Abstract
Static magnetic fields are a type of electromagnetic fields used in clinical practice. To ascertain what effect a static magnetic intramedullary device implanted in the rabbit femur had on fracture healing, 20 male New Zealand white rabbits with magnetic/nonmagnetic intramedullary implants were examined histologically, radiologically and for bone mineral density. Three groups were constituted according to the poles of the magnets. During surgery the intramedullary device was driven into the medulla. A femoral osteotomy was created with a mini Gigli wire at the centre point of the rod. Radiographs were obtained at the second and fourth weeks. Histological examination and bone mineral density were evaluated at the fourth week. The results of this study verified that an intramedullary implant with a static magnetic field improves bone healing in the first two weeks radiologically and that the configuration difference in magnetic poles has an effect on bone quality. Static magnetic fields have minor effects on bone mineral density values.
Introduction
The use of specific electromagnetic fields for the treatment of nonunions is well known [2, 14, 20]. The union rates of ununited fractures treated with pulsed electromagnetic fields (PEMF) are greater than 80% [1, 11, 20]. Originally, the use of electricity to stimulate osteogenesis was based on the idea of simulating the natural endogenous streaming potentials in bone with microampere currents applied directly via electrodes or induced by external electromagnetic fields [3].
Static magnetic fields (SMF) are another type of electromagnetic field used in clinical practice [9, 19]. Animal experiments have shown that long-term local SMF stimulation by permanent magnets can increase bone strength [6]. There is evidence that SMF affect osteoblastic maturation by up-regulating local factors [14]. There are some potential advantages for SMF. When a permanent magnet is used for stimulation, a power device supplied by external energy is not necessary. To complicate matters additionally, bone tissue invariably is surrounded by muscle tissue, the contraction of which also induces relatively large electric fields in the underlying bone tissue. Therefore, to ascertain what effect a static magnetic intramedullary device implanted in the rabbit femur has on healing fracture, it should be evaluated histologically, radiologically and for bone mineral density. This article is the first report of an intramedullary implant with static magnetic field.
Materials and methods
Experimental groups
The rabbit model was selected and the protocol was reviewed and approved by the Animal Care and Use Committee. Twenty male New Zealand white rabbits weighing approximately 3,930 g (range 3,750–4,250 g) were obtained. All rabbits were quarantined for 14 days and examined by a veterinarian to eliminate those with skeletal defects or poor health. Anteroposterior and lateral radiographs of the rabbit femurs were obtained. The intramedullary width and length was measured to design the intramedullary nail for the rabbit femur. The mean femoral length was 55 mm and the mean diameter of femur was 4 mm. The rabbits which did not fit this length and diameter were excluded and new rabbits were added to the study.
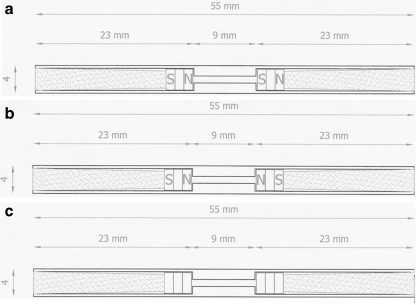
Three groups were constituted according to the poles of the magnets. The magnets were placed south-north versus south-north in group 1, and south-north versus north-south in group 2. Group 3 was a nonmagnetic control group without magnets in the rods (Fig. 1). The poles of the magnets were marked on the rods with a laser writer. The magnetic fields of the rods were measured individually, and the ones that did not have the magnetic strength mentioned were excluded from the study.
Fig. 1.
Technical drawings of the intramedullar rods in group 1 (a), group 2 (b), and group 3 (c)
Preparation of the magnetic intramedullary device
Stainless, 316 L steel rods with an outer diameter of 4 mm and inner diameter of 1 mm were prepared. The rods were drilled 23 mm with a 3.2 mm drill from both sides. Samarium Cobalt magnets, 3 mm in diameter, were placed on the outer ends of the rods. The magnets were inserted into the end of the tunnel from both sides. A Gaussmeter was used to measure the magnetic fields which were found to be 220–260 G. The magnets were secured by a teflon material inserted from both ends of the rods (Fig. 1).
Surgical technique
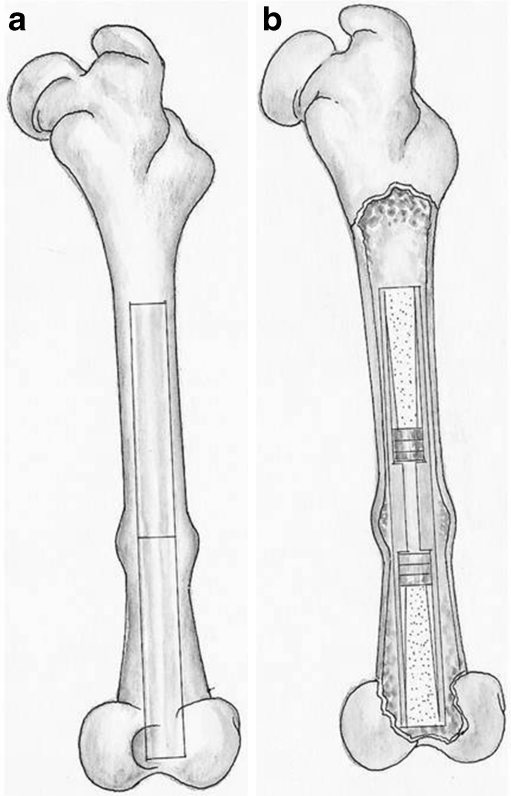
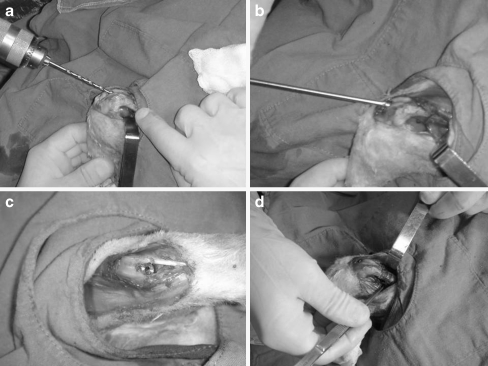
The rabbits were reared and the experiments were conducted at the animal research centre of our university hospital. The rabbits were anaesthetised with 35 mg/kg Ketamin and 5 mg/kg Xsilazin. The left leg of the rabbit was shaved, prepared with povidone-iodine solution, and draped for surgery. The surgery was performed under sterile conditions. A longitudinal skin incision was made on the antero-lateral aspect of the patella. After retraction of the patella to the medial side, a distal anterior entrance to the medulla of the femur was prepared. Two drills with diameters of 2.3 and 4 mm were sequentially used to prepare the medullary canal. A curette was used to empty the medullary canal. The rod was driven into the medulla. A femoral osteotomy was created with a mini Gigli wire at the centre point of the rod. The gap at the osteotomy site was the width of the Gigli wire (approximately 1.0 mm). The osteotomy site was washed with sterile saline, and the periosteum and skin were closed with 3–0 polyglycolic acid sutures (Fig. 2). The wound was dressed at the end of the procedure. Cephalotin (40 mg/kg) was given 30 minutes preoperatively and intramuscularly twice daily for 48 hours postoperatively. All rabbits were permitted immediate unrestricted weight-bearing in a standard sized rabbit cage after surgery. Radiographs were taken postoperatively and every two weeks thereafter for the duration of the study (Fig. 3).
Fig. 2.
Illustration of the implanted rods
Fig. 3.
Surgical implantation of the rods. a Drilling the medulla. b Implanting the rod. c Final position of the rod. d Osteotomy line
Method of examination
After euthanasia with an overdose of intravenous Pentothal Sodium 100 mg/kg at the fourth week, femurs were removed and dissected free of soft tissue. Before measuring bone mineral density, each rod was removed carefully so as not to damage the femur.
Radiographic evaluation
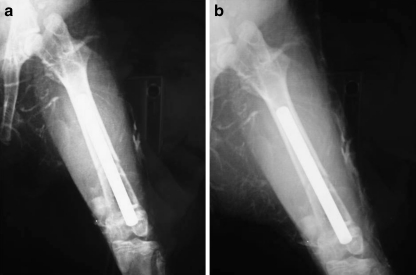
In this study, the following method for radiographic evaluation was used. Radiographs of each rabbit in the anteroposterior and lateral views were transmitted to an interfaced computer and then magnified eight times for measurement of cortical thickness by a digitiser. The degree of healing was assessed radiologically and histologically (Fig. 4). The radiographs were scored on a rating system from 0 through 6 points in the following manner: 0 points, no evidence of callus formation; 1–3 points, varying degrees of callus formation near the osteotomy site; 3–6 points, varying degrees of callus formation bridging the osteotomy site; 6 points, advanced union. All radiographs were scored by the principal investigator and a radiologist. When scores differed, they were averaged [10].
Fig. 4.
X-ray of the implanted femur in the second week (a) and fourth week (b)
Histological techniques
The specimens were removed and fixed in 10% phosphate buffered formalin solution and decalfication was performed by tissue decalcifier (Shandon TBD-1 Rapid Decalcifier, USA). The specimens were dehydrated in serial ethanol concentrations and embedded in paraffin. The specimen was cut with a band saw and thinned to a thickness of 3–5 µm with a grinding machine for microradiogram and stained with Masson's trichrom. Images were taken using a photomicroscope (Olympus BH 50, Tokyo, Japan). The specimens were blindly evaluated by a histologist and the assesment of the degree of the fracture healing was based upon the numeric grading system shown in Table 1. The parameters for the fracture healing were as follows: fibrous clot, 1 point; fibrous connecting tissue and cartilage, 2 points; cartilage only, 3 points; cartilage and cancellous bone, 4 points; cancellous bone, 5 points; compact bone, 6 points; and remodelled compact bone, 7 points. The parameters for the cartilage values were as follows: none, 0 points; healthy, small amount, 1 point; healthy, large amount, 2 points; hypertrophic, small amount, 3 points; hypertrophic, large amount, 4 points; and extensive resorption, 5 points. The parameters for the bone values were as follows: none, 0 points; thin trabecula, cartilage cores, 1 point; thin trabecula without cartilage, 2 points; thick lamellar trabecula, 3 points; compact bone, 4 points; and remodelled compact bone, 5 points. This method provides a systemic means for analysing the healing process and produces a general healing profile in which union represents the most general description of the fracture repair process [16].
Table 1.
Histological grading system used in evaluation of the samples [14]
| Histological evaluation | Numeric value |
|---|---|
| Fracture healing | |
| Fibrous clot | 1 |
| Fibrous connective tissue and cartilage | 2 |
| Cartilage only | 3 |
| Cartilage and cancellous bone | 4 |
| Compact bone | 6 |
| Remodelled compact bone | 7 |
| Cartilage values | |
| None | 0 |
| Healthy, small amount | 1 |
| Healthy, large amount | 2 |
| Hypertrophic, small amount | 3 |
| Hypertrophic, large amount | 4 |
| Extensive resorption | 5 |
| Bone values | |
| None | 0 |
| Thin trabecula, cartilage cores | 1 |
| Thin trabecula without cartilage | 2 |
| Thick lamellar trabecula | 3 |
| Compact bone | 4 |
| Remodelled compact bone | 5 |
Dual energy X-ray absorptiometric (DEXA) study
DEXA studies of femurs were done using a densitometer scan (L’accesorio Nucleare, Cerro Maggiore, Italy). The instrument includes an operating X-ray tube producing effective energies of 27 and 53 keV for a coupled beam. This model contains software that enables calculation of bone mineral content at any predetermined area. The specimens were positioned anteroposteriorly, and the bone mineral content was sampled in 2.0-mm increments transaxially. Bone mineral content and density osteotomy area of femurs were measured.
Statistical analysis
One-way ANOVA and Tukey Kramer multiple comparison tests were used for the statistical analysis. P < 0.05 was accepted as an indicator of a statistically meaningful difference.
Results
At four weeks, one of the rabbits had bilateral subtrochanteric femoral fracture, which was not associated with the osteotomy line. This rabbit was excluded from the study even though there was a callus formation at the osteotomy line. Three rabbits died during the intramedullary nailing process of the operation. Four rabbits died in the first postoperative week in their cages. Seven new rabbits were added to the groups. There was a fissure line from the osteotomy area to the proximal femur in two femurs which were well healed. All osteotomy lines were fused. No implant failure was detected.
Radiological findings
In the second week the mean radiological scores were 2.75 points (range 2–3 points, SD 0.46) in group 1, 3.25 points (range 3–4 points, SD 0.46) in group 2, and 1.75 points (range 1–3, SD 0.70) in group 3. There were statistically significant higher radiological scores in groups 1 and 2 compared to group 3 (p < 0.005). No significant difference was detected between groups 1 and 2.
At week four the radiological scores were 5.50 points (range 4–6, SD 0.75) in group 1, 6.0 points (SD 0) in group 2, and 5.62 points (range 5–6, SD 0.51) in group 3 (Table 2). No significant differences were detected between all groups at the fourth week.
Table 2.
Radiological scores by group
| Radiological scores | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Second week | 2.75a(range 2–3, SD 0.46) | 3.25a (range 3–4, SD 0.46) | 1.75 (range 1–3, SD 0.70) |
| Fourth week | 5.50 (range 4–6, SD 0.75) | 6.00 (SD 0) | 5.62 (range 5–6, SD 0.51) |
aThere were statistically higher scores in groups 1 and 2 at the second week (P < 0.005)
Histological findings
At four weeks, the osteotomies were consolidated in all groups with different stages. The mean histological fracture healing scores were 5.37 (range 4–7, SD 1.18) in group 1, 6.0 (range 4–7, SD 1.06) in group 2, and 4.62 (range 4–6, SD 0.74) in group 3. The cartilage values were 1.00 (range 0–4, SD 1.85) in group 1, 0.50 (range 0–4, SD 1.41) in group 2, and 2.0 (range 0–5, SD 2.20) in group 3. No statistically significant difference was detected between the three groups in fracture healing scores and cartilage values. Bone values were 3.12 (range 1–5, SD 1.55) in group 1, 4.12 (range 3–5, SD 0.83) in group 2, and 2.5 (range 1–4, SD 1.06) in group 3. The bone values in group 2 were statistically significantly higher than in groups 1 and 3 (Table 3).
Table 3.
Histological results by group
| Histological scores | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Fracture healing | 5.37 (range 4–7, SD 1.18) | 6.0 (range 4–7, SD 1.06) | 4.62 (range 4–6, SD 0.74) |
| Cartilage values | 1.0 (range 0–4, SD 1.85) | 0.50 (range 0–4, SD 1.41) | 2.0 (range 0–5, SD 2.20) |
| Bone values | 3.12 (range 1–5, SD 1.55) | 4.12 (range 3–5, SD 0.83) | 2.50 (range 1–4, SD 1.06) |
Dual energy X-ray absorptiometric (DEXA) findings
At four weeks, the mean bone scan was 0.35 g/cm2 (range 0.22–0.55 g/cm2, SD 0.10) in group 1, 0.31 g/cm2 (range 0.18–0.71 g/cm2, SD 0.17) in group 2, and 0.22 g/cm2 (range 0.11–0.44, SD 0.13) in group 3. Even though the DEXA scan values in groups 1 and 2 were higher than in group 3, no statistically significant difference was detected between the groups (Table 4).
Table 4.
Bone mineral density values
| Bone mineral density | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| DEXA score (g/cm2) | 0.35 (range 0.22–0.55) | 0.31 (range 0.18– 0.71) | 0.22 (range 0.11–0.44) |
DEXA scan values in groups 1 and 2 were higher than group 3
No statistically significant difference was detected between the groups
Discussion
Many factors have been investigated in relation to the influence of bone healing including electric stimulation, electromagnetic fields, piezoelectricity, ultrasound and mechanical factors such as intermittent tension, immobilisation, continuous passive motion and hormonal factors; most factors demonstrated limited effects in selected series, but none showed universal success [4, 5, 13]. The role of electromagnetic stimulation on delayed and nonunions of fractures is suggested by a large number of preclinical in vitro and in vivo studies which, collectively, show that exposure to electromagnetic energy stimulates the synthesis of extracellular matrix molecules [15, 18]. Magnetic field treatment is a therapeutic modality that has shown promise as a nonsurgical treatment for nonunions of long bones.
Static magnetic fields (SMF) are another type of electromagnetic field used in clinical practice [10, 21]. Although in vitro studies have shown that pulsed electromagnetic fields are more effective on bone formation than SMF, there are some potential advantages for SMF [19]. When a permanent magnet is used for stimulation, a power device supplied by external energy is not necessary. This makes SMF stimulation more suitable for long-term local healing. The major effects of SMF on osteoblast differentiation involve inducing membrane reorientation and distortion, which alter the diamagnetic isotropic properties [17, 20]. The SMF did not induce electric currents or movement of the charge. Because SMF are not dependant on electric energy, there are no heat and electric hazards on tissues. This makes SMF a potential orthopaedic tool for long-term local stimulation [23].
The results of the experiments suggest that there was an effect when an implant with a static magnetic field was applied. The healing was accelerated, initially at the second week, shown radiologically. But there was no difference at the fourth week radiologically. This may show that a static magnetic field is effective during the initial healing period. It seems likely that the total period of time required for union is not significantly altered by the magnetic effect radiologically. Hinsenkamp et al. also observed early significant mechanical and radiological effects on day 20 but no more statistical difference at day 45 on tibial rat osteotomy with PEMF [12]. The latest study by Cebrian et al. compared the use of electromagnetic fields in patients with pseudoarthrosis of tibia treated by intramedullary nailing with good clinical results. But in this study the treatment method was PEMF and not a static magnetic field. The effects of an intramedullary implant with static magnetic field have not been established. The difference in our study is the application of the magnetic field in an implant [7].
Generally, dual energy X-ray absorptiometry is accepted as a reliable measurement technique. It is more accurate than single or dual-photon absorptiometry and is recognised to reflect closely local material properties [8, 21]. Our report is the first describing temporary changes in bone mineral density determined using dual energy absorptiometry in combination with magnetic material. In this study, bone mineral content values were higher in groups 1 and 2. There was no statistically significant difference between the three experimental groups.
Histologically, in groups 1 and 3, the new bone had much thinner trabeculae—most with cartilaginous cores—than in group 2. Large blocks of cartilage were evident throughout the callus. In some instances, a cartilage plate was seen at the union site in the region of the uniting callus. This cartilage exhibited typical epiphyseal plate organisation with zones of resting, proliferating, hypertrophic, and calcifying cartilage, often at both the superior and inferior margins. New compact bone was strictly limited to the anchoring callus and was never extensively developed. No difference was detected between all groups in fracture healing and cartilage scores. In group 2 the bone score was higher than the other groups. This may show that the configuration of magnetic field is effective in bone quality. Actually bone strength is an important measure because it best describes the mechanical property and quality of bone [22]. Unfortunately, in this study mechanical bone strength was not evaluated nor were peak load, peak stress or modulus of elasticity. This is one of the short-comings of this study.
The results verified that an intramedullary implant with static magnetic field improves bone healing in the first two weeks radiologically and that the configuration difference in magnetic poles has an effect on bone quality. Static magnetic fields have minor effects on bone mineral density values.
References
- 1.Basset CAL, Mitchell SN, Gaston SR. Treatment of ununited tibial diaphyseal fractures with pulsing electromagnetic fields. J Bone Joint Surg. 1981;63:511–523. [PubMed] [Google Scholar]
- 2.Basset CAL, Mitchell SN, Hernandez E. Modification of fracture repair with selected pulsing electromagnetic fields. J Bone Joint Surg. 1982;64(A):888–895. [PubMed] [Google Scholar]
- 3.Bassett CAL. Beneficial effects of electromagnetic fields. J Cell Biochem. 1993;51:387–393. doi: 10.1002/jcb.2400510402. [DOI] [PubMed] [Google Scholar]
- 4.Brighton CT, Friedenberg ZB, Mitchell EI, Booth RE. Treatment of nonunion with constant direct current. Clin Orthop. 1977;124:106–123. [PubMed] [Google Scholar]
- 5.Brighton CT, Solomon R, Pollack SR. Treatment of recalcitrant non-union with a capacitatively coupled electrical field. J Bone Joint Surg Am. 1985;67:577–585. [PubMed] [Google Scholar]
- 6.Bruce GK, Howlett CR, Huckstep RL. Effect of a static magnetic field on fracture healing in rabbit radius: preliminary results. Clin Orthop Relat Res. 1987;222:300–306. [PubMed] [Google Scholar]
- 7.Cebrian J, Gallego P, Frances A, Sanches P, Manrique E, Marco F, Lopez- Duran L. Comparative study of the use of electromagnetic fields in patients with pseudoarthrosis of tibia treated by intramedullary nailing. Int Orthop. doi:10.1007/s00264-009-0806-1 [DOI] [PMC free article] [PubMed]
- 8.Cullum ID, Ell PJ, Ryder JP. X-ray dual-photon absorptiometry. Br J Radiol. 1989;62:587–592. doi: 10.1259/0007-1285-62-739-587. [DOI] [PubMed] [Google Scholar]
- 9.Darendeliler MA, Sinclair PM, Kusy RP. The effects of samarium cobalt magnets and pulsed electromagnetic fields on tooth movement. Am J Orthod Dentofacial Orthop. 1995;107:578–588. doi: 10.1016/S0889-5406(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 10.Haas WG, Lazarovici MA, Morrison DM. The effect of low frequency magnetic fields on the healing of the osteotomized rabbit radius. Clin Orthop Relat Res. 1979;145:245–251. [PubMed] [Google Scholar]
- 11.Gossling HR, Bernstein RA, Abbott J. Treatment of ununited tibial fractures: a comparison of surgery and pulsed electromagnetic fields (PEMF) Orthopedics. 1992;15:711–719. doi: 10.3928/0147-7447-19920601-08. [DOI] [PubMed] [Google Scholar]
- 12.Hinsenkamp M, Bourgois R, Basset CAL, Chiabrera A, Burny F, Ryaby J. Electromagnetic stimulation of fracture repair. Influence on healing of fresh fractures. Acta Orthop Belg. 1978;44:671–698. [PubMed] [Google Scholar]
- 13.Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing. A preliminary report. Clin Orthop. 1999;366:258–263. doi: 10.1097/00003086-199909000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Huang HM, Lee S-Y, Yao W-C, et al. Static magnetic fields up-regulate osteoblast maturity by affecting local differentiation factors. Clin Orthop Relat Res. 2006;447:201–208. doi: 10.1097/01.blo.0000203464.35561.be. [DOI] [PubMed] [Google Scholar]
- 15.Kotani H, Kawaguchi H, Shimoaka T, et al. Strong static magnetic stimulates bone formation to a definite orientation in vitro and in vivo. J Bone Miner Res. 2002;17:1814–1821. doi: 10.1359/jbmr.2002.17.10.1814. [DOI] [PubMed] [Google Scholar]
- 16.Marino AA, Cullen JM, Reichmanis M, Becker RO. Fracture healing in rats exposed to extremely low- frequency electric fields. Clin Orthop Relat Res. 1979;145:239–244. [PubMed] [Google Scholar]
- 17.McLeod KJ, Rubin CT. The effect of low-frequency electrical fields on osteogenesis. J Bone Joint Surg. 1992;74:920–929. [PubMed] [Google Scholar]
- 18.Parkinson WC. Comments on the use of electromagnetic fields in biological studies. Calcif Tissue Int. 1985;37:198–207. doi: 10.1007/BF02554842. [DOI] [PubMed] [Google Scholar]
- 19.Riley MA, Walmsley AD, Harris IR. Magnets in prosthetic dentistry. J Prosthet Dent. 2001;86:137–142. doi: 10.1067/mpr.2001.115533. [DOI] [PubMed] [Google Scholar]
- 20.Sharrard WJW, Sutcliffe ML, Robson MJ, Maceachern AG. The treatment of fibrous non-union of fractures by pulsing electromagnetic stimulation. J Bone and Joint Surg. 1982;64B(2):189–193. doi: 10.1302/0301-620X.64B2.6978339. [DOI] [PubMed] [Google Scholar]
- 21.Tiedeman JJ, Lippiello L, Connolly J, Strates BS (1990) Quantitative roentogenographic densitometry for assessing fracture healing. Clin Orthop 279–286 [PubMed]
- 22.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-K. [DOI] [PubMed] [Google Scholar]
- 23.Yan QC, Tomita N, Ikada Y. Effects of static magnetic field on bone formation of rat femurs. Med Eng Phys. 1998;20:397–402. doi: 10.1016/S1350-4533(98)00051-4. [DOI] [PubMed] [Google Scholar]