Abstract
Whether selective cyclo-oxygenase-2 (COX-2) inhibitors are equally effective compared to nonselective NSAIDs for the prevention of heterotopic ossification (HO) after total hip arthroplasty (THA) is still unclear. We carried out a comprehensive search strategy, in which only randomised controlled trials were included. Two reviewers independently assessed methodological quality and extracted outcome data. Analyses were performed using Stata version 10.0. Four eligible randomised controlled trials totalling 808 patients were included. Meta-analysis results showed that no statistically significant difference was found in overall incidence of HO (RR 1.08; 95% CI 0.71–1.64), incidence of moderate severe HO (Brooker II and III) (RR 0.83; 95% CI 0.48–1.42) and any grade of Brooker classification between two groups. In summary, the selective COX-2 inhibitors are equally effective as nonselective NSAIDs for the prevention of HO after THA. Considering the gastrointestinal side effects of nonselective NSAIDs, we recommend selective COX-2 inhibitors for the prevention of HO after THA. However, future well-designed, randomised controlled trials are still needed to further confirm our results.
Introduction
Heterotopic ossification (HO) is a frequent complication after surgical procedures such as total hip arthroplasty (THA) and acetabular trauma surgery. The exact aetiology of HO is still unclear. Many factors are associated with the incidence of HO, even the surgical approach [1]. The incidence has been reported as high as 60–75% without prophylaxis [2].
Many prophylaxis measures have been used, including radiotherapy, non-steroidal anti-inflammatory drugs (NSAIDs) and diphosphonates [3, 4]. Among them, NSAIDs have been recommended as a general prophylaxis after surgery [5]. However, the common gastrointestinal side effects of traditional NSAIDs trouble the patients and limit their application. The exact mechanism of NSAIDs on inhibition of HO is not exactly clear.
The cyclo-oxygenase (COX) enzyme includes two isoforms, COX-1 and COX-2. COX-1 is associated with gastrointestinal side effects of nonselective inhibitors of NSAIDs [6]. Selective COX-2 inhibitors seem without the disadvantages of gastrointestinal side effects associated with COX-1 inhibition. However, whether selective COX-2 inhibitors are equally effective compared to nonselective NSAIDs for the prevention of heterotopic ossification after THA is still unclear. Several randomised control trials have addressed this issue, but the results seem inconclusive [7–10].
In order to summarise available randomised control trials and make this issue clear, we performed a meta-analysis of available evidence comparing selective COX-2 inhibitors with nonselective COX inhibitors of NSAIDs for prevention of HO after THA.
Methods and materials
We searched Medline (1966–June 2009), Embase (1980–June 2009), Science Citation Index (1981–June 2009), Cochrane Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews (Cochrane Library, Issue 2, 2009) for randomised clinical trials that compared selective COX-2 inhibitors with nonselective COX-1 and COX-2 inhibitors in the prevention of HO after total hip replacement. We also searched for unpublished trials and those in progress using clinical trials repositories, including the National Institute of Health (June 2009), the National Research Register (June 2009), and Current Controlled Trials (June 2009). The following terms were used: “heterotopic ossification”, “heterotopic bone formation”, “total hip arthroplasty” and “fractures”. Searches were not restricted by year of publication or language. Reference lists of all included studies were scanned to identify additional potentially relevant studies. Two reviewers independently screened the titles and abstracts of identified papers, and full text copies of all potentially relevant studies were obtained.
Study selection and outcomes
We included studies if they were randomised trials of the selective COX-2 inhibitor compared with the nonselective COX-1 and COX-2 inhibitors in the prevention of HO, regardless of the daily dose and duration of inhibitors.
The extent of HO was graded according to the classification of Brooker et al. [11] as follows:
Grade 0: no ossification
Grade I: islands of bone in the soft tissues about the hip
Grade II: bone spurs from the pelvis or proximal end of the femur, with at least 1 cm between opposing bone surfaces
Grade III: bone spurs from pelvis or proximal end of the femur, reducing the space between opposing bone surfaces to less than 1 cm
Grade IV: apparent bone ankylosis of the hip
The primary outcome was the incidence of HO according to Brooker’s classification. The secondary outcomes were gastrointestinal side effects and hip joint function.
Data extraction
Two reviewers independently extracted information concerning trial characteristics, patient data, outcome measures, and study quality using a standardised protocol and reporting document. Disagreements were resolved by consensus. To quantify the level of agreement between reviewers, a κ statistic was calculated. The κ statistic is a chance-corrected proportional index, with values ranging from +1 (perfect agreement) to −1 (complete disagreement). Information extracted included personal information, methodology, details on interventions, and reported outcomes.
Study quality assessment
We assessed the methods of every study according to the Cochrane Handbook for Systematic Reviews of Interventions, including reporting of the randomisation method, allocation concealment, blinding of outcome assessment, and completeness of follow-up.
Statistical analysis
The meta-analysis was done in line with recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-analyses guidelines (QUOROM) [12] with standard software (Stata version 10.0) [13]. Analyses were on an intention-to-treat basis. Heterogeneity was assessed with I2 statistics [14]. I2 is the proportion of total variation observed between the trials which is attributable to differences between trials rather than sampling error (chance). Relative risk (RR) was used as the summary statistic to perform statistical analysis of dichotomous variables. A fixed-effect model was used for calculations of summary estimates and their 95% CI. However, when the heterogeneity was significant, a random-effects model was used.
Results
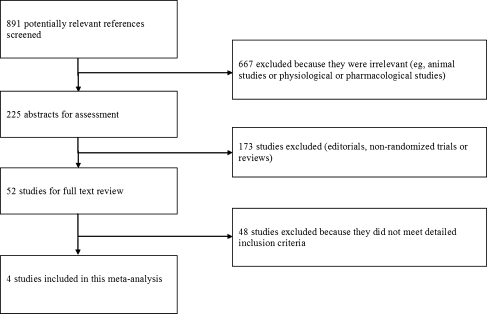
Of the 891 references screened, four randomised controlled trials were included in the final analysis (Fig. 1) [7–10]. Tables 1 and 2 summarise the baseline characteristics and quality assessment, respectively. All the included studies compared selective COX-2 inhibitors with nonselective COX inhibitors for prevention of HO after total hip arthroplasty, which involved 808 patients. Among the included patients, 22 patients from the study of Barthel et al. [7], 12 patients from the study of van der Heide et al. [8], four patients from Grohs et al. [9] and ten patients from Saudan et al. [10] failed to finish the study due to discontinuation of medication or were lost to follow-up; none of these patients were included in the final analysis in respective studies. In the study of Barthel et al. [7], assignment of patients to the 7.5 mg meloxicam group was discontinued due to high incidence of HO (8/24) after six months; thus this group was not comparable (the other group had a follow-up for 12 months). We excluded this group in our meta-analysis. Therefore, 736 patients were included in the final analysis. All groups in the included studies were comparable. All the studies evaluated HO with Brooker’s classification. After adjustment for chance concerning the agreement between reviewers, the κ coefficient on the agreement of the included studies was 0.89 (95% CI 0.68–0.94), suggesting good agreement between reviewers in data extraction.
Fig. 1.
Selection of studies
Table 1.
| Study (country) | Year | Total (n) | Mean age (y) | Setting | Selective COX-2 inhibitor | Nonselective COX inhibitor | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose (per day) | Duration of treatment (days) | Drug | Dose (per day) | Duration of treatment (days) | |||||
| Barthel et al. [7] (Germany) | 2002 | 272 | 63 | THA | Meloxicam | 7.5 mg and 15 mga | 14 | Indomethacin | 2 × 50 mg | 14 |
| Van der Heide et al. [8] (Netherlands) | 2007 | 186 | Not reported | THA | Rofecoxib | 2 × 25 mg | 7 | Indomethacin | 3 × 50 mg | 7 |
| Grohs et al. [9] (Austria) | 2007 | 100 | 60 | THA | Rofecoxib | 1 × 25 mg | 7 | Indomethacin | 2 × 25 mg + 1 × 50 mgb | 7 |
| Saudan et al. [10] (Switzerland) | 2007 | 250 | 69.5 | THA | Celecoxib | 2 × 200 mg | 10 | Ibuprofen | 3 × 400 mg | 10 |
THA total hip arthroplasty
a Two groups received meloxicam with 7.5 mg and 15 mg, respectively
b 100 mg per day in doses of 25, 25, and 50 mg
Table 2.
| Study | Randomization | Allocated concealment | Blinding | Length of follow-up | Withdrawal or lost to follow-up (%) |
|---|---|---|---|---|---|
| Barthel et al. [7] | Adequate | Unclear | Unclear | 12 months | 7 |
| Van der Heide et al. [8] | Adequate | Adequate | Adequate | 12 months | 6 |
| Grohs et al. [9] | Adequate | Adequate | Adequate | 15 months | 4 |
| Saudan et al. [10] | Adequate | Adequate | Unclear | 3 months | 4 |
The results of the comparison between these two groups (selective COX-2 inhibitor vs. nonselective COX inhibitor) as as shown in Table 3.
Table 3.
Meta-analysis results
| Outcomes | Number of studies | Number of participants (n) | Test of homogeneity | RR (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| S-COX-2 | NS-COX | I2(%) | P value | ||||
| Overall incidence of HO | 4 | 113/367 | 122/369 | 64.5 | 0.04 | 1.0 (0.80, 1.24)a | 0.98a |
| 64.0 | 0.04 | 1.08 (0.71, 1.64)b | 0.73b | ||||
| Incidence of moderate severe HO ( Brooker II and III) | 4 | 22/367 | 28/369 | 27.0 | 0.25 | 0.83 (0.48, 1.42)a | 0.49a |
| Incidence of Brooker I | 4 | 90/367 | 94/369 | 55.4 | 0.08 | 1.02 (0.79, 1.32)a | 0.89a |
| 54.5 | 0.09 | 1.08 (0.69, 1.68)b | 0.74b | ||||
| Incidence of Brooker II | 3 | 13/250 | 10/246 | 0.0 | 0.38 | 1.32 (0.59, 2.96)a | 0.50a |
| Incidence of Brooker III | 3 | 3/250 | 2/246 | 30.6 | 0.23 | 1.43 (0.29, 7.13)a | 0.66a |
| Incidence of Brooker IV | 4 | 1/367 | 0/369 | NA | NA | 3.19 (0.13, 76.36)a | 0.47a |
HO heterotopic ossification, S-COX-2 selective COX-2 inhibitor, NS-COX nonselective COX inhibitor, NA not available
a Fixed-effect model
b Random-effects model
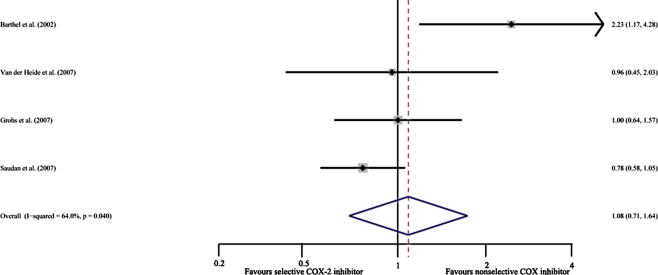
Overall incidence of HO
This result was based on four studies [7–10] (736 participants). Because the examinations of overall incidence of HO were heterogeneous among the included trials, we used a random effects model. The pooled RR was 1.08 (95% CI 0.71–1.64). This result was not statistically significant (P = 0.73) (Fig. 2).
Fig. 2.
Overall incidence of heterotopic ossification (HO) in selective COX-2 inhibitor prophylaxis group and nonselective COX inhibitor prophylaxis group
Incidence of moderate severe HO (Brooker II and III)
This result was based on four studies [7–10] (736 participants). The test for homogeneity showed that results were consistent across trials (P = 0.25, I2 = 27.0%). Meta-analysis showed the pooled RR was 0.83 (95% CI 0.48–1.42). This result was not statistically significant (P = 0.49).
Incidence of Brooker I HO
This result was based on four studies [7–10] (736 participants). Because the examinations of overall incidence of HO were heterogeneous among the included trials, we used a random effects model. The pooled RR was 1.08 (95% CI 0.69–1.68). This result was not statistically significant (P = 0.74).
Incidence of Brooker II HO
This result was based on three studies [7–9] (496 participants). The test for homogeneity showed that results were consistent across trials (P = 0.38, I2 = 0.0%). Meta-analysis showed the pooled RR was 1.32 (95% CI 0.59–2.96). This result was not statistically significant (P = 0.50).
Incidence of Brooker III HO
This result was based on three studies [7–9] (496 participants). The test for homogeneity showed that results were consistent across trials (P = 0.23, I2 = 30.6%). Meta-analysis showed the pooled RR was 1.43 (95% CI 0.29–7.13). This result was not statistically significant (P = 0.66).
Incidence of Brooker IV HO
This result was based on three studies [7–9] (496 participants). There was only one study reporting one case of Brooker IV HO. Meta-analysis showed RR 3.19 (95% CI 0.13–76.36). This result was not statistically significant (P = 0.47).
Gastrointestinal side effects
Across the included studies, 16 patients receiving nonselective COX inhibitors (4.4%) discontinued treatment because of gastrointestinal side effects, whereas ten patients in the selective COX-2 inhibitor group (2.7%) discontinued for gastrointestinal side effects.
Hip joint function
Only one study [9] reported the hip joint function according to the Harris hip score, though it only provided median score and range. At 12-month follow-up, the median Harris hip score was 100 (63–100) in the selective COX-2 inhibitor group and 96 (46–100) in the nonselective COX inhibitors group. No correlation between Harris hip score and grade of ossification was found.
Discussion
The main objective of this meta-analysis was to assess whether selective COX-2 inhibitors were equally effective compared to nonselective NSAIDs for the prevention of HO after THA. Meta-analysis results showed that no statistically significant difference was found in overall incidence of HO, incidence of moderate severe HO (Brooker II and III) and any grade of Brooker classification between the two groups.
The prophylactic effect of traditional (nonselective) NSAIDs on HO after THA has been proven [5]. However, the main disadvantages are well-known gastrointestinal side effects, including gastric perforations, ulcers and bleeding. In order to reduce side effects, the period of prophylaxis has been shortened from six weeks to two weeks, and even one week, all with similar results [15, 16]. However, side effects were still frequently observed [17]. There are three types of gastric side effects: symptoms, endoscopic findings and complications. With short-term prophylactic use of NSAID, we expected the side effects of NSAID to be limited to mainly symptoms and superficial erosions. Gastro protection is COX-1 mediated, so selective COX-2 inhibitors provide a promising approach. Our analysis was consistent with the above hypothesis. In the COX-2 selective inhibitor group, only 2.7% of patients discontinued their medication due to gastrointestinal side effects.
Increasing intraoperative blood loss is another problem of traditional nonselective NSAIDs. Kristensen et al. [18] found that the use of indomethacin increased perioperation blood loss, which was mainly due to the reduction in platelet aggregation. Weber et al. [19] found that perioperative blood loss in the meloxicam group (selective COX-2 inhibitor group) was 17% less than that observed in the indomethacin group. So this is another advantage of selective COX-2 inhibitors.
There was rising concern about the effects of this class of drugs on fracture repair and bone ingrowth in both bone grafts and uncemented prostheses. There was both experimental and clinical evidence of inhibition of new bone formation by NSAIDs (both selective and non-selective) [20, 21]. However, a study in a bone chamber model in goats found no difference in bone ingrowth when comparing goats treated with either meloxicam or ketoprofen (nonselective NSAIDs) and goats not receiving any NSAID [22]. Wurnig et al. [23] found comparable results six years after uncemented THA implantation in two groups—one receiving indomethacin, the other not receiving any NSAID. So the effect of NSAIDs on bone ingrowth appears to be limited. Besides, some studies have suggested that long-term prescription of selective COX-2 inhibitors increased the risk of cardiovascular events [24]. The use of these drugs for prophylaxis of HO was mainly short term, so the risk of cardiovascular events would be extremely low.
Thus, we can see that the therapeutic effects of NSAIDs are largely the result of inhibition of the enzyme COX-2, whereas adverse effects are primarily due to the inhibition of COX-1. Our meta-analysis confirmed that the selective COX-2 inhibitors were equally effective as nonselective NSAIDs for the prevention of HO after THA. With fewer side effects than nonselective NSAIDs, the compliance of patients may be better in the selective COX-2 inhibitors treatment group.
All meta-analyses are subject to potential bias because of systematic and random errors [25]. Our meta-analysis of randomised controlled trials may have several limitations. First, the number of included studies and participants was relatively small. Second, two included studies [7, 9] used mucoprotection, so the gastrointestinal side effects were rare in both groups. The comparison of drugs safety was limited. Third, only one study [9] reported the hip joint function using the Harris Hip Score. Functional analyses could provide complementary clinical information on the impact of HO. Unfortunately, there was not sufficient information for meta-analysis.
In summary, although there are some limitations in our meta-analysis, based on available data, the selective COX-2 inhibitors are equally effective as nonselective NSAIDs for the prevention of HO after THA. Considering the side effects of traditional nonselective NSAIDs, we recommend selective COX-2 inhibitors for the prevention of HO after THA. However, future well-designed, randomised controlled trials are still needed to further confirm our results.
References
- 1.Guo JJ, Tang N, Yang HL, Qin L, Leung KS. Impact of surgical approach on postoperative heterotopic ossification and avascular necrosis in femoral head fractures: a systematic review. Int Orthop. 2009 doi: 10.1007/s00264-009-0849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt SA, Kjaersgaard-Andersen P, Pedersen NW, Kristensen SS, Pedersen P, Nielsen JB. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. A randomized, double-blind clinical trial. J Bone Jt Surg Am. 1988;70:834–838. [PubMed] [Google Scholar]
- 3.Thomas BJ. Heterotopic bone formation after total hip arthroplasty. Orthop Clin North Am. 1992;23:347–358. [PubMed] [Google Scholar]
- 4.Pakos EE, Pitouli EJ, Tsekeris PG, Papathanasopoulou V, Stafilas K, Xenakis TH. Prevention of heterotopic ossification in high-risk patients with total hip arthroplasty: the experience of a combined therapeutic protocol. Int Orthop. 2006;30:79–83. doi: 10.1007/s00264-005-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal BC, Rodgers A, Clark T, Gray H, Reid IR, Dunn L, MacMahon SW. A systematic survey of 13 randomized trials of non-steroidal anti-inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand. 2000;71:122–128. doi: 10.1080/000164700317413076. [DOI] [PubMed] [Google Scholar]
- 6.Rahme E, Barkun AN, Adam V, Bardou M. Treatment costs to prevent or treat upper gastrointestinal adverse events associated with NSAIDs. Drug Saf. 2004;27:1019–1042. doi: 10.2165/00002018-200427130-00004. [DOI] [PubMed] [Google Scholar]
- 7.Barthel T, Baumann B, Noth U, Eulert J. Prophylaxis of heterotopic ossification after total hip arthroplasty: a prospective randomized study comparing indomethacin and meloxicam. Acta Orthop Scand. 2002;73:611–614. doi: 10.1080/000164702321039543. [DOI] [PubMed] [Google Scholar]
- 8.Heide HJ, Rijnberg WJ, Sorge A, Kampen A, Schreurs BW. Similar effects of rofecoxib and indomethacin on the incidence of heterotopic ossification after hip arthroplasty. Acta Orthop. 2007;78:90–94. doi: 10.1080/17453670610013475. [DOI] [PubMed] [Google Scholar]
- 9.Grohs JG, Schmidt M, Wanivenhaus A. Selective COX-2 inhibitor versus indomethacin for the prevention of heterotopic ossification after hip replacement: a double-blind randomized trial of 100 patients with 1-year follow-up. Acta Orthop. 2007;78:95–98. doi: 10.1080/17453670610013484. [DOI] [PubMed] [Google Scholar]
- 10.Saudan M, Saudan P, Perneger T, Riand N, Keller A, Hoffmeyer P. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement: a prospective randomised trial. J Bone Jt Surg Br. 2007;89:155–159. doi: 10.1302/0301-620X.89B2.17747. [DOI] [PubMed] [Google Scholar]
- 11.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Jt Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Bradburn M, Deeks J, Altman D. Sbe24: metan−an alternative meta-analysis command. Stata Tech Bull Reprints. 1998;8:86–100. [Google Scholar]
- 14.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 15.Cella JP, Salvati EA, Sculco TP. Indomethacin for the prevention of heterotopic ossification following total hip arthroplasty. Effectiveness, contraindications, and adverse effects. J Arthroplast. 1988;3:229–234. doi: 10.1016/S0883-5403(88)80020-2. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann S, Trnka HJ, Metzenroth H, Frank E, Ritschl P, Salzer M. General short-term indomethacin prophylaxis to prevent heterotopic ossification in total hip arthroplasty. Orthopedics. 1999;22:207–211. doi: 10.3928/0147-7447-19990201-09. [DOI] [PubMed] [Google Scholar]
- 17.Heide HJ, Koorevaar RT, Schreurs BW, Kampen A, Lemmens A. Indomethacin for 3 days is not effective as prophylaxis for heterotopic ossification after primary total hip arthroplasty. J Arthroplast. 1999;14:796–799. doi: 10.1016/S0883-5403(99)90027-X. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen SS, Pedersen P, Pedersen NW, Schmidt SA, Kjaersgaard-Andersen P. Combined treatment with indomethacin and low-dose heparin after total hip replacement. A double-blind placebo-controlled clinical trial. J Bone Jt Surg Br. 1990;72:447–449. doi: 10.1302/0301-620X.72B3.2111327. [DOI] [PubMed] [Google Scholar]
- 19.Weber EW, Slappendel R, Durieux ME, Dirksen R, Heide H, Spruit M. COX 2 selectivity of non-steroidal anti-inflammatory drugs and perioperative blood loss in hip surgery. A randomized comparison of indomethacin and meloxicam. Eur J Anaesthesiol. 2003;20:963–966. doi: 10.1097/00003643-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, Fox N, Genovese M, Regula D, Smith RL. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20:1164–1169. doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 21.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Jt Surg Am. 2007;89:500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 22.Heide HJ, Hannink G, Buma P, Schreurs BW. No effect of ketoprofen and meloxicam on bone graft ingrowth: a bone chamber study in goats. Acta Orthop. 2008;79:548–554. doi: 10.1080/17453670710015562. [DOI] [PubMed] [Google Scholar]
- 23.Wurnig C, Schwameis E, Bitzan P, Kainberger F. Six-year results of a cementless stem with prophylaxis against heterotopic bone. Clin Orthop Relat Res. 1999;361:150–158. doi: 10.1097/00003086-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 25.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]