive diffusion of glucose, fructose and methyl-glucose. Finally, all sleeves were rinsed for 20 s in ice-cold KRb1 buffer with stirring.
For L-proline uptake, the segment was incubated in 50 mM L-proline, 0.2 µM L-[3H]proline, and 20 µM inulin [carboxyl-14C]; L-carnosine in 25 mM L-carnosine, 0.9 µM L-[3H]carnosine and 20 µM inulin [carboxyl-14C]; and taurocholic acid in 0.6 mM taurocholic acid, 0.07 µM [3H]taurocholic acid, and 20 µM inulin [carboxyl-14C]. In all cases, inulin [carboxyl-14C] was used to correct for adherent fluid.
Each 1 cm piece of intestine was transferred to a pre-weighed glass vial, weighed, and dissolved in 1 mL of Solvable® (Perkin Elmer, Boston, MA) at 37°C overnight. Following tissue digestion, a 10 mL volume of Ecolume® (MP, Irvine, CA) was added to each vial, vortexed and 3H and 14C activity determined by dual-label counting with a manually calibrated LS 6500 Multi-purpose Scintillation Counter (Beckman Coulter, Fullerton, CA). Quench curves were created using the LS 6500 software and counting samples with a range of tissue weights, each spiked with the same amount of 3H alone, 14C alone, or 3H and 14C. Uptakes of nutrients are expressed as nmoles per min per cm or per mg of wet weight of intestine.
In the vitamin ACE experiment, glucose uptake was determined in the duodenum, jejunum and ileum of each intestine, in order to estimate total intestinal glucose uptake. Uptake of all other nutrients was determined in the jejunum. Since almost all of the intestine was used for uptakes, levels of antioxidant expression could not be determined in the vitamin ACE experiment.
mRNA analysis
In the oxidative stress experiment, a 2 cm piece of jejunum was scraped to remove the mucosa which was then immediately frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted with Trizol® (Invitrogen, Carlsbad, CA) and subjected to reverse transcriptase PCR using an iCycler® thermal cycler (Biorad, Hercules, CA) and a SuperScript III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The cDNA obtained was subjected to real-time PCR for the SOD1, SOD2, catalase, glutathione peroxidase 1 and β-actin genes using the Mx3000P® QPCR System (Stratagene, La Jolla, CA). The real-time PCR was performed with Brilliant® SYBR® Green QPCR Master Mix Reagent (Stratagene, La Jolla, CA). Earlier work showed β-actin expression to remain unaltered within the range of radiation doses used in this study, thereby serving as our housekeeping gene [7]. Thermal cycling proceeded with 40 cycles (initial activation step: 10 min at 95°C, 40 cycles: 30 s at 95°C, 1 min annealing temperature of primers, 30 s at 72°C and terminal step: 1 min at 95°C and 30 s at 55°C). The amount of RNA was calculated with relative standard curves for RNA of the specific genes studied. Normalization to β-actin was conducted to account for variability in concentration of total RNA. To examine for genomic contamination, the reaction was also carried out in samples that did not receive reverse transcriptase amplification reagents. A reverse transcriptase PCR was then carried out on total RNA, followed by three determinations by real-time PCR with the following primer sequences: (1) SOD1 (forward 5′-CAA GCG GTG AAC CAG TTG TGT TGT-3′, reverse 5′-TCA CAT TGC CCA GGT CTC GGT CTC CAA CAT-3′); SOD2 (forward 5′-TGG AGA ACC CAA AGG AGA GTT GCT-3′, reverse 5′-TGT TGT TCC TTG CAA TGG GTC CTG-3′); (2) catalase (forward 5′-AGA GGA AAC GCC TGT GTG AGA ACA-3′, reverse 5′-AGT CAG GGT GGA CGT CAG TGA AAT-3′); and (3) glutathione peroxidase 1 (forward 5′-TTT CCC GTG CAA TCA GTT CGG ACA-3′, reverse 5′-AGC CTT CTC ACC ATT CAC TTC GCA-3′); β-actin (forward 5′-TTG TTA CCA ACT GGG ACG ACA TGG-3′, reverse 5′-CTG GGG TGT TGA AGG TCT CAA ACA-3′). These primers were designed to amplify only the mRNAs.
Western blot
A 2 cm piece was excised from the jejunum, scraped to separate the mucosa from muscle tissue, and the mucosa immediately frozen in liquid nitrogen and stored at −80°C. Frozen tissue (~100 mg) was homogenized in 1 ml of T-PER® Tissue Protein Extraction Reagent (Pierce, Rockford, IL) with 10 µl of cØmplete Protease Inhibitor Cocktail (Roche, Mannheim, Germany), incubated in ice (10 min) and centrifuged (20 min) at 12,000 rpm at 4°C. The supernatant was kept for protein quantification by the Bicinchoninic Acid CA™ Protein Assay Kit assay (Thermo Scientific, Rockford, IL). Samples were prepared with a Lane Marker Reducing Sample Buffer 5× (Thermo Scientific, Rockford, IL) by boiling for 7 min. Samples and ladder kaleidoscope pre-stained standards (Biorad, Hercules, CA) were analyzed using a precast 12% Tris-HCl gel. The gel proteins were then transferred to a 0.45 µm pore nitrocellulose membrane (Millipore, Billerica, MA) with a Mini Trans-Blot Cell (Biorad). The membrane was incubated in 5% non fat milk in Tris-buffered saline – Tween 20 (TBST) followed by overnight incubation with a primary antibody (4°C). After washing, the membrane was incubated for 1 h with the secondary antibody. The primary antibodies were: rabbit anti-mouse superoxide dismutase 1 (Millipore), rabbit anti-mouse superoxide dismutase 2 (Millipore), and goat anti-mouse catalase (Santa Cruz Biotechnology, Santa Cruz, CA). We were unable to find an anti-glutathione peroxidase 1 antibody that worked. The secondary antibodies were all horseradish peroxidase conjugated antibodies: donkey anti-rabbit antibodies, sheep anti-mouse antibodies (GE Healthcare, Piscataway, NJ) and rabbit anti-goat antibodies (Millipore, Billerica, MA). Horseradish peroxidase was developed using a western blotting detection kit (GE Healthcare), and autoradiography film (Denville Scientific, Metuchen, NJ).
Preparation of intestinal mucosa for immunohistochemistry staining
Intestinal tissue (0.5 cm long) was fixed in 4% buffered paraformaldehyde, embedded in paraffin, and sectioned (6 µm thick). Sections were deparafinnized, rehydrated, and epitope retrieval achieved in 10 mM citrate buffer (pH 6.0) in an autoclave. After initial incubation in 1% (for SOD1 sections) or 5% (for 4HNE sections) normal goat serum in PBS, sections were subsequently exposed to the primary antibody overnight at 4°C.
While there are no specific biomarkers for radiation-induced damage, oxidative stress arising from it has been detected by monitoring changes in levels of antioxidants, antioxidant enzymes, and biomarkers of oxidative stress like 4HNE. The latter is a toxic unsaturated aldehyde formed during lipid peroxidation that can also bind to proteins to form carbonyls [8]. Thus we tracked the location of tissues that experienced radiation-induced oxidative stress, with 4HNE.
Primary antibodies were rabbit anti-mouse SOD1 (1:1000 dilution in PBS) or rabbit anti-4HNE (1:200 dilution in PBS). Slides were rinsed with PBS and then incubated with a secondary antibody (Cy3 conjugated rabbit antigoat IgG (1:200) or Alexa 488 conjugated goat antirabbit (1:200)). Sytox Green Nucleic Acid stain (1:300 dilution with deionized water), was applied to SOD1 sections and incubated for 15 min, followed by rinsing with distilled water. Cover slips were mounted to each slide using Vectashield® mounting medium (Vector Laboratories, Burlingame, CA). Similar slides were prepared with the substitution of PBS for the primary antibody to check for nonspecific binding and extraneous excitation of the secondary antibody. Processing of tissues from different treatments was done using, where applicable, exactly the same solutions. Slides were viewed through a Zeiss LSM510 confocal microscope with argon laser (458, 488, 514 nm) and HeNe laser (543 nm) for simultaneous collection of two fluorescent signals. To facilitate comparison of images, acquisition of images was accomplished using exactly the same confocal settings (see Supplemental Fig. S3).
Statistics
Values were expressed as means ± standard error. Two-way ANOVA or General Linear Models (GLM) analysis for unbalanced data was used to determine the statistical significance. Pairwise differences were evaluated subsequent to 2-way ANOVA or GLM data by comparison of least-square means. Statistical evaluations were performed using Statview 5.0 (Abacus Concepts, Berkeley, CA) and SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Nutrient uptake at 24 and 48 h postirradiation
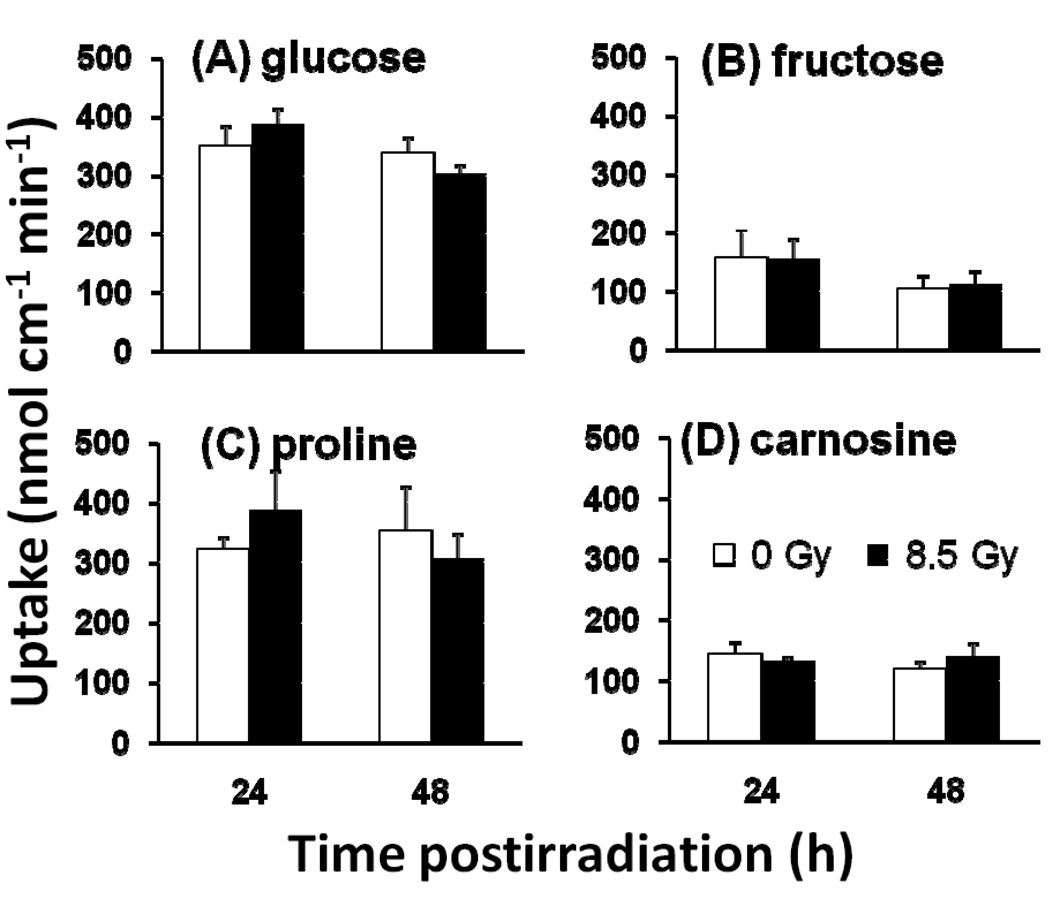
We have previously shown that radiation-induced decreases in intestinal sugar uptake and transporter mRNA levels were observed mainly 8 – 15 d after acute whole-body γ-irradiation with absorbed doses of 7 – 10 Gy [7]. We now find that there was little or no change in antioxidant mRNA expression in the intestinal mucosa during this time interval (Supplemental Fig. S1). Conversely, to demonstrate that the intestine was absorbing nutrients within 48 h post irradiation when most changes in antioxidant expression described below occurred, we show that glucose uptake did not change significantly with dose or time postirradiation (Fig. 2A). Fructose, proline, and carnosine uptakes (Fig. 2B–D) also did not change significantly with radiation dose (P > 0.80 by two-way ANOVA for all nutrients) and time postirradiation (P > 0.17 for all nutrients).
Fig. 2.
In vitro measurements of nutrient uptake rate in isolated jejunum of unirradiated (open bars) and irradiated (8.5 Gy, filled bars) mice 24 and 48 h postirradiation. (A) D-Glucose, (B) D-fructose, (C) proline, and (D) carnosine uptakes per cm. Results are means ± SE (n = 6). By two way ANOVA, there were no significant effects of radiation and of time postirradiation on all nutrient uptakes (see text for P values).
Time course of changes in mRNA expression of antioxidant enzymes
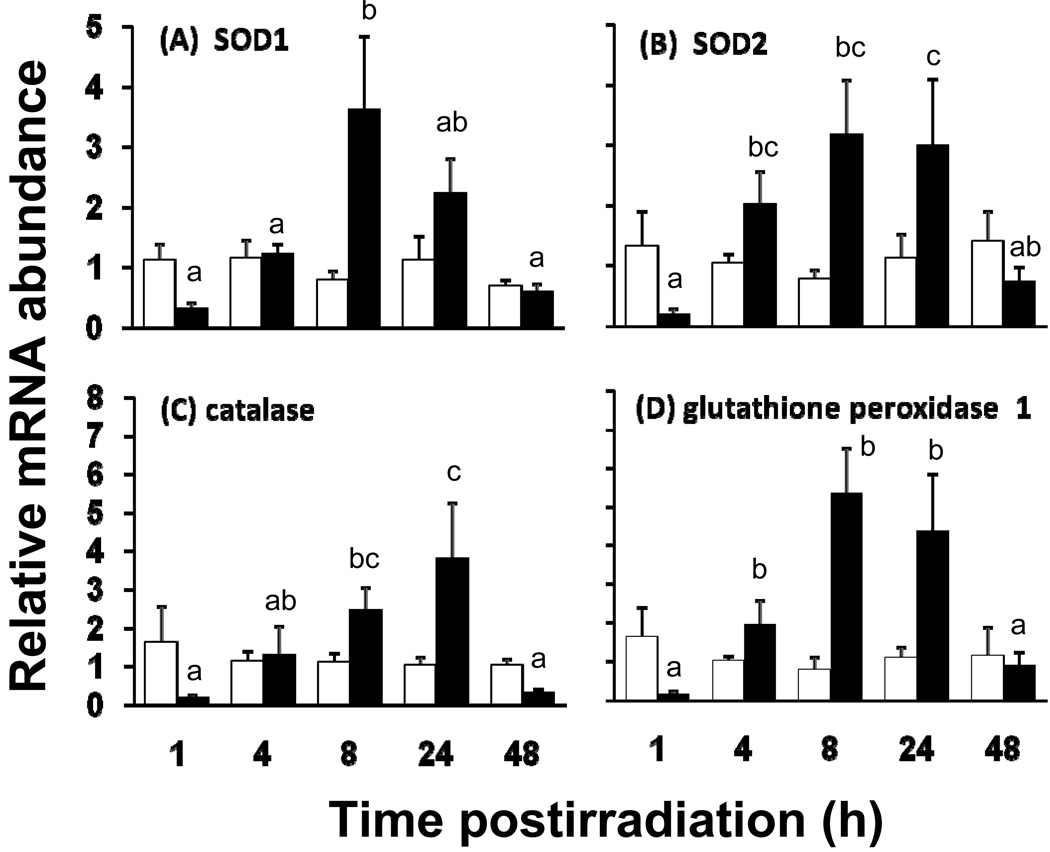
In contrast to the absence of radiation-induced reduction of nutrient transport at t<48 h, levels of the cytosolic antioxidant enzyme SOD1 mRNA varied with postirradiation time (P = 0.02 by two way ANOVA) and dose (borderline, P = 0.06), with a highly significant interaction between postirradiation time and dose (P = 0.01) (Fig. 3A). The statistically borderline dose dependence is based on the fact that mRNA abundance was not expected to and actually did not change significantly in unirradiated mice. The significant interaction indicated that the dependence of mRNA abundance on dose depended on postirradiation time (Fig 3A). For example, SOD1 expression was similar for 0 and 8.5 Gy both at 4 and 48 h postirradiation. At 8 and 24 h, mucosal SOD1 mRNA abundance was 2 – 4× greater in 8.5 Gy mice compared to the abundance in control mice sacrificed at the same time. In contrast, at 1 h postirradiation, the SOD1 mRNA abundance in unirradiated mice was several-fold greater than in irradiated mice. It should be noted that because the RNA was extracted only from the mucosa, these irradiation-induced changes reflected changes mainly in the intestinal absorptive cells.
Fig. 3.
Relative mRNA abundance of antioxidant enzymes in isolated jejunum of unirradiated (open bars) and irradiated (8.5 Gy, filled bars) mice. (A) SOD1, (B) SOD2, (C) catalase, and (D) glutathione peroxidase 1 as measured by real time PCR. mRNA was collected from the mucosa of the proximal small intestine of mice fed with control diet and sacrificed 1, 4, 8, 24 and 48 h post-irradiation. Results are means ± SE (n = 6). Filled bars with different superscripts are significantly different (P < 0.05 by one-way ANOVA). Thus, there is a marked decrease in levels of mRNA at 1irradiation, followed by a dramatic increase, and then a decline to levels similar to unirradiated mice. Open bars are similar throughout, indicating that expression in unirradiated mice did not change over time.
Patterns of expression were similar for the other antioxidant enzymes. For mRNA levels of mitochondrial SOD2, their dependence on postirradiation time (P = 0.11) and dose (P = 0.06) were borderline but the interaction was quite robust (P = 0.01) (Fig. 3B). For SOD2, differences were observed mainly at 4, 8 and 24 h when expression increased by 2 – 3× in irradiated mice. The mRNA abundance of catalase varied with postirradiation time (P = 0.03) but not dose (P = 0.22), and the interaction of these two variables was highly significant (P = 0.007) (Fig. 3C). Catalase mRNA levels were greatest at 24 h postirradiation, and decreased markedly by 48 h to levels observed at 1 h postirradiation. For glutathione peroxidase 1 (Fig. 3D), mRNA levels depended significantly on postirradiation time (P = 0.007), dose (P = 0.002), and there was an interaction between dose and postirradiation time (P = 0.0005). Glutathione peroxidase 1 mRNA abundance peaked at 8 h to levels 6× greater than control, and then decreased gradually.
It is important to point out that mRNA levels of all antioxidant enzymes, in unirradiated mice, did not vary as a function of time (P > 0.50 by one way ANOVA). This strongly supports consistency in sampling and analysis. Moreover, the levels of mRNA for all antioxidant enzymes in irradiated mice were always much less (3 – 8× decrease, P < 0.02) than in controls at 1 h. This indicates that the effect of acute whole-body irradiation on antioxidant enzyme expression was triphasic: i) rapid decrease of steady state levels of mRNA occurring in minutes, ii) dramatic upregulation occurring within hours, and iii) another downregulation occurring within a day or two after irradiation. Expression of antioxidant mRNA in irradiated mice was 10 – 20× less at 1 h compared to that at peak expression occurring 7 – 23 h later. Finally, like the findings at 48 h, it was determined that at 8 d postirradiation, mRNA expression levels of SOD1, SOD2, catalase, and glutathione peroxidase 1 were similar in irradiated and unirradiated mice (Supplemental Fig. S1).
Abundance of antioxidant enzymes following acute irradiation
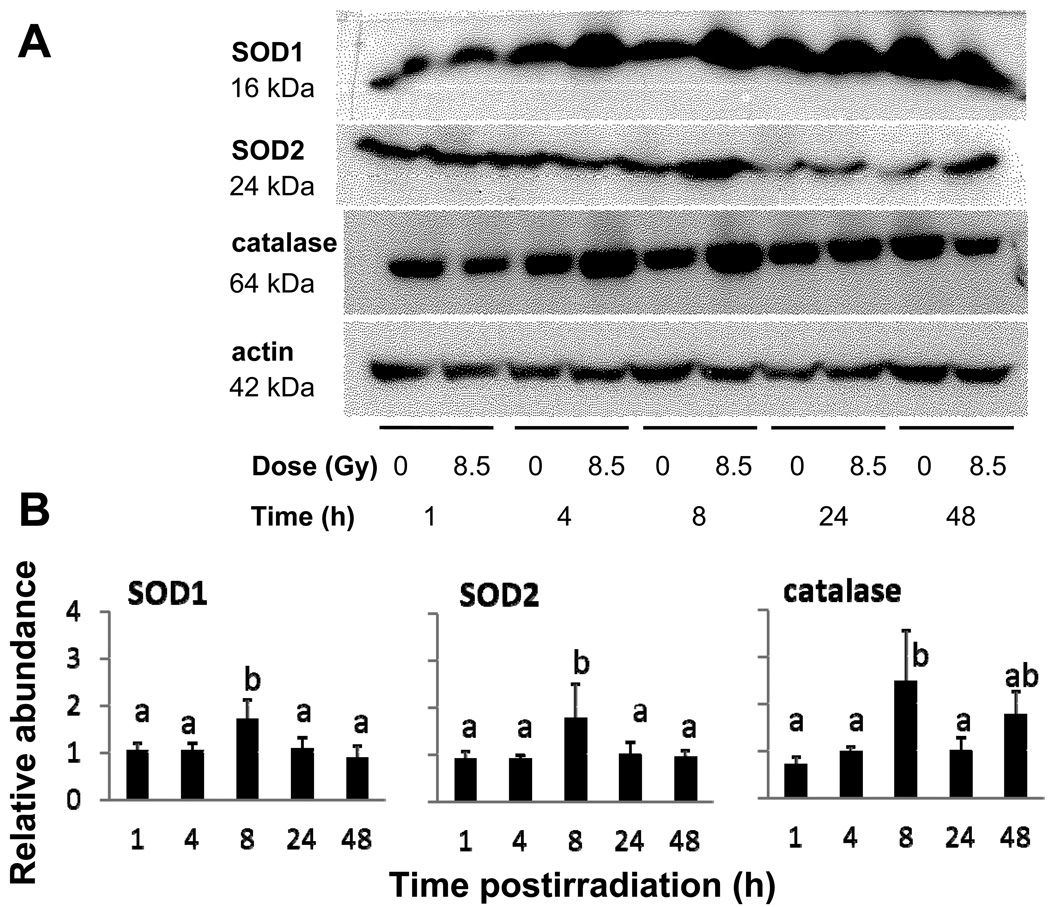
The pattern of radiation-induced changes in protein abundance of antioxidant enzymes was less pronounced but generally followed that for mRNA abundance (Fig. 4a). Like their mRNA abundance, intestinal SOD1, SOD2 and catalase protein levels in irradiated mice tended to peak 8 – 24 h postirradiation (P < 0.10 by one way ANOVA), but generally by only about two-fold when compared to unirradiated mice (Fig. 4b). Since protein homogenates were derived from mucosal scrapes, these changes reflect alterations in levels of antioxidant enzymes in the absorptive cells.
Fig. 4.
(A) Effect of γ-irradiation on protein abundance of the antioxidant enzymes SOD1, SOD2, and catalase as determined by western blotting of mucosal homogenates. Homogenates were obtained from the jejunum of mice fed with control diet and sacrificed 1, 4, 8, 24 and 48 h postirradiation. (B) Mean relative antioxidant enzyme protein levels based on densitometry scans of western blots. Results are the ratio of the densities corresponding to 8.5Gy and 0Gy from homogenates obtained from the same trial. Results are means ± SE (n = 5 for SOD1 n = 5 for SOD2, and n = 4 for catalase). Bars with different superscripts are significantly different (P < 0.05 by one-way ANOVA).
Levels and immunolocalization of 4HNE and SOD1
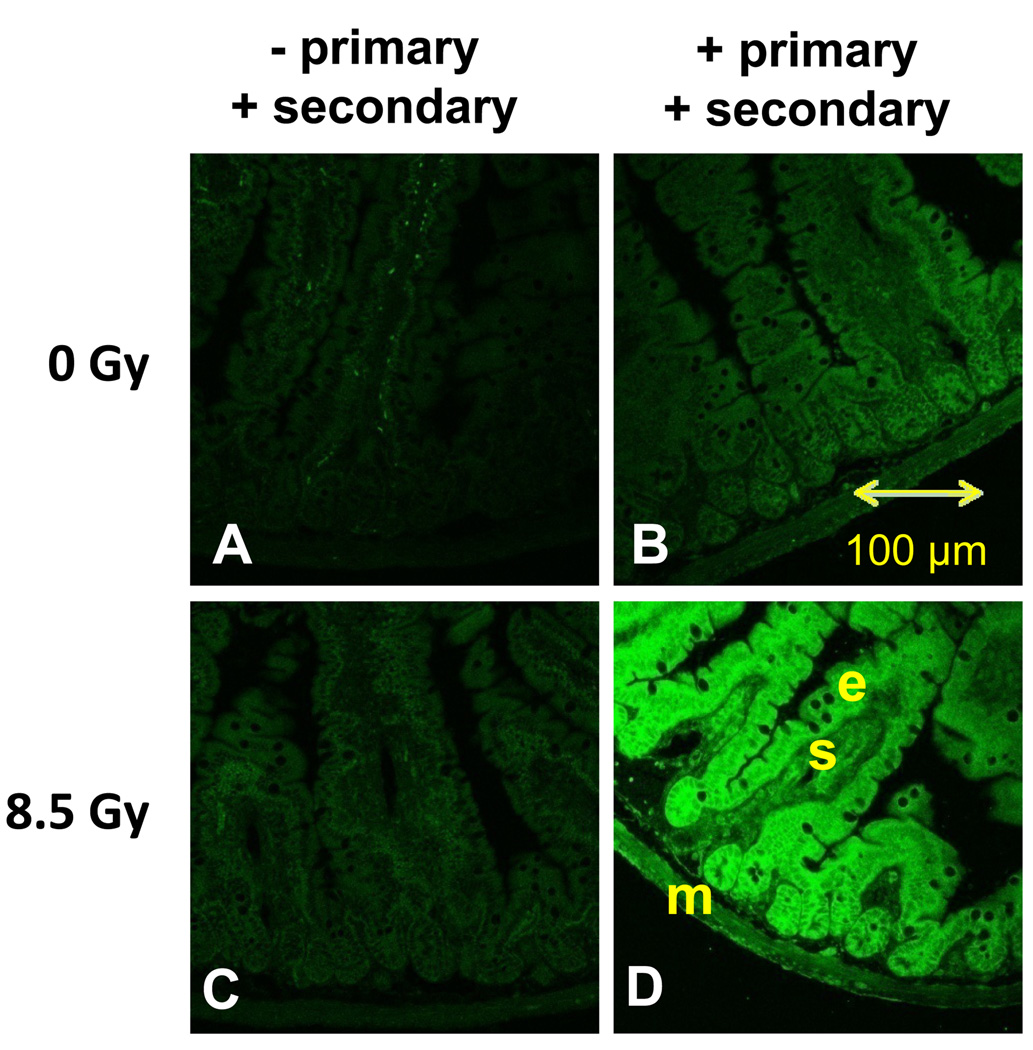
Levels of 4HNE were apparently greater in the small intestine of mice 8 h after acute whole-body irradiation with 8.5 Gy (Fig. 5). The 8 h time-point was chosen because the greatest antioxidant enzyme response was observed at this time postirradiation. In unirradiated mice, there was little fluorescence evident in the absence of primary antibody (Fig. 5A), and there was little evidence of endogenous 4HNE when probed with anti 4HNE (Fig. 5B). Absence of primary antibody also produced little fluorescence in the intestine of irradiated mice (Fig. 5C). Levels of 4HNE were much greater in epithelial cells (e) lining the intestinal lumen as opposed to the submucosa (s) and muscle (m) layers underneath (Fig. 5D). Even when irradiated, there was negligible fluorescence with no antibodies or with only the primary antibody thereby suggesting no endogenous fluorescence (Supplemental Fig. S2 A, C). Different sets of unirradiated and irradiated mice showed similar results (Supplemental Fig. S2 B, D).
Fig. 5.
Immunolocalization of the oxidative stress biomarker 4HNE in the small intestine of mice 8 h after acute whole-body irradiation with 8.5 Gy. (A) Intestinal section from unirradiated mice probed with a secondary (Alexa 488 goat antirabbit) antibody but no primary (rabbit anti-4HNE) antibody. (B) Section from unirradiated mice probed with both primary and secondary antibodies. (C) Section from irradiated mice probed with a secondary antibody only. (D) High levels of 4HNE were found in the intestine of irradiated mice probed with primary and secondary antibodies. Epithelial cells (e) lining the mucosa seemed to have greater amounts of 4HNE than the submucosal (s) or muscle (m) layers.
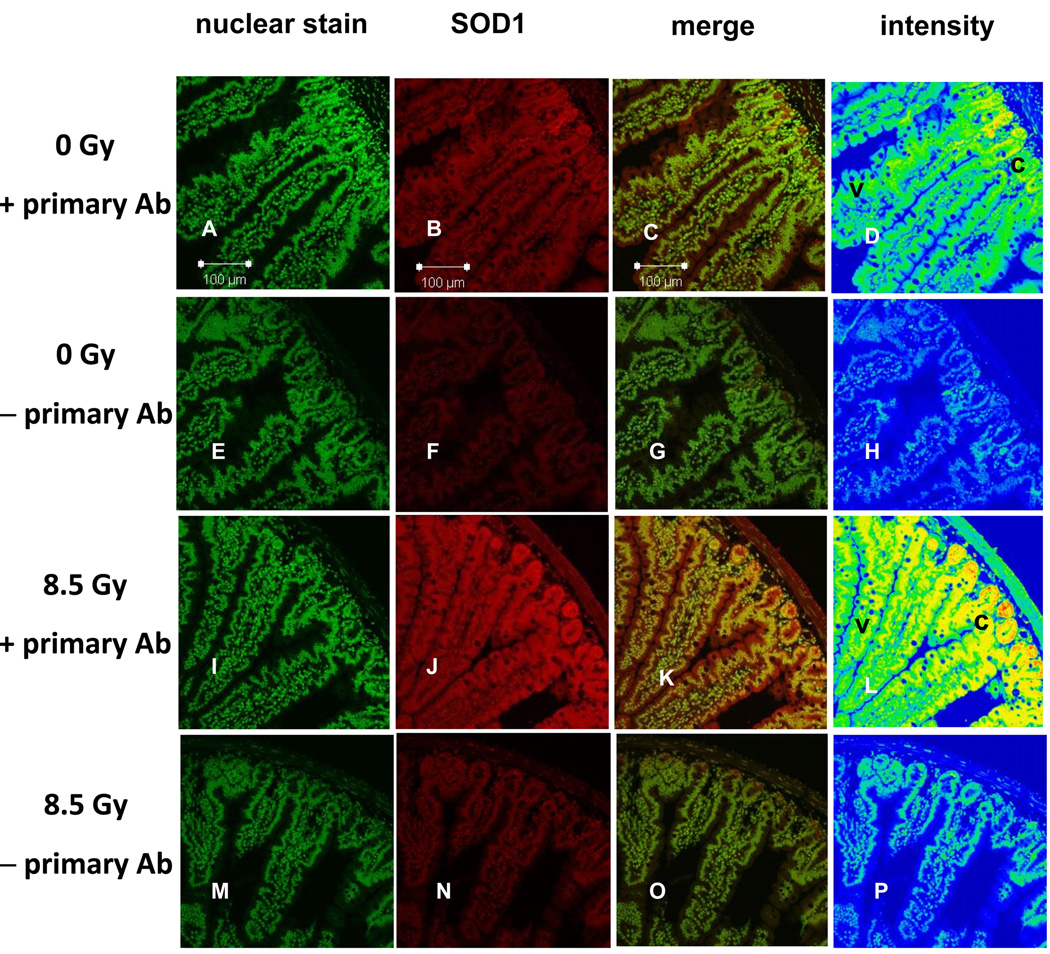
Increases in levels of SOD1 seemed much greater in the intestinal epithelial cells relative to the submucosa and muscle layers (Fig. 6). Intestines of unirradiated mice had low levels of SOD1 (Figs. 6A–D), with greatest concentrations in the crypt regions. Constitutive SOD1 expression seems to be mainly in the cytosol and appears to be higher in the crypt region (c) where cells are rapidly dividing, compared to upper villus regions (v). There is almost no fluorescence in the absence of SOD1 antibody (Figs. 6E–H). Intestines of mice 8 h after irradiation with 8.5 Gy seemed to show increases in levels of SOD1 (Figs. 6I–L) – these results confirm findings of western blots (Fig. 4) obtained from different mice which indicated greater SOD1 expression in irradiated mice. There was little SOD1 expression in the basal lamina right below the crypt. Most increases in SOD1 expression appeared to be in the crypt region (Supplemental Fig. S3). In the absence of SOD1 antibody, there was little fluorescence (Figs. 6M–P) in intestinal sections obtained from irradiated mice. The villus-crypt difference in fluorescence is markedly greater when the antibody is present than when it is absent (compare Fig. 6L and 6D to 6P and 6H), suggesting that the radiation-induced villus-crypt gradient in fluorescence without the antibody cannot fully explain the marked, radiation-induced difference in fluorescence villus-crypt gradient with the antibody. Some compounds like NADH exhibit autofluorescence [17], and radiation-induced perturbations in the level of these autofluorescent compounds in the crypt may be the source of the small villus-crypt gradient in endogenous autofluorescence shown in Fig. 6P. While there was little change in intensity of nuclear staining upon irradiation (Figs. 6A,E,I,M), there are marked radiation-related changes in intensity of SOD1 fluorescence (Figs. 6B,F,J,N and Figs. 6D,H,L,P). SOD1 levels in epithelial cells seemed greater than those in the submucosa and muscle (Figs. 6D and L).
Fig. 6.
Immunolocalization of SOD1 (red) in the small intestine after acute whole-body irradiation with 0 Gy and 8.5 Gy. Sections in column 1 were stained with Sytox Green (nuclear stain). Column 3 represents merged images from columns 1 and 2, while column 4 depicts staining intensity where blue = low, green = modest, yellow = high and red = highest intensity. Sections from unirradiated mice with primary antibody (row 1, panels A – D) and without (row 2, panels E – H). Irradiated mice with primary antibody (row 3, panels I – L) and without (row 4, panels M – P). SOD1 expression is greater in irradiated mice (compare panels B, C with J, K), confirming western blots in Fig. 4. Control panels H and P show no difference in intensity of staining of cytosol and nuclei along the crypt-villus axis when no anti-SOD1 is present. Before irradiation, endogenous SOD1 expression seems already greater in the crypt (top right, c) as opposed to upper villus (v) regions (panel D). After irradiation, there is a dramatic increase in SOD1 throughout the mucosa, and little increase in SOD1 in the submucosal and muscle layers (panel L). SOD1 expression seems greatest in the crypt region (c, panel, L) of intestines from irradiated mice.
Radioprotection of intestinal nutrient transport
Dietary intake, body weight, and intestinal weight
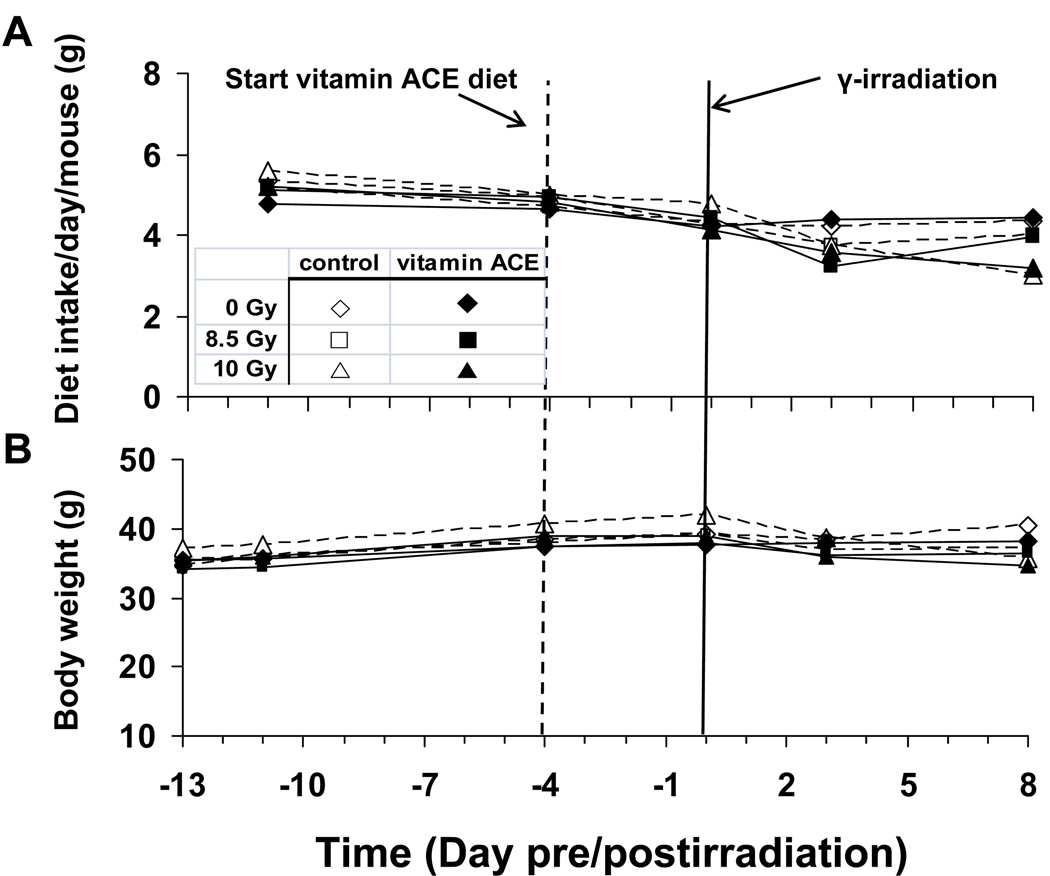
Consumption of the vitamin ACE diet was not significantly different from mice fed the control diet (Fig. 7). However, at 8 d postirradiation, food intake in the 10 Gy mice was reduced by ~ 25% (P = 0.0001) in comparison to both the 0 Gy and the 8.5 Gy exposed mice whose food intakes did not differ or differed modestly. Our earlier studies in calorie restricted mice indicate that acute (2 d to 2 wk) reductions in daily food consumption by up to 30% have no effect on intestinal nutrient transport [18]. Body weights were independent of dose except at 8 d postirradiation (P = 0.03). Total intestinal weight is largely unchanged 8 d after irradiation with 8.5 Gy (Table 2). However, irradiation with 10 Gy results in a small but significant (P < 0.05) reduction in total intestinal weight. This reduction is mitigated significantly by the vitamin ACE diet (Table 2).
Fig. 7.
As diagrammed in Fig. 1B, this is the effect of diet and radiation dose on (A) dietary intake (g per day per mouse), and (B) body weight as a function of time pre and postirradiation. Here, the mice were irradiated with 0 (control), 8.5 or 10 Gy at day 0 (see solid vertical line). The mice were housed one per cage and fed with either control (open symbols) or vitamin ACE enriched diet (closed symbols). Mean results are plotted, n = 6. Standards errors have been omitted for clarity. Mice were fed with the control diet from day −13 to day −4. Then, half of the mice were switched to the vitamin ACE-supplemented diet (dashed vertical line). Diet and radiation dose had modest effects on feeding rate and body weight.
Table 2.
Lengths and weights of mouse intestines 8 d postirradiation†.
| Absorbed dose |
Segment weight (mg/cm) |
Total intestinal weight |
Total intestinal length |
||
|---|---|---|---|---|---|
| Diet | (Gy) | proximal | distal | (g) | (cm) |
| Control | 0 | 64 ± 2 | 23 ± 1 | 1.65 ± 0.08 | 46 ± 1 |
| ACE | 0 | 59 ± 2 | 26 ± 2 | 1.61 ± 0.08 | 46 ± 1 |
| Control | 8.5 | 61 ± 3 | 28 ± 5 | 1.56 ± 0.11 | 47 ± 1 |
| ACE | 8.5 | 59 ± 1 | 22 ± 1 | 1.65 ± 0.06 | 48 ± 0 |
| Control | 10 | 50 ± 1* | 20 ± 1 | 1.40 ± 0.09* | 46 ± 1 |
| ACE | 10 | 57 ± 2 | 21 ± 1 | 1.54 ± 0.10 | 48 ± 1 |
Values are means ± SE of 6 independent experiments.
Data obtained from parallel studies.
= significantly different (P < 0.05)
Sugar transport
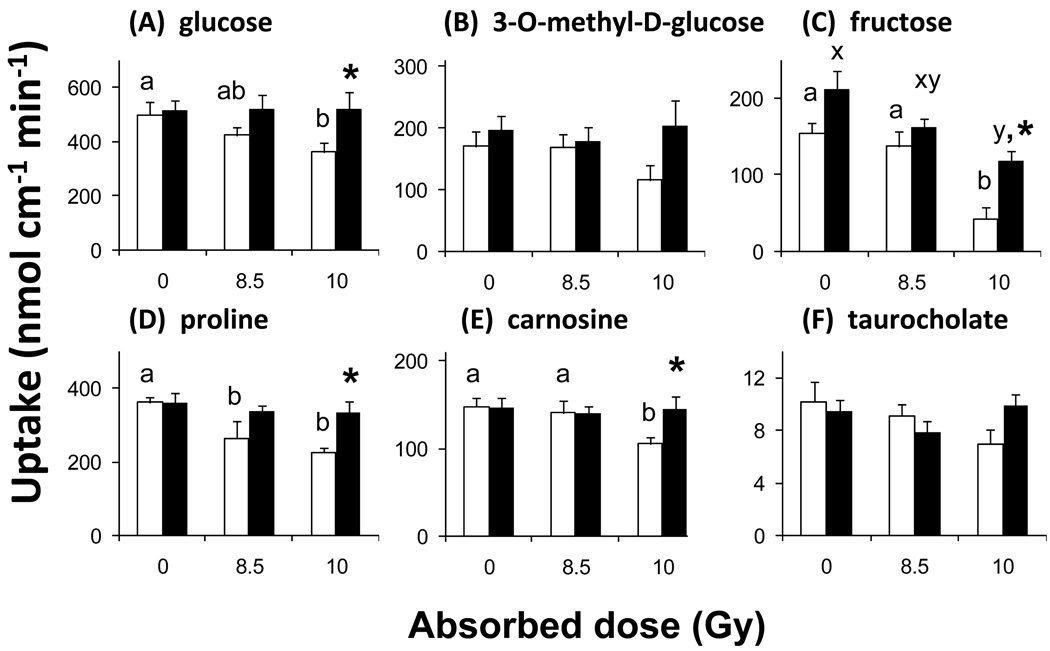
To clearly demonstrate protection, we first tested by one way ANOVA if radiation reduced intestinal uptake of sugars in mice fed the control diet, and if it did, whether a cocktail of dietary vitamins A, C and E prevented radiation-induced decreases in uptake. D-glucose uptake decreased with dose in control (P = 0.05) but not in vitamin-supplemented (P = 0.80) mice (Fig. 8A). Uptake of the non-metabolizable analog 3-O-methyl-D-glucose tended to decrease with dose (P = 0.20), but not when mice were consuming vitamins A, C and E (P = 0.85) (Fig. 8B). The 30% decrease in D-glucose and 3-O-methyl-D-glucose uptake at 10 Gy was clearly prevented by the antioxidant vitamins. Fructose uptake decreased with dose by 3-fold (P = 0.0009) in mice fed control diets (Fig. 8C). Fructose uptake also decreased in irradiated mice that were fed vitamin-supplemented diets (P = 0.02), however at 10 Gy, fructose uptake in vitamin-supplemented mice was much greater (3-fold) than in unsupplemented mice. Likewise, D-glucose (P = 0.04) and 3-O-methyl-D-glucose (P = 0.09) uptakes were also greater in vitamin-treated mice compared with those in control mice at 10 Gy. Treatment with vitamins also tended to rescue D-glucose uptake at 8.5 Gy (P = 0.17).
Fig.8.
In vitro measurements of (A) D-Glucose, (B) 3-O-methyl-D-glucose, (C) D-fructose, (D) proline, (E) carnosine uptake rate in the jejunum of mice irradiated with 0, 8.5, or 10 Gy and sacrificed 8 d postirradiation. Taurocholate (F) uptake was determined in the distal ileum. Mice were fed AIN-76A diet (control, open bars) or an AIN-76A diet supplemented with a cocktail of vitamins A, C, and E (filled bars). Results are means (± SE) of 6 independent experiments. Open bars with different letter superscripts are significantly different from other open bars (P < 0.05 by one-way ANOVA), suggesting that increasing radiation dose reduce uptake rate. With the exception of fructose, filled bars did not decrease with increasing radiation dose, suggesting that consumption of the vitamin ACE-supplemented diet prevented the radiation-induced reduction in uptake. Filled bars with asterisks are significantly greater (P < 0.05 by one-way ANOVA) than open bars at the same radiation dose, suggesting that uptake is greater in tissues from vitamin-treated mice. Similar results are obtained when uptakes are expressed per mg of intestine.
Amino acid, peptide and bile acid transport
Intestinal uptake of nutrients other than sugars also decreased with dose. In mice fed control diets, proline absorption decreased in a dose-dependent manner with 30% and 40% reductions at 8.5 and 10 Gy, respectively (P = 0.008) (Fig. 8D). Supplementing the diet with vitamins ACE again prevented the decrease (P = 0.68). Absorption of the dipeptide carnosine also decreased with dose in dietary control (P = 0.04) but not in vitamin-supplemented (P = 0.95) mice (Fig. 8E). Taurocholate absorption was independent of radiation dose in mice eating both control (P=0.24) and vitamin-supplemented (P = 0.23) diets (Fig. 8F). In all cases, patterns of nutrient uptake, when expressed per mg (not shown), mirrored those expressed per cm of intestine.
At 10 Gy, proline, carnosine and taurocholate uptakes were greater (P = 0.01 to 07) in vitamin-treated compared with those in control mice, suggesting that antioxidant vitamins increased uptake rates at absorbed doses that compromised nutrient transport.
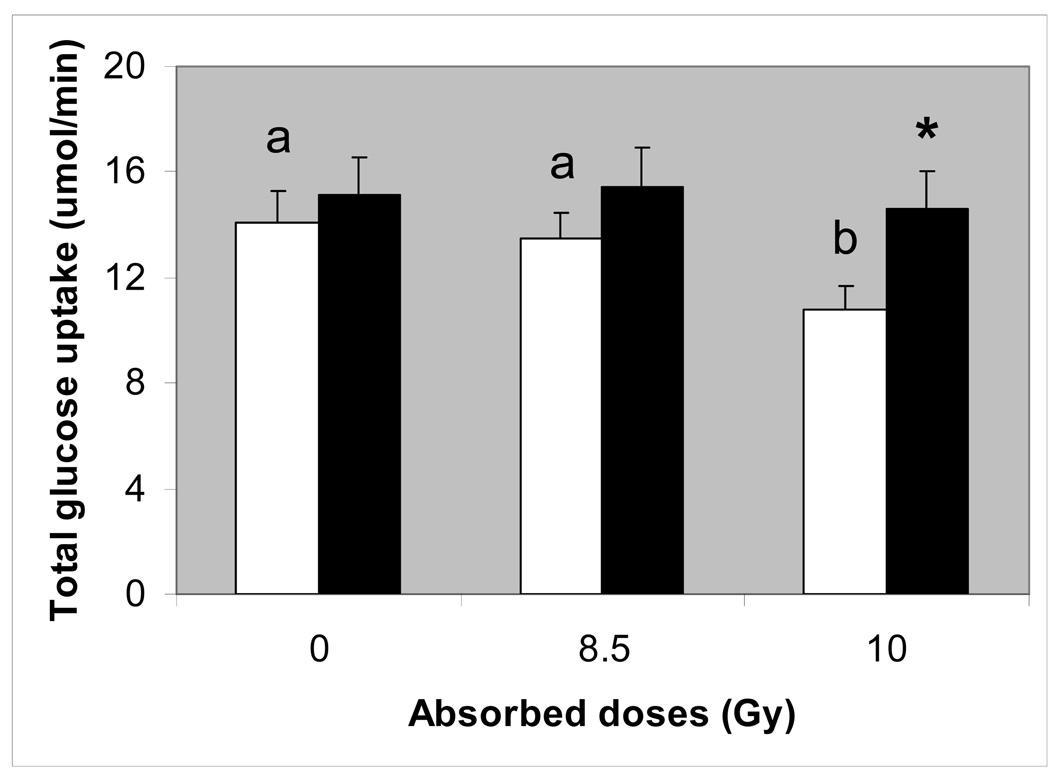
Total glucose uptake
To portray the effect of acute γ-irradiation on nutrient absorptive function of the entire intestine, glucose uptake was determined not only in the jejunal (Fig. 8A) but also in the duodenal and ileal regions of the small intestine (not shown). When uptake was integrated from proximal through distal regions, the total uptake capacity of the intestine could be estimated (Fig. 9). Total absorptive capacity of the intestine for glucose decreased with dose (P < 0.05) but not in mice fed the vitamin ACE diet (P > 0.50).
Fig. 9.
Total glucose uptake for the entire small intestine, estimated by integrating uptakes from the duodenal, jejuna and distal regions. Open bars with different letter superscripts are significantly different (P < 0.05 by one-way ANOVA), suggesting that increasing radiation dose reduced uptake rate. Filled bars did not change with radiation dose, suggesting that consumption of the vitamin ACE-supplemented diet prevented the radiation-induced reduction in uptake. The filled bar with asterisk is significantly greater (P < 0.05 by one-way ANOVA) than the open bar at 10 Gy, suggesting that uptake was greater in tissues from vitamin-treated mice.
Intestinal permeability
L-glucose uptake, a measure of intestinal permeability, was neither affected by diet nor radiation (Supplemental Fig. S4).
Summary of nutrient uptake studies
In summary, 8 d after acute irradiation with 8.5 and 10 Gy, intestinal absorption of most nutrients decreased, but consumption of antioxidant vitamins prevented these radiation-induced decreases in nutrient uptake.
DISCUSSION
About 600,000 cancer patients undergo radiation therapy each year, of which over 200,000 involve the abdominal and pelvic areas. Intestinal dysfunction occurs in 60–90 % of patients receiving abdominal radiation therapy, and a conservative estimate of the number of patients with radiation-induced intestinal dysfunction living in the USA is 2 million [4]. Prognosis is poor, and some patients require prolonged parenteral nutrition and about 10% eventually succumb. The side effects of irradiation on intestinal structure and function, termed radiation enteritis, dictate the upper limits of radiation therapy involving abdominal organs. While radiation enteritis is a severe problem in patients receiving abdominal or pelvic radiation therapy, there is no standardized strategy for medical prevention and therapy [19]. A major gap in knowledge pertaining to radiation enteritis is the absence of studies linking changes in oxidative stress and adaptations of the antioxidant network with alterations in intestinal function. Additional information about this link may shed light on methods that may increase the radiation tolerance of normal intestinal tissue to improve the quality of life of cancer survivors and to enhance radiation efficacy by dose escalation [20].
The main and novel findings in this study are that (1) the time course of radiation-induced changes in expression of antioxidant enzymes differs from that of radiation-induced reductions in intestinal absorptive function, (2) the cell type and location expressing the greatest changes in levels of oxidative stress and in antioxidant enzymes are, respectively, epithelial cells responsible for nutrient absorption and the crypt region comprised mainly of undifferentiated cells, and (3) a cocktail of antioxidant vitamins A, C and E is an effective radioprotector of intestinal absorptive function when consumed before irradiation. Since nutrient transporters are expressed only in epithelial cells, the tissue type with the greatest levels of 4HNE, it is not surprising to detect reductions in nutrient uptake several days after intestines are exposed to radiation. Two minor findings reported here for the first time are that (1) the radiation-induced decline in glucose transport is likely due to effects on the transport step, since radiation also decreased the transport of the non-metabolizable glucose analog, 3-Omethylglucose, and (2) the absorption of the dipeptide carnosine decreased as a function of radiation absorbed dose.
Oxidative stress and antioxidant enzymes in intestinal mucosa
There are only a few studies that have focused on understanding radiation-induced oxidative stress on the small intestine. Repeated low dose exposure (0.25 Gy monthly irradiation for a total of 4.5 Gy) of the whole body increases the concentration of thiobarbituric acid reactive substances (a biomarker of oxidative stress) and activities of glutathione peroxidase in the small intestine but not in most other organs [21], indicating the highly radiosensitive nature of the small intestine relative to other organ systems.
Radiation-induced changes were observed in small intestinal mRNA and protein expression, as well as activity of several antioxidant enzymes in antibiotic-treated mice exposed to high dose (14 Gy) abdominal irradiation [22]. Of the nine antioxidant enzymes examined, only SOD2 and thioredoxin 2 (both localized in the mitochondria) responded 6 h after exposure of the small intestine to 14 Gy. These interesting findings were similar to our results, and provided additional evidence of the rapid response of intestinal antioxidant enzymes to acute high dose irradiation. No other time points were examined by Haton et al [22]. However, it may be essential to track the time course of the antioxidant response to irradiation, because the magnitude of change in mRNA expression of these enzymes may be transient and seems dependent on time after irradiation. In mouse skin, the redox-sensitive transcription factor NFκB and a group of NFκB-related proteins are activated within 15 min of exposure to ionizing radiation [23], leading to increases in SOD2 expression and activity 24 h later [24]. Thus, it will be interesting to determine whether NFκB inhibitors will prevent the rapid and transient radiation-induced increases in expression of antioxidant enzymes in mouse small intestine in vivo.
Four days after acute abdominal irradiation of mice with 14 Gy, intestinal structural damage was evident, and it was paralleled by reductions in SOD1, SOD2 and catalase mRNA expression and activity [22]. These findings are not surprising because anatomical destruction of the intestinal tissue would reduce if not outright prevent the expression of many genes, and likely indicate that the antioxidant network of the intestine has been overwhelmed. We have earlier shown, by tracking nutrient uptake for several days after irradiation, that at 8.5 – 10 Gy, nutrient absorption decreases ~ 8 – 14 d after irradiation [7]. Reductions in absorptive function decrease before overt morphological damage can be observed.
Other in vivo studies have used the approach of providing antioxidants supplements, as well as potentially radioprotective compounds, and then evaluating radiation-induced changes in the levels of antioxidants and antioxidant enzymes in animal models. In these studies, lipid peroxides have generally been shown to increase with absorbed dose. However, it was not consistently clear for these studies whether irradiation increases endogenous antioxidant enzyme expression or activity, and whether glutamine in humans [25], selenium in rats [26], vitamin E in rats [26], Triphala in mice [27], and melatonin in rats [28], could enhance endogenous antioxidant activity in the small intestine after irradiation. Perhaps the failure to clearly observe effects of exogenous antioxidants on endogenous antioxidant enzyme expression or activity is due to the transient nature of the response of endogenous antioxidants. Regardless of their inconsistent effect on endogenous antioxidant expression or activity, these exogenous antioxidants were consistently shown to offer radioprotection for the small intestine by reducing levels of oxidative damage.
Location of radiation-induced antioxidant enzyme expression
The crypt-villus site of radiation-induced changes in intestinal antioxidant enzyme expression is apparently unknown, even though this information is critical because it identifies the cell types or locations that respond to radiation insults regardless of whether other overt changes are apparent. Here we show that, although radiation-induced increases in 4HNE levels are remarkable in almost all tissue types, the greatest increase seem localized mainly in the innermost mucosal layer comprised of epithelial cells. Since 4HNE levels seem homogenous along the crypt-villus axis of epithelial cells, oxidative damage (as indicated by 4HNE location) may be similar among epithelial cells at various stages of development. In contrast, the crypt-villus location of antioxidant enzyme adaptation clearly indicates that SOD1 expression first increases markedly in crypt cells. Epithelial cells along the crypt-villus axis not only differ in terms of development, but also in terms of radiosensitivity [1, 29], with differentiated villus cells being relatively much less radiosensitive. Actively proliferating intestinal stem cells in the crypt region are among the most radiosensitive cells in the body, and their sensitivity (LD50 < 1 Gy, [30]) is an order of magnitude lower than those of other tissues in the mucosa (e.g., endothelium, > 10 Gy, [31]; neuronal tissue, > 20 Gy [32].
Intestinal irradiation slows down the rate of mitosis and proliferative activity of crypt cells and therefore the replacement of villus cells within 12 – 48 h postirradiation [29, 33]. Replacement of villus cells is dependent on number of and rate of division of surviving stem cells in the crypt. If no crypt cells survive at very high doses, then the villus is denuded and ulcers occur and nutrient transport decreases without recovering. If some crypt cells survive, the villus is eventually regenerated and the time of regeneration is dependent on the severity of the insult to the crypt. When the villus is repopulated with functionally mature enterocytes, nutrient transport recovers.
There have been reports that microvascular endothelial apoptosis in the submucosal and muscle layers is the primary lesion leading to intestinal dysfunction [31]. However, at absorbed doses above and below the threshold for death from the GI syndrome, recent work has demonstrated that vascular endothelial cell apoptosis does not appear to be the cause of the GI syndrome [34]. Most cells affected by ionizing radiation actually reside in the epithelium.
The gastrointestinal glutathione peroxidase-2 is highly expressed in the intestinal crypt region [35], and it will be interesting to evaluate its role in radioprotection, since irradiation seems to markedly affect the crypt region. Glutathione peroxidase-2 is protective against intestinal inflammation in vivo [36] and its expression, as well as activity, are induced by intestinal luminal microflora [37].
Radioprotection of intestinal transport function
Although there has been a large number of studies evaluating potential radioprotectors for the small intestine, there has only been a few that focused on radioprotection for nutrient absorptive function. When given before irradiation, a diet high in saturated, but not polyunsaturated, fatty acids prevented the radiation-associated decline in intestinal uptake of glucose and some fatty acids [38]. A synthetic prostaglandin (enprostil) did not prevent the radiation-associated decline in intestinal glucose uptake when given before or after irradiation [39]. Pre-radiation treatment with antioxidant selenium led to a lower incidence of diarrhea in patients receiving pelvic radiation [40], suggesting that consumption of antioxidants prior to irradiation can diminish the adverse effects of radiation on intestinal function.
The top five nutraceuticals in terms of U.S. sales were vitamin C, coenzyme Q, vitamin E, vitamin A/beta-carotene, and noni juice. We have previously shown that vitamin A alone does not protect the small intestine from damage caused by acute whole-body irradiation [7], hence we here chose to provide a cocktail of vitamins A, C and E – a mixture that turned out to be an excellent radioprotector. The experimental level of 0.3 g or 150,000 IU/kg diet of vitamin A has been shown by Friedenthal et al. to be highly protective of the GI tract against radiation damage [41]. This level is non-toxic to rats even when consumed for 1 y [42]. Humans consuming 1,000,000 IU/d for 5 y have not experienced vitamin A toxicity [43]. About 100× RDA is the likely dietary limit [44].
The control diet did not contain vitamin C which mice synthesize. The optimal level of vitamin C for radioprotection of testes is about 10 g/kg diet [45], hence we added an equivalent (in terms of activity) amount (29 g/kg diet) of vitamin C phosphate (only 35% active). When injected into target tissue or peritoneum, vitamin E is a highly effective radioprotector particularly for the small intestine [26, 46], but injections are often impractical. Vitamin E may be added at 3× the minimum required in the diet to be effective in intestinal and hepatic radioprotection [26, 47]. Dietary vitamin E, at 30× control values, suppresses lipid peroxidation in cancerous tissue [48]. Even higher levels (250×, 10,000 IU/kg diet) protected crypt cell numbers, mucosal height, and goblet cell numbers against radiation effects [13]. Although very high, these levels consumed for 2 wk did not cause vitamin E toxicity in rats [13].
Dietary antioxidant vitamins can potentially protect both normal and malignant gut tissues from irradiation. Thus, patients who take antioxidant vitamins during radiation therapy of their malignant tumor may have fewer bouts of diarrhea, but may also experience reductions in the anti-tumor efficacy of ionizing radiation. However, Blumenthal et al. have shown that, in the context of radioimmunotherapy of GW-39 human colon cancer xenografts in mice with 131I-MN14 anti-CEA IgG, a cocktail of vitamins A, C, and E facilitated a 42% increase in maximum tolerated dose and protected against reductions in body weight and myelosuppression [14]. They also observed that, while this cocktail of vitamins afforded considerable protection of normal tissues, no protection was extended to the tumor. These findings are consistent with recent epidemiological studies conducted by Simone et al. that show patients who underwent radiation therapy and took non-prescription antioxidants actually had increased survival [49, 50]. Taken together, these and our studies auger well for the use of vitamins as radioprotectors of normal tissue in the context of radiotherapy of cancer.
Perspective
In summary, using radiation absorbed doses that clearly result in marked reductions in nutrient transport and that acutely increase levels of oxidative stress and expression of antioxidant enzymes in intestinal epithelial cells responsible for nutrient absorption, we found that ingestion of high levels of antioxidants for a few days prior to irradiation may blunt radiation-induced oxidative stress and allow epithelial cells of the small intestine to retain its absorptive function. While the implications of these findings to radiation oncology were already alluded to in the first paragraph of this discussion, they also have implications for radiological emergencies or terrorism. The gastrointestinal (GI) injuries that arise in casualties from such radiation exposures can lead to temporary debilitation or death. While death is catastrophic indeed, the economic and social implications of temporary debilitation can also be enormous. Accordingly, development of medical products capable of ameliorating GI damage caused not only by otherwise lethal radiation exposures, but also by sublethal exposures that may adversely impact important GI functions such as nutrient transport is necessary. In this way, one can reduce the debilitating effects of sublethal doses of radiation to keep the general population maximally productive under emergency conditions. Specialized agents can be manufactured and stockpiled for use by emergency responders, however, making a fresh supply of such agents immediately available to the potentially large number of victims in the general public is complex. Therefore, identifying radioprotectors that are readily available to the general public, without the need for stockpiling, is desirable. The chemotoxicity of the most effective synthetic radioprotectors often limits their use in humans [51]. Accordingly, Weiss and Landauer [52] have suggested the use of naturally occurring antioxidants and related agents. The present studies provide support for this premise and argue in favor of using a combination of vitamins A, C, and E to protect nutrient transport against radiation-induced intestinal damage caused by accidents or terrorist events. These vitamins are inexpensively manufactured, easily administered, safe for repeated doses, relatively stable, and readily available to the general public. Additional studies are under way to assess the capacity of this cocktail to protect intestinal transport against chronic irradiation, and to ameliorate reductions in transport when administered after the advent of an acute or chronic radiation insult. It is recommended that the consequences of intake of high doses of this vitamin cocktail be studied in humans.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Veronique Douard for help with experiments and for valuable discussion. We also would like to extend our gratitude to all of the staff at the NJMS Comparative Medicine Resources facility, especially Jose Espinal, Sabrina Jung, Ana Henriquez, and Peter Rodriguez.
GRANTS
This study was supported, in part, by National Institute of Allergy and Infectious Diseases Grant RC1-AI-078518 (to R. W. Howell). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The laboratory of Dr. Ferraris also receives support from National Science Foundation Grant IOS-0722365 and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-075617A and that of Dr. Howell from National Cancer Institute Grant RO1-CA-83838.
ABBREVIATIONS
- ANOVA
analysis of variance
- GLM
general linear models
- GI
gastrointestinal tract
- LET
linear energy transfer
- SOD
superoxide dismutase
- 4HNE
4-hydroxy-nonenal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Potten CS, Merritt A, Hickman J, Hall P, Faranda A. Characterization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiat Biol. 1994;65:71–78. doi: 10.1080/09553009414550101. [DOI] [PubMed] [Google Scholar]
- 2.Somosy Z, Horvath G, Telbisz A, Rez G, Palfia Z. Morphological aspects of ionizing radiation response of small intestine. Micron. 2002;33:167–178. doi: 10.1016/s0968-4328(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 3.Miholic J, Vogelsang H, Schlappack O, Kletter K, Szepesi T, Moeschl P. Small bowel function after surgery for chronic radiation enteritis. Digestion. 1989;42:30–38. doi: 10.1159/000199822. [DOI] [PubMed] [Google Scholar]
- 4.Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 5.Strup-Perrot C, Mathe D, Linard C, Violot D, Milliat F, Francois A, Bourhis J, Vozenin-Brotons MC. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G875–G885. doi: 10.1152/ajpgi.00088.2004. [DOI] [PubMed] [Google Scholar]
- 6.MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14:523–528. doi: 10.1046/j.1365-2036.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Roche M, Neti PV, Kemp FW, Agrawal A, Attanasio A, Douard V, Muduli A, Azzam EI, Norkus E, Brimacombe M, Howell RW, Ferraris RP. Radiation-induced reductions in transporter mRNA levels parallel reductions in intestinal sugar transport. Am J Physiol Regul Integr Comp Physiol. 2010;298:R173–R182. doi: 10.1152/ajpregu.00612.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harapanhalli RS, Narra VR, Yaghmai V, Azure MT, Goddu SM, Howell RW, Rao DV. Vitamins as radioprotectors in vivo. II. Protection by vitamin A and soybean oil against radiation damage caused by internal radionuclides. Radiat Res. 1994;139:115–122. [PubMed] [Google Scholar]
- 10.Sarma L, Kesavan PC. Protective effects of vitamins C and E against gammaray-induced chromosomal damage in mouse. Int J Radiat Biol. 1993;63:759–764. doi: 10.1080/09553009314552161. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 12.Empey LR, Papp JD, Jewell LD, Fedorak RN. Mucosal protective effects of vitamin E and misoprostol during acute radiation-induced enteritis in rats. Dig Dis Sci. 1992;37:205–214. doi: 10.1007/BF01308173. [DOI] [PubMed] [Google Scholar]
- 13.Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol) Ann Surg. 1995;222:504–508. doi: 10.1097/00000658-199522240-00008. discussion 508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenthal RD, Lew W, Reising A, Soyne D, Osorio L, Ying Z, Goldenberg DM. Anti-oxidant vitamins reduce normal tissue toxicity induced by radio-immunotherapy. Int J Cancer. 2000;86:276–280. doi: 10.1002/(sici)1097-0215(20000415)86:2<276::aid-ijc19>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy M, Bruninga K, Mutlu EA, Losurdo J, Choudhary S, Keshavarzian A. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol. 2001;96:1080–1084. doi: 10.1111/j.1572-0241.2001.03742.x. [DOI] [PubMed] [Google Scholar]
- 16.Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- 17.Shuttleworth CW. Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem Int. 56:379–386. doi: 10.1016/j.neuint.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraris RP, Cao QX, Prabhakaram S. Chronic but not acute energy restriction increases intestinal nutrient transport in mice. J Nutr. 2001;131:779–786. doi: 10.1093/jn/131.3.779. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerer T, Bocker U, Wenz F, Singer MV. Medical prevention and treatment of acute and chronic radiation induced enteritis--is there any proven therapy? a short review. Z Gastroenterol. 2008;46:441–448. doi: 10.1055/s-2008-1027150. [DOI] [PubMed] [Google Scholar]
- 20.Haydont V, Bourgier C, Vozenin-Brotons MC. Rho/ROCK pathway as a molecular target for modulation of intestinal radiation-induced toxicity. Br J Radiol 80 Spec No. 2007;1:S32–S40. doi: 10.1259/bjr/58514380. [DOI] [PubMed] [Google Scholar]
- 21.Siems W, Gartner C, Kranz D, Schneider W, Grune T, Schimke J, Gau S, Wege U, Gerber G. Long term effects of monthly low dose whole body irradiation on the glutathione status and thiobarbituric acid-reactive substances in different organs of male Wistar rats. Radiobiol Radiother (Berl) 1990;31:257–263. [PubMed] [Google Scholar]
- 22.Haton C, Francois A, Vandamme M, Wysocki J, Griffiths NM, Benderitter M. Imbalance of the antioxidant network of mouse small intestinal mucosa after radiation exposure. Radiat Res. 2007;167:445–453. doi: 10.1667/RR0581.1. [DOI] [PubMed] [Google Scholar]
- 23.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factorkappaB and manganese superoxide dismutase mediate adaptive radioresistance in lowdose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 24.Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giris M, Erbil Y, Oztezcan S, Olgac V, Barbaros U, Deveci U, Kirgiz B, Uysal M, Toker GA. The effect of heme oxygenase-1 induction by glutamine on radiation-induced intestinal damage: the effect of heme oxygenase-1 on radiation enteritis. Am J Surg. 2006;191:503–509. doi: 10.1016/j.amjsurg.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Mutlu-Turkoglu U, Erbil Y, Oztezcan S, Olgac V, Toker G, Uysal M. The effect of selenium and/or vitamin E treatments on radiation-induced intestinal injury in rats. Life Sci. 2000;66:1905–1913. doi: 10.1016/s0024-3205(00)00516-6. [DOI] [PubMed] [Google Scholar]
- 27.Sandhya T, Lathika KM, Pandey BN, Bhilwade HN, Chaubey RC, Priyadarsini KI, Mishra KP. Protection against radiation oxidative damage in mice by Triphala. Mutat Res. 2006;609:17–25. doi: 10.1016/j.mrgentox.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Sener G, Jahovic N, Tosun O, Atasoy BM, Yegen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003;74:563–572. doi: 10.1016/j.lfs.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Becciolini A, Balzi M, Potten CS. Radiation effects on proliferation and differentiation in the rat small intestine. In: Potten CS, Hendry JH, editors. Radiation and gut. Vol. 1995. Amsterdam: Elsevier; pp. 85–143. [Google Scholar]
- 30.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 31.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 32.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Stone HB, Tofilon PJ, Wong RS. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res. 2003;159:812–834. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 33.Becciolini A, Cremonini D, Fabbrica D, Balzi M. Cell proliferation and differentiation in the small intestine after irradiation with multiple fractions. Acta Radiol Oncol. 1986;25:51–56. doi: 10.3109/02841868609136378. [DOI] [PubMed] [Google Scholar]
- 34.Schuller BW, Rogers AB, Cormier KS, Riley KJ, Binns PJ, Julius R, Hawthorne MF, Coderre JA. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int J Radiat Oncol Biol Phys. 2007;68:205–210. doi: 10.1016/j.ijrobp.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 35.Kipp A, Banning A, Brigelius-Flohe R. Activation of the glutathione peroxidase 2 (GPx2) promoter by beta-catenin. Biol Chem. 2007;388:1027–1033. doi: 10.1515/BC.2007.137. [DOI] [PubMed] [Google Scholar]
- 36.Esworthy RS, Yang L, Frankel PH, Chu FF. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr. 2005;135:740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 37.Esworthy RS, Binder SW, Doroshow JH, Chu FF. Microflora trigger colitis in mice deficient in selenium-dependent glutathione peroxidase and induce Gpx2 gene expression. Biol Chem. 2003;384:597–607. doi: 10.1515/BC.2003.067. [DOI] [PubMed] [Google Scholar]
- 38.Thomson AB, Keelan M, Lam T, Cheeseman CI, Walker K, Clandinin MT. Saturated fatty acid diet prevents radiation-associated decline in intestinal uptake. Am J Physiol. 1989;256:G178–G187. doi: 10.1152/ajpgi.1989.256.1.G178. [DOI] [PubMed] [Google Scholar]
- 39.Thomson AB, Keelan M, Clandinin MT, Tavernini M, Lam T, Walker K, Cheeseman CI. Lack of protective effect of oral enprostil, a synthetic prostaglandin E2, on intestinal transport and morphology following abdominal irradiation in the rat. Can J Physiol Pharmacol. 1989;67:1351–1356. doi: 10.1139/y89-215. [DOI] [PubMed] [Google Scholar]
- 40.Dennert G, Horneber M. Selenium for alleviating the side effects of chemotherapy, radiotherapy and surgery in cancer patients. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD005037.pub2. CD005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedenthal E, Mendecki J, Davis L, Seifter E. The role of vitamin A and analogues in prevention of radiation toxicity during radiotherapy of cancer. In: Jacobs MM, editor. Vitamins and minerals in the prevention and treatment of cancer. Boca Raton, FL: CRC Press; 1991. pp. 275–278. [Google Scholar]
- 42.Levenson SM, Gruber CA, Rettura G, Gruber DK, Demetriou AA, Seifter E. Supplemental vitamin A prevents the acute radiation-induced defect in wound healing. Ann Surg. 1984;200:494–512. doi: 10.1097/00000658-198410000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werback MR. Nutritional influences on illness. New Canaan, CO: Keats Publishing, Inc; 1987. [Google Scholar]
- 44.Bendich A, Langseth L. Safety of vitamin A. Am J Clin Nutr. 1989;49:358–371. doi: 10.1093/ajcn/49.2.358. [DOI] [PubMed] [Google Scholar]
- 45.Narra VR, Howell RW, Sastry KS, Rao DV. Vitamin C as a radioprotector against iodine-131 in vivo. J Nucl Med. 1993;34:637–640. [PubMed] [Google Scholar]
- 46.Satyamitra M, Devi PU, Murase H, Kagiya VT. In vivo radioprotection by alpha-TMG: preliminary studies. Mutat Res. 2001;479:53–61. doi: 10.1016/s0027-5107(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan V, Jacobs AJ, Simpson SA, Weiss JF. Radioprotection by vitamin E: effects on hepatic enzymes, delayed type hypersensitivity and postirradiation survival of mice. In: Meyskens F, Prasan KN, editors. Modulation and mediation of cancer by vitamins. Basel: Karger; 1983. pp. 119–131. [Google Scholar]
- 48.Cameron IL, Munoz J, Barnes CJ, Hardman WE. High dietary level of synthetic vitamin E on lipid peroxidation, membrane fatty acid composition and cytotoxicity in breast cancer xenograft and in mouse host tissue. Cancer Cell Int. 2003;3:3. doi: 10.1186/1475-2867-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simone CB, 2nd, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, Part 2. Altern Ther Health Med. 2007;13:40–47. [PubMed] [Google Scholar]
- 50.Simone CB, 2nd, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 1. Altern Ther Health Med. 2007;13:22–28. [PubMed] [Google Scholar]
- 51.Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect 105. 1997;6 Suppl:1473–1478. doi: 10.1289/ehp.97105s61473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.