Abstract
Injudicious reaming of the tibial shaft can lead to extreme local hyperthermia, which in turn can result in the rare but catastrophic complication of segmental bone and soft tissue necrosis (osteocutaneous thermal necrosis). This is a retrospective study showing osteocutaneous thermal necrosis occurring after tibial intramedullary reaming salvaged by Ilizarov reconstruction in seven patients from the collective experience of four limb reconstruction centres. All patients were males, with an average age of 51.8 years (range, 30–70 years), who had undergone intramedullary reaming during the treatment of closed tibial fractures. In all patients, circumferential bone and variable contiguous soft tissue necrosis developed a few days after reaming. Bone and soft tissue reconstruction was subsequently performed using a circular external fixator (Ilizarov apparatus or Taylor spatial frame) a mean of four months after injury in six patients; in one case, reconstruction was undertaken four years after the original injury. Two complications (secondary tissue breakdown at a bone transport site; premature consolidation) necessitated cessation of bone transport at one of two bone transport levels in two patients. All patients eventually healed with a good functional result after an average of 11.5 months in the fixator (range, 10–13 months).
Introduction
The deleterious consequences of local hyperthermia induced by intramedullary reaming as a complication of intramedullary fixation of tibial fractures are occasionally noted in the literature [11, 13, 15, 16]. Friction forces involved in reaming of the intramedullary canal generate a rise in temperature of the surrounding tissues that, under controlled conditions, is reported in vivo between 0.5°C and 16°C for a maximum of 15 seconds [7]. The mechanical work per unit during the friction process is determined by the contact pressure, the relative speed of reaming, local accumulation of reaming debris, instrument design, and by the roughness of the surface [1]. When safe reaming practices designed to avoid excessive heat generation are violated, impressive temperature increases can occur. Temperatures as high as 175–225°C after two minutes of reaming have been estimated with subsequent development of irreversible thermal damage to bone and surrounding tissues [1]. We are aware of six reported patients with osteocutaneous thermal necrosis, all involving the tibia [11, 13, 15]. A seventh case of segmental bone necrosis without associated soft tissue necrosis has been reported after intramedullary reaming of the humerus [13]. Secondary exposure of a devitalised fracture site and the intramedullary device within necrotic tissues with subsequent osteomyelitis was noted in all of these patients [11, 13, 15]. The lower leg is probably at higher risk for the development of osteocutaneous thermal necrosis as a result of its trifoil shape of tibia and relatively thin soft tissue envelope. Segmental bone necrosis, combined with soft tissue necrosis and deep infection, creates a therapeutic challenge of osteocutaneous thermal necrosis. Various reconstructive procedures have been described in these patients, but the outcome of such treatments have not been comprehensively reported [11, 13, 15, 16]. Our aim in this study was to (1) identify patients whose condition could be attributable to osteocutaneous thermal necrosis after reaming of the tibial shaft, (2) ascertain whether circular external fixation using Ilizarov bifocal and trifocal segmental bone transport methods [2, 14] could be used to reconstruct these patients’ limbs, (3) identify complications of these reconstructions, and (4) perform preoperative screening of the high risk group.
Materials and methods
To identify patients with osteocutaneous thermal necrosis after reaming of the tibial shaft, we reviewed the entire history of treatment of complicated tibial fractures using Ilizarov methods in four centres from January 1995 to December 2006. Criteria for inclusion in the study group included (1) treatment by tibial intramedullary reaming for a closed tibial fracture (Fig. 1) without major soft tissue injury, (2) development of full-thickness soft tissue necrosis in the early postoperative period, (3) subsequent exposure of bone secondary to the soft tissue necrosis, (4) presence of circumferential necrosis of a segment of the tibia at the time of reconstructive Ilizarov treatment and (5) presence of systematic and local disease (Fig. 2). We identified seven patients who met these criteria. All patients were males with a mean age of 51.8 years (range, 30–70 years) at the time of injury. Tibial fractures were the result of domestic trauma in two patients, road accidents in four and a work accident in one. Reamed nailing was performed after a mean of nine days (range, 5–30 days) after initial injury. Although details regarding the reaming techniques were not reported on the surgical records, the main procedures have been described. All tibial fractures were reamed. Two of the patients were heavy smokers, and one had diabetes mellitus. Two further patients had associated vascular compromise. One patient had an acute complete post-traumatic lesion of the distal anterior tibial artery associated with a complete post-traumatic lesion of the interosseous artery documented by arteriography. Another patient had chronic femoral arterial insufficiency and underwent insertion of a femoral arterial prosthesis before surgery for the tibial fracture. In that particular case, an unsuccessful attempt at tibial nailing was performed 30 days after injury followed by open reduction and internal osteosynthesis with a plate performed during the same surgical session. The reasons the nailing procedure failed were not reported on the surgical record. A very large area of osteocutaneous necrosis (15 × 4 cm) ensued, which extended still further after a posterolateral local rotational flap failed. In one patient, the fracture was temporarily stabilised with an external fixator and converted to a reamed intramedullary nail 12 days after injury. Worsening of skin conditions after surgery was reported in various forms on all clinical records, and a large area of necrosis of soft tissues was noted after a mean of 13 days (range, 7–28 days) from the reaming procedure. Deep infection after intramedullary fixation or attempted fixation was documented in five of the seven patients. In four, the causative organism was Staphylococcus aureus and in one case Pseudomonas aeruginosa. Intramedullary fixation was removed in five of six patients immediately before initiation of reconstruction using the Ilizarov apparatus, during the same surgical procedure. Ilizarov salvage reconstruction treatment was initiated an average of four months (range, 49 days to nine months) after initial injury in six patients, and in one patient reconstruction was initiated four years after the original injury. A conventional Ilizarov circular apparatus (Plustek Amplimedical Orthocare, Assago, Milano, Italy) was used in five patients and a Taylor spatial frame (Smith and Nephew, Memphis TN, USA) was used in two patients [3, 5]. The basic reconstructive strategy consisted of segmental resection of the necrotic bone, application of a stabilising circular fixator to the residual limb, and osteotomy of the remaining viable tibial segment at one or two levels for bone transport into the segmental defect. In five of the seven patients, some necrotic soft tissues were still present at the time of Ilizarov surgery and were debrided at the time of bone resection. In two patients, soft tissue debridement preceded Ilizarov treatment. “Bifocal” treatment (osteotomy at one level) was used in one patient; “trifocal” treatment (osteotomy at two levels: one proximal and one distal to the segmental defect) was used in the other six. In trifocal treatment, the two transport segments of bone are usually moved towards each other, which shortens treatment time (Fig. 3). We routinely debrided the intramedullary canal in all cases. The extent of bone resection was determined on the basis of the presence of viable bleeding bone on the resected surfaces. A temporary shortening was performed in only two cases. In one of them, in spite of acute shortening of 2.5 cm, the resection site initially remained partly open. The amount of resected tibia varied from 4 to 12 cm (mean 8 cm). In four patients the resected bone segment was examined histologically. In each of these patients, circumferential, full-thickness segmental necrosis of bone was confirmed. No plastic surgical reconstruction of the soft tissues was performed in any case. In five of the seven patients, a “docking site” procedure, consisting of debridement of bone ends at the contact site and iliac crest autologous bone grafting, was performed when bone fragments came into contact at the end of transport. One patient had resection of an intact fibula performed in conjunction with the “docking site” procedure. Follow-up controls were carried out at one, two, three, six, 12 and 24 months after frame removal. We did not employ a clinical score to quantify our results.
Fig. 1.
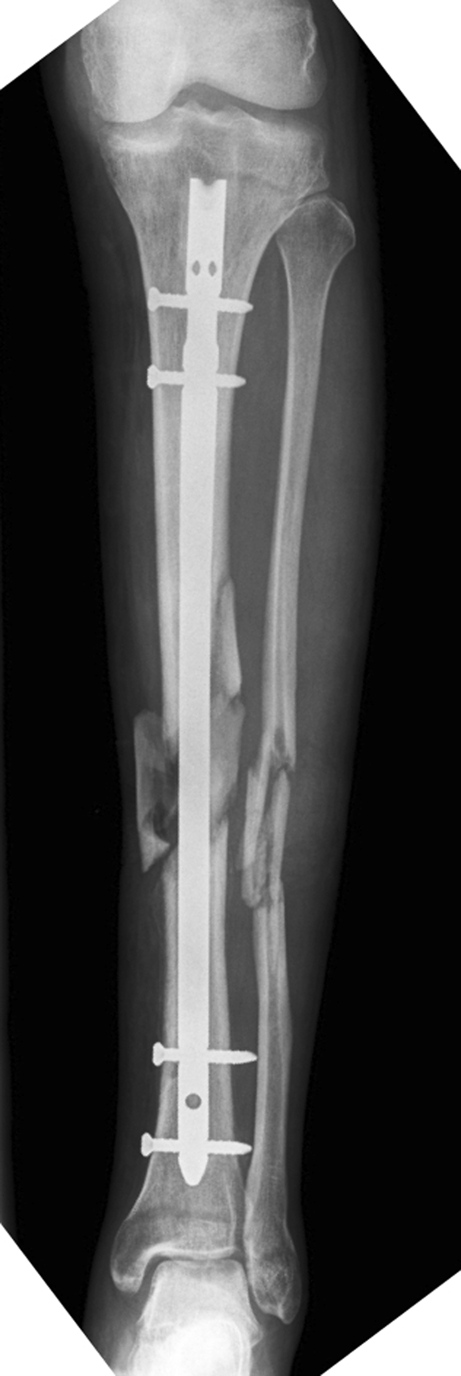
A 38-year-old male with closed tibial fracture (AO classification 42C31; Injury Severity Score [ISS] 18) after a pedestrian versus motor vehicle crush injury. Anteroposterior radiograph two weeks after insertion of reamed intramedullary nail
Fig. 2.

Intraoperative appearance of leg after wound debridement and resection of dead bone showing a defect with the nail
Fig. 3.

Anteroposterior X-rays after docking site procedure during trifocal treatment with a Taylor Spatial Frame (TSF); bone defect was 10.3 cm
Results
Complete soft tissue healing, resolution of intramedullary infection, and tibial consolidation occurred in all seven patients (Figs. 4 and 5). Soft tissues and skin healing progressed spontaneously during bone transport to the extent that no patient required an intermediate procedure of plastic surgical reconstruction for skin coverage. The apparatus was removed an average of 11.5 months after application (range, 10–13 months). Partial weight bearing with crutches was allowed immediately after frame removal and full weight bearing 30 days after frame removal. Routine weight bearing X-rays of the whole leg after healing were performed in five of seven patients. Average transportation time was 112 days (range, 52–190), and average consolidation time was 222 days (range, 121–295). Complications included partial wound breakdown with exposure of the anterior portion of the proximal regenerated bone, which occurred in the patient who had required the femoral artery prosthesis before tibial fracture treatment. This patient developed septic occlusion of the arterial prosthesis during tibial reconstructive treatment. The arterial prosthesis was replaced, bone transport at this level discontinued, and hyperbaric oxygen therapy instituted. Under this treatment protocol, the soft tissue defect healed and consolidation of the posterior portion of the new bone occurred; the bone transport needed to complete the segmental reconstruction was performed at the distal osteotomy site. In a second patient, premature consolidation of the regenerate bone developed at one level. Bone transport was continued uneventfully at the other level. One patient developed posterior bowing of the proximal segmental regenerate bone after frame removal and was treated with cast immobilisation. The tibia consolidated with a residual radiographic deformity of 6° of procurvatum without functional problems. Another patient had consolidation in 5° of valgus. Range of motion of the knee remained normal in all patients. Five patients lost 10–20° of ankle dorsiflexion, but none developed a true equinus contracture. Limb length discrepancy at the end of treatment was within 1 cm in 6/7 cases; in the patient who had a septic occlusion of the femoral artery prosthesis, we observed a shortening of 2 cm of the involved leg.
Fig. 4.
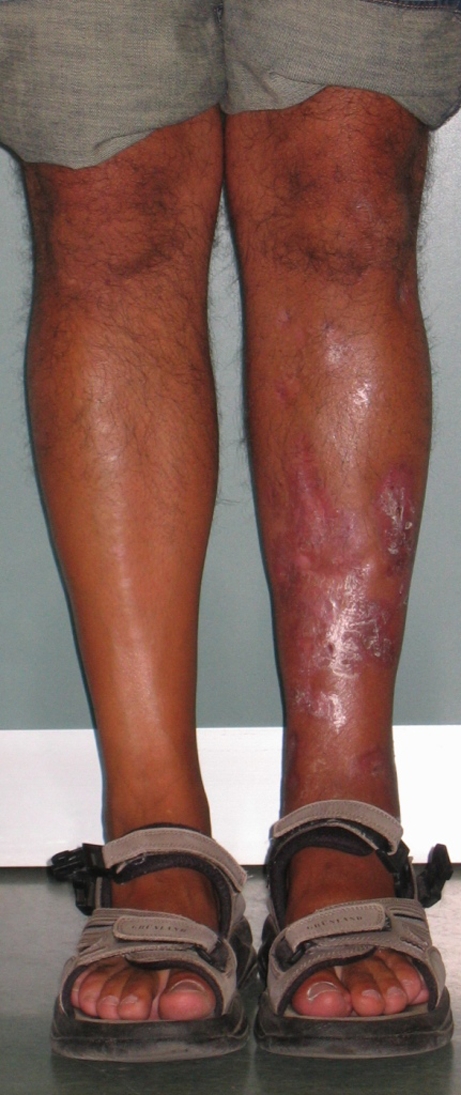
Standing front view three years after frame removal. Complete healing of soft tissues without any plastic surgery
Fig. 5.

Anteroposterior radiograph of the leg at two years after Taylor spatial frame (TSF) removal showing excellent alignment, closure of the defect, bony healing of the docking site and the proximal and distal tibia lengthening regenerate sites. Total time in frame was 330 days. Index lengthening (months/cm) was 1.06
Discussion
The incidence of soft tissue ulcerations after reamed nailing of closed tibia fractures has been attributed by some authors to heat transmission consequent to incorrect reaming procedures [11, 13]. Only a few cases [11, 13] have been reported in the past, and recently a new one has been reported [15]. Although the heating of cortical bone during reaming has been evaluated in theoretical models [1], animal [4, 12] and human cadaveric bone [9], and in vivo in both animals [3, 10] and humans [6–8], the clinical relevance of these data has been debated. An in vivo assessment [6] of heat production during reaming in humans demonstrated peak temperatures of 36.1–51.6°C, with the highest value lasting no more than 15 seconds, without evidence of clinical manifestation related to excessive heat production. This suggests that correct reaming procedures limit heat generation to biologically tolerable levels. Conclusive evidence of the relationship between incorrect reaming and osteocutaneous necrosis would require hypothetical unrealistic routine thermocouple cortical temperature records during the reaming procedures or routine Doppler perfusion scan of all diabetic atherosclerotic patients planned for reamed intramedullary reaming. In the patients reported in the literature, clinical difficulties during reaming of closed tibial fractures have been suspected as a cause of heat transmission and necrosis of tissues. Our inclusion criteria for patients suspected of osteocutaneous thermal necrosis were clinical, related to appearance of an all layer soft tissue necrosis of tibia after reaming of a closed tibia fracture, with circumferential necrosis of bone documented at the time of Ilizarov surgery. These criteria do not prove conclusively that tissue damage has been the result of thermal damage; nevertheless, the fact that in seven patients a closed tibia fracture, apparently without soft tissue problems, developed a circumferential necrosis of bone and an all layer soft tissue destruction after a reamed nailing procedure is a matter of concern and needs explanation if the thermal pathogenesis is rejected. Necrosis of bone and soft tissues after a reaming procedure of a tibial fracture can be ascribed in our patients to causes other than thermal damage, as local trauma, infection and vascular compromise. Local trauma can provoke soft tissue necrosis that would become evident only days after the initial injury, so the necrosis could be incorrectly attributed to the reaming procedure. In five of the six cases presented in the literature, this objection can be raised, because those patients underwent reamed nailing the same day as the injury. Our patients underwent reamed tibial nailing after a mean of eight days, during which the conditions of soft tissues of the leg remained satisfactory. Thus, a local damage of tissues related to the procedure can be postulated. Infection can lead to severe necrosis of bone, but an all layer necrosis of soft tissues surrounding a fracture site and developing in a few days, if caused by infection, is usually associated with critical general conditions, such as necrotising Clostridial fasciitis. Therefore, if our patients had only infected nails, the rapid development of soft tissue compromise has yet to be explained. Furthermore, in all the cases reported in the literature related to thermal damage due to reaming, intramedullary infection has been documented [11, 13, 15]. Some acute or chronic vascular compromise was present in five out of seven of our patients and may have enhanced tissue sensitivity to traumatic or thermal damage. We assume that defective perfusion of tissues could have precipitated and extended the thermal tissue damage, and we do not consider the presence of vascular compromise as an argument against the thermal hypothesis. One limitation of our study is the lack of details of the reaming techniques we found in patient’s clinical records. The segmental nature of the necrosis in the osteocutaneous syndrome, involving circumferential bone and soft tissues, made the segmental reconstruction proposed by Ilizarov the most appropriate treatment [3]. Treatments that do not deal with circumferential bone necrosis, but limit themselves to soft tissue coverage, require about one year in the frame. The strong regenerative effect of the controlled tensile forces on soft tissues obviated the need for reconstructive surgery. No donor site morbidity related to plastic flaps or vascularised fibula transfer was incurred. Trifocal lengthening could be used for the two complications of regenerate open breakdown and regenerate premature consolidation, and the trifocal mode was used in this condition. Our study was intended to confirm occasional reports in the literature of the insurgence of an all layer ulceration of soft tissues after reamed nailing of closed tibial fractures. It is likely that the data we collected were insufficient for definitive correlation of such clinical pictures with thermal damage; nevertheless, the clinical problem exists, and if the damage factor is not related to heat transmission, other mechanisms of tissue injury that occur in conjunction with the reaming procedure must be identified. Finally, we are aware that most of the patients had diabetes mellitus in the osteocutaneous necrosis group. Osteocutaneous thermal necrosis is a rare complication of intramedullary reaming and fixation, probably related to injudicious reaming techniques combined with a number of potential local and host factors, especially patients with poor vascular perfusion such as diabetics with arthrosclerosis. We believe that preoperative scanning of high risk groups can identify the potential complication and be effectively managed by the use of Ilizarov bone transport techniques [2, 3, 14].
Acknowledgments
We thank John Birch, MD, from the Texas Scottish Rite Hospital for Children in Dallas, Texas, USA, for great care in providing excellent revision writing.
Conflict of interest statement None of the authors have received financial support related to this study. This study was approved by the ethical review boards of A. Manzoni Hospital (Lecco), Menaggio Hospital (Como), Banaras Hindu University (Varanasi), Benha Medical School (Egypt) and Niguarda Hospital (Milan).
References
- 1.Baumgart F, Kohler G, Ochsner PE. The physics of heat generation during reaming of the medullary cavity. Injury. 1998;29(Suppl 2):B11–B25. doi: 10.1016/S0020-1383(98)80058-2. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo R, Catagni M, Johnson EE. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop Relat Res. 1992;280:143–152. [PubMed] [Google Scholar]
- 3.El-Alfy B, El-Mowafi H, El-Moghazy N (2010) Distraction osteogenesis in management of composite bone and soft tissue defects. Int Orthop 34(1):115–118. doi:10.1007/s00264-008-0574-3 [DOI] [PMC free article] [PubMed]
- 4.Frolke JP, Peters R, Boshuizen K, Patka P, Bakker FC, Haarman HJ. The assessment of cortical heat during intramedullary reaming of long bones. Injury. 2001;32:683–688. doi: 10.1016/S0020-1383(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 5.Ganger R, Radler C, Speigner B, Grill F (2009) Correction of post-traumatic lower limb deformities using the Taylor spatial frame. Int Orthop. doi:10.1007/s00264-009-0839-5 [DOI] [PMC free article] [PubMed]
- 6.García OG, Mombiela FL, Fuente CJ, Aránguez MG, Escribano DV, Martín JV. The influence of the size and condition of the reamers on bone temperature during intramedullary reaming. J Bone Joint Surd Am. 2004;86:994–999. doi: 10.2106/00004623-200405000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis PV, Snowden S, Matthews SJ, Smye SW, Smith RM. Temperature rise during reamed tibial nailing. Clin Orthop Relat Res. 2002;395:255–261. doi: 10.1097/00003086-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Giannoudis PV, Snowden S, Matthews SJ, Smye SW, Smith RM. Friction burns within the tibia during reaming. Are they affected by the use of a tourniquet? J Bone Joint Surg Br. 2002;84:492–496. doi: 10.1302/0301-620X.84B4.12563. [DOI] [PubMed] [Google Scholar]
- 9.Henry HSL, Adcock RA, Fraunhofer JA, Seligson D. Heat of intramedullary reaming. South Med J. 1987;80:173–176. doi: 10.1097/00007611-198702000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Karunakar MA, Frankenburg EP, Le TT, Hall J. The thermal effects of intramedullary reaming. J Orthop Trauma. 2004;18:674–679. doi: 10.1097/00005131-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Leunig M, Hertel R. Thermal necrosis after tibial reaming for intramedullary nail fixation. A report of three cases. J Bone Joint Surg Br. 1996;78:584–587. [PubMed] [Google Scholar]
- 12.Muller C, McIff T, Rahn BA, Pfister U, Weller S. Intramedullary pressure strain on the diaphysis and increase in cortical temperature when reaming the femoral medullary cavity: a comparison of blunt and sharp reamers. Injury. 1993;24(Suppl 3):S22–S30. doi: 10.1016/0020-1383(93)90003-O. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner PE, Baumgart F, Kohler G. Heat-induced segmental necrosis after reaming of one humeral and two tibial fractures with a narrow medullary canal. Injury. 1998;29(Suppl 2):B1–B10. doi: 10.1016/S0020-1383(98)80057-0. [DOI] [PubMed] [Google Scholar]
- 14.Paley D, Catagni MA, Argnani F, Villa A, Benedetti GB, Cattaneo R. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989;241:146–165. [PubMed] [Google Scholar]
- 15.Saldua NS, Kuhn KM, Mazurek MT. Thermal necrosis complicating reamed intramedullary nailing of a closed tibial diaphysis fracture: a case report. J Orthop Trauma. 2008;22(10):737–741. doi: 10.1097/BOT.0b013e31818ccddf. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JE, Weil YA, Khoury A, Liebergall M, Mosheiff R (2009) Thermal tibial osteonecrosis: A diagnostic challenge and review of the literature. Injury. doi:10.1016/j.injury.2009.09.041 [DOI] [PubMed]


