Abstract
The bactericidal and phagocytic capacities of monocytes for E. coli, Staphylococcus, Salmonella, and Listeria, and factors that influence these functions were evaluated and compared with those of the polymorphonuclear leukocytes of 30 normal human subjects. Monocytes killed a significantly smaller proportion of each of the bacterial species than did neutrophils from the same individuals. Whereas the neutrophils of all individuals demonstrated the ability to kill significant numbers of the four bacterial species, there was a marked variation in the effect of monocytes of different individuals on the growth curves of these same bacteria. When the bactericidal capacity of an individual's monocytes to more than one species of bacteria was examined in the same experiment, a significant difference in the effect of monocytes on the growth curve of one bacterial species as opposed to another was noted in 4 of 17 subjects. The bactericidal ability of monocytes of single individuals was consistent on different days in 9 of the 11 subjects whose monocytes were examined more than once against the same bacteria.
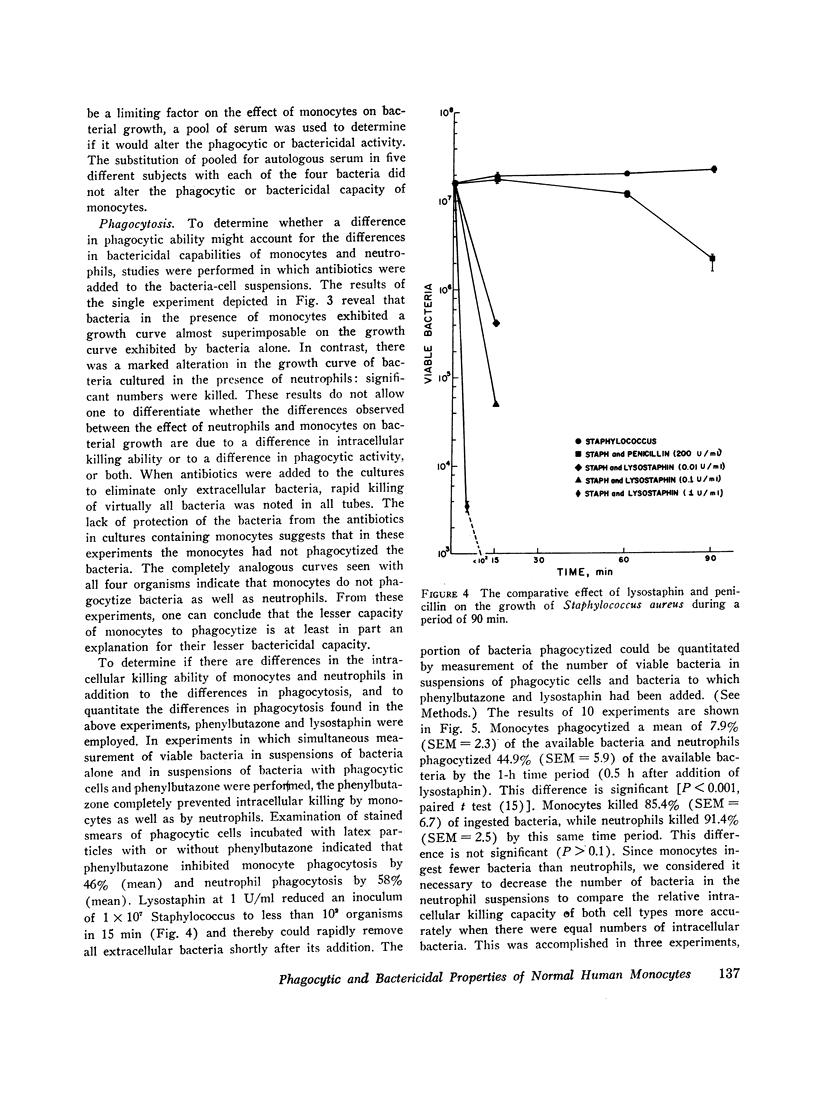
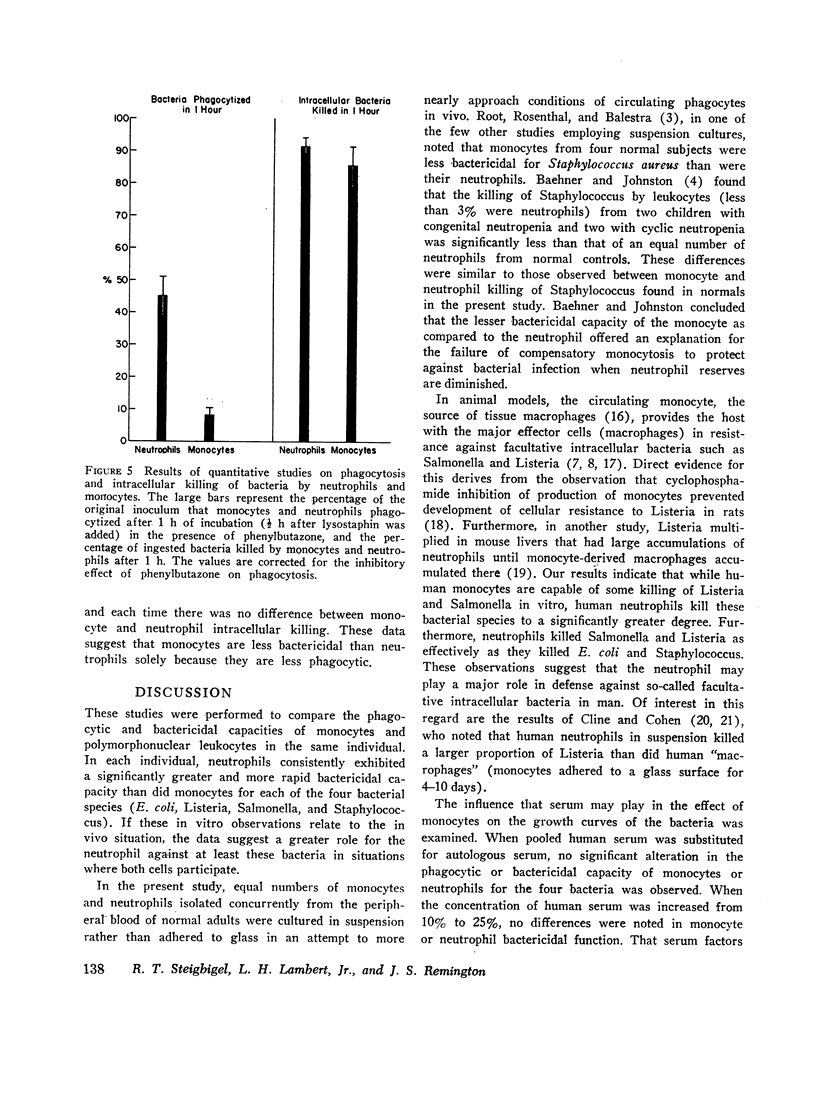
Studies were performed to determine if the lesser bactericidal capability of monocytes was due to a difference in the ability of monocytes and neutrophils to phagocytize or to a difference in the ability of these cells to kill ingested bacteria or both. The results demonstrated that monocytes phagocytize bacteria significantly less well than neutrophils, but the intracellular killing capacity of both cell types is equal. Addition of phenylbutazone to cell suspensions completely inhibited intracellular killing by both monocytes and neutrophils, suggesting the possibility that the bactericidal mechanisms in both cell types might be similar.
Monocyte killing of E. coli, Salmonella, and Listeria, but not of Staphylococcus, was significantly diminished in heat-inactivated autologous serum. Neither increasing the concentration of autologous serum from 10% to 25% nor replacement of autologous serum with pooled human serum had any effect on monocyte killing of any of the four bacteria.
These studies demonstrate that peripheral blood monocytes are less bactericidal for the four bacterial species than neutrophils, solely because monocytes are less phagocytic. A baseline for further study of factors that influence monocyte function and for study of this cell in selected patient populations is provided.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Windhorst D. B., Good R. A. Improved tests for the evaluation of neutrophil function in human disease. J Lab Clin Med. 1968 Jul;72(1):136–148. [PubMed] [Google Scholar]
- BRAUDE A. I., FELTES J. Studies in the destruction of staphylococci by human leukocytes; effect of clumping of intracellular and extracellular bacteria on the results obtained with the agar-plate method. J Lab Clin Med. 1953 Aug;42(2):289–298. [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. C., Huber H., Douglas S. D., Fudenberg H. H. A defect in circulating mononuclear phagocytes in chronicgranulomatous disease of childhood. J Immunol. 1968 Nov;101(5):1093–1095. [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Gardner D. E., Graham J. A., Miller F. J., Illing J. W., Coffin D. L. Technique for differentiating particles that are cell-associated or ingested by macrophages. Appl Microbiol. 1973 Mar;25(3):471–475. doi: 10.1128/am.25.3.471-475.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Pollara B., Good R. A. Protection of phagocytized bacteria from the killing action of antibiotics. Nature. 1966 Jun 11;210(5041):1131–1132. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALDONADO J. E., HANLON D. G. MONOCYTOSIS: A CURRENT APPRAISAL. Mayo Clin Proc. 1965 Mar;40:248–259. [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T. The mediator of cellular immunity. IV. Cooperation between lymphocytes and mononuclear phagocytes. Cell Immunol. 1971 Aug;2(4):317–325. doi: 10.1016/0008-8749(71)90066-9. [DOI] [PubMed] [Google Scholar]
- North R. J. The action of cortisone acetate on cell-mediated immunity to infection. Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971 Dec 1;134(6):1485–1500. doi: 10.1084/jem.134.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodey G. E., Park B. H., Windhorst D. B., Good R. A. Defective bactericidal activity of monocytes in fatal granulomatous disease. Blood. 1969 Jun;33(6):813–820. [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Melly M. A., Hash J. H., Koenig M. G. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J Biol Med. 1967 Feb;39(4):215–229. [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Sbarra A. J. Effect of phenylbutazone on phagocytosis and intracellular killing by guinea pig polymorphonuclear leukocytes. J Bacteriol. 1968 Dec;96(6):1982–1990. doi: 10.1128/jb.96.6.1982-1990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]




