Although oral chemotherapy is associated with ease of administration, it has the same exposure risks as intravenous formulations. However, the general misconception seems to be that exposure risk is low, therefore oral chemotherapeutic agents are safer to handle.
Abstract
Although there has been a significant increase in the availability and use of oral chemotherapeutic agents, the guidelines around their safe handling are still evolving. Although oral chemotherapy is associated with ease of administration, it has the same exposure risks to health care practitioners, patients, and their caregivers as intravenous formulations, and because it is administered in the home, to the families of patients. However, the general misconception appears to be that exposure risk is low and therefore oral chemotherapeutic agents present little risk and are safer to handle. In a series of three roundtable meetings, a team of international pharmacists from North America and Europe reviewed existing guidelines and identified gaps in recommendations that we believe are important for safe handling. The present article is a compilation of these gaps, especially applicable to manufacturers and distributors, storage and handling, and patient education regarding safe handling. These recommendations, on the basis of our experience and of best practices, provide an international perspective and can be adapted by institutions and practices for development of standardized procedures specific to their needs for the safe handling of oral chemotherapeutic agents.
Introduction
Traditionally, chemotherapy has been administered by intravenous infusion in an oncology inpatient unit or clinic or a physician's office. Over the past decade, however, self-administration of oral chemotherapy has increased because of the availability of novel therapeutic agents.1–3 Numerous advantages to the use of oral chemotherapy have been described, including increased control and convenience for the patient, potential increase in the quality of life, sustained medication exposure, and potential reduction in travel costs and use of health care resources.1,2,4 Despite these advantages, it is imperative to note that multiple factors associated with oral chemotherapy can compromise patient safety and contribute to medication errors, contamination, and inadvertent exposure to other individuals.5,6
Chemotherapy, because of its relatively narrow therapeutic index, is often associated with a greater risk of adverse events (AEs) than other medications, and when used in combination, may result in an even greater incidence of AEs.5,6 In contrast to administration in the institutional setting, where the prescribed medication, dose, regimen, and response to therapy are subject to several levels of assessment, patient or caregiver (defined as family members or friends who assist the patient) administration of oral chemotherapy is more likely to be susceptible to errors, nonadherence, and increased AEs as a result of a lack of coordinated care. Although there are no publications comparing chemotherapy errors that occur with oral versus intravenous administration, known issues with oral administration include incorrect dosing and limited monitoring, which can lead to underdosing or overdosing, serious toxicity, morbidity, and mortality.5–8 In addition, patient nonadherence to oral chemotherapy is a significant problem, which is less of a concern with parenteral therapy given in an institutional setting under the supervision of health care professionals.9,10 Finally, AEs may be difficult to monitor with the personal administration of oral chemotherapy if fewer clinic visits are needed for drug administration purposes; thus, it is crucial to inform the patient of the known AE profile associated with the medication.11
Accidental exposure to oral chemotherapeutic agents can occur at various stages during handling (ie, transport, unpacking, storage, handling, administration, and disposal).12–14 Thus, guidelines for safe and appropriate handling across the health care continuum are imperative. Some of the existing recommendations to ensure the safe storage, prescribing, dispensing, administration, and disposal of cytotoxic oral chemotherapy drugs are listed in the Appendix (online only).2,7,12,15–31 However, the recommendations have not been universally accepted or incorporated into practice. Recent surveys of health care practitioners as well as patients found that the perception of oral chemotherapy being safer than intravenous chemotherapy was prevalent.32,33 In addition, a survey of pharmacy directors of National Cancer Institute–designated cancer centers published in 2007 identified gaps in pharmacy practices, safety assessments, and prescribing methods and demonstrated the need for safe practice guidelines.34
During our review of existing guidelines, we found that none of the guidelines addressed all areas the panel deemed critical for the safe handling of oral chemotherapeutic agents. The purpose of the present article is to provide an outline of recommendations made by an international panel of pharmacists to address these critical areas that could serve as a starting point to build a framework for the safe storage, handling, administration, and disposal of oral chemotherapeutic agents for manufacturers and distributors, health care workers, and patients or their caregivers. We believe that these recommendations can be adapted by institutions and practices for development of standardized procedures specific to their needs regarding the safe handling of oral chemotherapeutic agents. We have focused on recommendations for oral chemotherapeutic agents; however, they may be applicable to all oral agents utilized in the treatment of cancer.
Methods
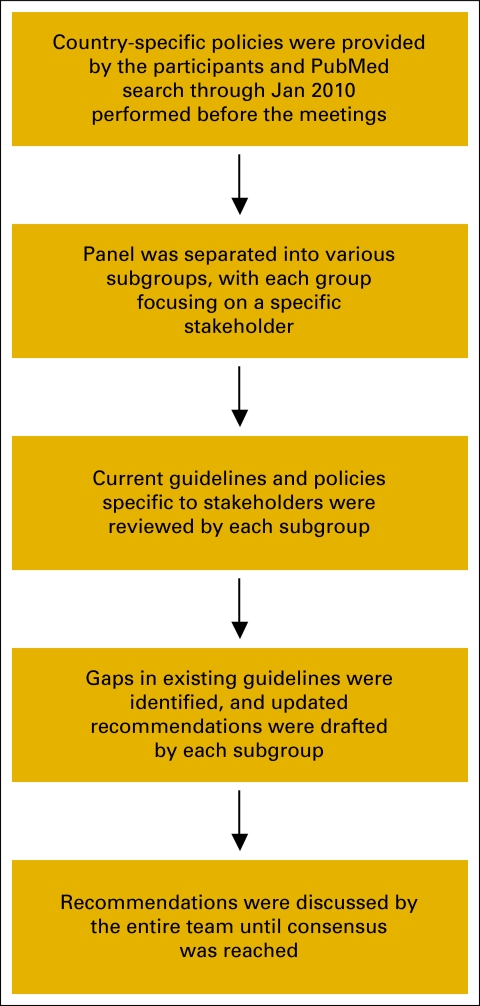
An expert panel comprising pharmacists from Austria, Canada, France, Germany, Spain, the United Kingdom, and the United States, representing hospital, community, ambulatory care, and specialty pharmacy, convened for a series of three roundtable meetings. Although the panel represented only one discipline, each pharmacist on the panel was chosen to represent a unique patient care setting. The participants were selected by the lead author on the basis of their active participation in international pharmacy societies as well as country-specific societies with a focus on oncology.
Before the meetings, representatives from each country provided any currently available recommendations for handling oral chemotherapy from their respective country, including guideline documents and institutional or country policies. In addition, a literature review was performed through PubMed to search for relevant publications and existing guidelines through January 2010. All the guidelines reviewed at the meetings are listed in the Appendix. In addition to existing guidelines, the panel also drew on best practices that were based on the professional experience of the panel members.
At the initial meeting, the panel formed subgroups. Each subgroup reviewed currently available guidelines for manufacturers, distributors, health care providers, and other individuals or groups involved in the handling of oral chemotherapeutic agents at various stages (stakeholders); identified the gaps in these existing guidelines; identified current best practices; and formulated recommendations for handling of oral chemotherapy drugs. The findings of each subgroup were presented to and commented on by the entire team. A working draft of recommendations for the safe handling of oral chemotherapeutic agents was developed and then discussed for consensus at subsequent meetings. Although no formal process was used to reach a consensus, discussion on each point was continued until consensus was reached, and in a few cases, until it was clear that no consensus would be reached. A flowchart of the methodology is shown in Figure A1 (online only). The goal was to develop a framework of recommendations that can be included in guidelines specific to individual institutions and practices.
Recommendations for Safe Handling
A number of stakeholders are involved in handling oral chemotherapeutic agents at various stages. Recommendations for safe handling by these stakeholders are outlined in the following sections.
Manufacturers and Distributors
There are well-defined regulations for manufacturers and distributors to ensure safe transport and handling of chemotherapy drugs. Although the initial step for safe handling of oral chemotherapy agents begins with the manufacturer, recommendations for manufacturers and distributors are not included in currently published safe handling guidelines (see Appendix for the list of guidelines), but the panel believes they play a pivotal role. Appropriate packaging could minimize the handling of chemotherapy drugs by health care providers and patients, thus contributing to safer handling. This includes clear labeling on the outside of the package indicating that the agent is cytotoxic. In addition, manufacturers should package only the amount of tablets or capsules needed for one cycle of therapy. Because new regimens are constantly being developed and approved, another approach for manufacturers is to use unit-of-use packaging, thereby reducing the need for packaging based on a cycle of therapy. Each of these steps will ensure limited handling of these agents. Finally, manufacturers are encouraged to develop a liquid formulation or provide information on compounding a liquid formation of their product. Additional recommendations for manufacturers and distributors are listed in Table 1. Health care professionals are encouraged to reinforce the importance of these points to stakeholders and regulatory agencies whenever possible.
Table 1.
Recommendations for Manufacturers and Distributors
| Consideration |
|---|
| Packaging and segregation |
| Effective packaging and segregation techniques should be used to avoid contamination prior to distribution. |
| Packaging should clearly state whether segregation techniques have been used so that individuals unpacking the medications can take additional precautions if necessary. |
| Packaging material should be durable, able to contain any accidental leakage during handling and transport, and tamper-proof. |
| Package label should indicate that the agent is cytotoxic (eg, size-appropriate modifications of the European Society of Oncology Pharmacy yellow hand, the Association paritaire pour la santé et la sécurité du travail du secteur sociale's “C” symbol”). |
| Distributors should ensure that the labeling on the packaging is intact and that oral cytotoxic agents are stored and transported separately from noncytotoxic agents. |
| Minimizing handling of oral chemotherapeutic agents |
| Manufacturers should provide the appropriate number of tablets or capsules per packing based on the amount needed for one cycle of therapy. If this approach is not an option, manufacturers should attempt to use unit packaging (ie, individual packaging for tablets or capsules). Additionally, based on treatment protocols for various diseases being treated and new data, manufacturers should consider preparing additional dosage strengths as appropriate. |
| Because many patients inherently, or as a result of their disease, have difficulty swallowing tablets or capsules, a liquid formulation, or information on how to compound a liquid formulation, should be provided by the manufacturer. |
| Educational materials |
| Manufacturers should provide educational material regarding safe handling to each stakeholder, including physicians, RNs, pharmacy personnel, patients, and caregivers. |
| Manufacturers should update patient education materials as new information becomes available. |
Health Care Providers
Health care providers have a major responsibility in ensuring safe handling of oral chemotherapeutic agents. Because of the significance of this responsibility, health care providers should be appropriately trained, ensure that their knowledge is current with developments in the field, and follow all applicable discipline-specific guidelines when handling oral chemotherapeutic agents. Because this panel consisted exclusively of pharmacists, our recommendations are focused on the roles typically undertaken by pharmacists, but they can be adapted to other health care professionals who perform similar roles, on the basis of systems existing in individual practice settings or medical centers.
Training and competencies.
As recommended in most safe handling guidelines, health care professionals should attend orientation programs and routine training courses specific to their roles. They should also complete competencies associated with these training programs, along with an accompanying assessment for licensing qualification if applicable. The training programs should be approved by an oncology organization or appropriate local organizations.35,36 In addition, within a health care institution, a primary educator should be established as a source of referral and continued education for training health care professionals on oral chemotherapy. This would ensure that patients receive consistent education, training, and monitoring across the multidisciplinary team.8,37
Health care workers should be trained and competent to treat individuals accidently exposed to chemotherapeutic agents and on the disposal of cytotoxic medications. All clinical staff who are likely to come in contact with oral chemotherapeutic agents or with waste from patients who have received these agents (eg, clerks, hygiene workers, and sanitation workers) should undergo appropriate training. The latter point of training non–health care professional staff was important to the panel because this recommendation is not included in the any guidelines. With the changing paradigm of oral chemotherapy, these individuals should be appropriately trained because the traditional systems of handling chemotherapy, and those involved, are more diverse with oral chemotherapy. A list of training recommendations for health care providers is shown in Table 2.
Table 2.
Recommendations for Health Care Providers
| Parameter |
|---|
| Storage |
| Proper storage and handling of oral chemotherapeutic agents should be ensured by health care professionals in order to prevent accidental exposure and to ensure the integrity of these medications. |
| In health care institutions and pharmacies, cytotoxic agents should be stored in a designated area per the manufacturer's instructions, and separate from noncytotoxic agents. |
| Some agents are air-, moisture-, and/or light-sensitive; therefore, storage specifications should be followed. |
| Handling |
| Correct use of personal protective clothing and equipment should be instituted to minimize exposure and health risks.13,14,21 |
| Oral chemotherapeutic agents should not be dispensed using automatic counting machines. |
| Disposable gloves should be used for dispensing. Hands must be washed before and after glove application. |
| Manipulations such as compounding, crushing, cutting, or splitting should be performed in a biological safety cabinet41 and should involve the use of personal protective equipment, which should be disposable to the extent possible. |
| Separate equipment should be used for cytotoxic and noncytotoxic agents. |
| The pharmacist (or other qualified professional) should attempt to limit additional handling of hazardous medications by other health care professionals. For oral chemotherapeutic agents in powder form, for instance, unit doses of the medications in the solution form (ie, reconstituted) should be prepared in the pharmacy and placed in an oral syringe, ready for administration. |
| Health care professionals who store and dispense oral chemotherapeutic agents must have a written emergency plan in the event of a spill or accidental exposure. It is recommended that annual spill simulation exercises be conducted. |
| An updated list of hazardous medications should be readily accessible to all health care personnel involved in handling of oral chemotherapeutic agents. |
| Disposal and cleaning of contaminated materials |
| All disposable protective clothing as well as any disposable materials used while handling oral chemotherapeutic agents should be disposed of as cytotoxic waste according to the local waste disposal regulatory guidelines. |
| All nondisposable materials exposed to chemotherapeutic agents including counting trays, tools, surfaces, etc should be washed or decontaminated* thoroughly after use. |
| Training and competencies for safe handling |
| Health care professionals should attend orientation programs and routine training courses specific to their roles, and should complete competencies associated with these training programs, along with an accompanying assessment for licensing qualification if applicable.35,36 |
| A primary educator within a health care institution should be established as a source of referral and continued education on oral chemotherapy for health care professionals, allowing for consistent education, training, and monitoring across the multidisciplinary team.8,37 |
| Health care workers involved in the handling of oral chemotherapeutic agents should be trained and competent to treat individuals accidently exposed to chemotherapeutic agents and on the disposal of cytotoxic medications. |
| All clinical staff who are likely to come in contact with oral chemotherapeutic agents or with waste from patients who have received these agents (eg, clerks, hygiene workers, and sanitation workers) should undergo appropriate training. |
In some European countries, limited data support the use of cleaning agents that are validated for the removal of cytotoxic agents.38
Storage and handling.
When handling oral chemotherapeutic agents, health care providers must adhere to good practice as defined by local standard operating procedures and national guidelines. Key recommendations are outlined in Table 2, many of which are targeted to retail pharmacies, particularly in the United States, because the panel felt there was a gap in practice in this setting.
Handling of oral chemotherapy by pregnant staff members initiated a broad discussion by the panel, and no consensus was reached regarding a recommendation. All members of the panel agreed the goal was to minimize or eliminate any role of pregnant staff in handling chemotherapy agents, oral or intravenous; however, this may not be feasible in all practice settings. Many panel members argued against a recommendation as long as appropriate protection (eg, gown, gloves) were used by pregnant staff.
Another issue for handling that generated a significant discussion was the cleaning of nondisposable materials exposed to chemotherapy drugs. This includes counting trays, tools, and surfaces. When intravenous chemotherapy drugs are mixed in a biologic safety cabinet, all the guidelines recommend terminal cleaning or cleaning with each shift. With the increase in the prescribing of oral chemotherapy, more pharmacies will be involved in fulfilling the prescription. Cleaning of the tools and surfaces exposed to these agents has been limited to washing the items and area thoroughly with soap and water, 70% alcohol, or sodium hypochlorite; in some settings, no cleaning occurs. The risk for contamination of other medications and patient exposure could be significant. Currently, there are limited options for cleaning of these surfaces, although in some European countries, limited data support the use of cleaning agents that have been validated for the removal of cytotoxic agents.38 The panel agreed further research is urgently needed to develop a valid, readily available, and affordable decontamination agent for use in the health care setting and the patient home.
Patient counseling.
Health care professionals should provide patients and caregivers with education and training to ensure their understanding of safe handling procedures as well as thorough knowledge of proper administration of all medications. Patient literature and other educational materials should be monitored and evaluated to ensure that current and accurate information is being delivered.
Patient consent for oral chemotherapy should be obtained. Patients should be consulted and assessed for their ability to take oral therapy and to comply with their treatment plan. Tools are available to assist with this evaluation.39 Patients should also be advised on all matters related to safe handling.
All current medications should be reviewed with the patient or caregiver to identify potential medication interactions or interference with dietary requirements, and clear dosing instructions should be provided, including what to do when a dose is skipped or when vomiting of a dose (spillage) occurs. During refill of prescriptions, any potential medication and food interactions must be reassessed and discussed with the patient or caregiver. The patient should be made aware of the required monitoring arrangements by being provided with access to the written protocol and treatment plan from the institution where the treatment was initiated. Patients who are pregnant or breast-feeding should be counseled on recommended medications and their risk-benefit profiles.
Patients and Caregivers
Recommendations for patients and caregivers are included in some guidelines but are limited in details, so the panel focused on these responsibilities and created a summary of dos and don'ts for patients that could be put into practice and provided to all patients (Table 3). Caregivers should understand all information given to patients, including the transport, storage, dispensing, and disposal requirements to ensure safe handling. They must work with the patient and health care provider to ensure appropriate dosing for patients in their care and report any treatment-related adverse effects. Caregivers who are pregnant, breast-feeding, or children should not handle any chemotherapy medications or waste products. Finally, to further ensure the safety of these individuals and others in the patient's home, guidelines from Australia and Canada recommend that patient's clothes and bed linen be handled with gloves and washed separately from other items and that toilets be double-flushed after use, during and 4 to 7 days after discontinuing chemotherapy.12,18 These recommendations are supported by a recent publication involving cyclophosphamide exposure that showed significant contamination on and around the toilet and that the use of gloves reduced personal contamination from changing bed linens one- to six-fold.40 Because drugs may be eliminated from the body as active or inactive metabolites in sweat, saliva, urine, or stool for five to seven half-lives, the panel agreed that these recommendations were important and should be implemented.
Table 3.
Specific Recommendations for Patients and Their Caregivers: Dos and Don'ts
| Dos for Oral Chemotherapy | Don'ts for Oral Chemotherapy |
|---|---|
| On receiving your prescription, review the package label, specifically checking medication name and dosage.27 | Leave medication in open areas, near sources of water, direct sunlight, or where they can be accessed by children or pets. |
| Ensure that you completely understand when and how to take the medication and ask questions if there is any confusion. | Store medications in the areas where food or drinks are stored or consumed. |
| Transport and store medicine as instructed and as outlined in the packaging label.27 | Crush, break, or chew tablets. |
| Use gloves if possible and wash hands thoroughly before and after glove application.* If gloves are not worn, tip tablets and capsules from their container/blister pack directly into a disposable medicine cup. | Double-up on doses, unless instructed by a health care professional. |
| Administer the medication as instructed. | Share prescriptions or medication. |
| Keep a journal of adverse effects. Make a list of adverse effects for which the health care professional has to be contacted immediately. | Assume that oral chemotherapy is safer than intravenous chemotherapy. |
| Consider using adherence devices. Use separate devices for cytotoxic and noncytotoxic agents. | Skip doses unless instructed by your physician. |
| Report any overdosing immediately. | Discard medication down the toilet or in the garbage. |
| Keep information ready for necessary action in the event of accidental exposure (including emesis and accidental ingestion).23,27 | |
| Return wet, damaged, unused, discontinued, or expired medications to the pharmacist or hospital for disposal.27 | |
| Report all medications (prescription and nonprescription as well as complementary and alternative medicines) and any specific dietary requirements to the health care provider/prescriber, at the time of assessment and consultation. Inform other health care professionals that you are on oral chemotherapy (eg, surgeons and dentists).42,43,45 | |
| Minimize the number of individuals coming in contact with the cytotoxic medications.27 | |
| Wash the patient's clothes and bed linen separately from other items.12,18* | |
| Double-flush the toilet after use, during use of and 4 to 7 d after discontinuing oral chemotherapy.12,18 |
It is recommended that caregivers wear gloves at all times while handling oral chemotherapeutic agents as well as contaminated items in order to minimize risk of exposure.
Summary
In this article, our international team of oncology pharmacists identified gaps in existing guidelines for the safe handling of oral chemotherapeutic agents, especially as it applies to manufacturers and distributors, storage and handling, and patient education. Although the limitations of our approach include informal methods of panel selection, the lack of a voting method for consensus agreement, and a nonsystematic literature review, there are significant strengths to the recommendations. First, our recommendations are relevant to multiple stakeholders, beginning with the manufacturer. In addition, although the panel was composed solely of pharmacists, this group has significant experience with safe handling of hazardous agents coupled with managing oral agents for all disease types. On the basis of our international experience and best practices, we compiled key recommendations to fill the gaps of existing guidelines. Therefore, this article, which provides an international perspective, is timely and is ideally suited to be a framework for the development of safe handling guidelines specific to individual institutions and practices.
Oral chemotherapy has distinct advantages and disadvantages compared with intravenous chemotherapy. Although the responsibility for safe handling and administration of oral chemotherapy ultimately lies with the patient or their caregiver, it is the responsibility of all members of the health care team to ensure they are informed regarding the safe and appropriate use of their chemotherapy. All stakeholders should follow established guidelines when handling oral chemotherapeutic agents and continually review and assess their standards and compliance with agreed procedures. In addition, all facilities that handle oral chemotherapy drugs should develop standard operating procedures that are specific to their practice.
Acknowledgment
This article is a result of roundtable meetings to discuss the safe handling of oral chemotherapeutic agents. Although the meetings were supported by Merck (formerly Schering Corp.), only the authors had control over the content of this article. Medical writing assistance was provided by Philip Reigan, PhD, and Meenakshi P. Subramanian, PhD, Evidence Scientific Solutions, which was supported by Merck.
Appendix
Figure A1.
Flowchart depicting the methodology involved in the development of the recommendations reported in this article.
List of Available Guidelines and Policies for Safe Handling of Oral Chemotherapeutic Agents
The US Department of Labor Occupational Safety and Health Administration technical manual15
Guidelines from the American Society of Health-System Pharmacists21
National Comprehensive Cancer Network (NCCN) Task Force Report on Oral Chemotherapy2
Recommendations from the National Institute for Occupational Safety and Health (NIOSH)17
American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards28
Association paritaire pour la santé et la sécurité du travail du secteur affaires sociales (ASSTSAS) document (Canada)12
Guidelines from the Canadian Association of Pharmacy in Oncology25
The European Society of Oncology Pharmacy (ESOP) declaration (http://www.esop.li/downloads/oral_antineoplastic_therapy.pdf)
National Patient Safety Agency. Risks of incorrect dosing of oral anti-cancer medications. NPSA/2008/RRR0017
The Merseyside & Cheshire Cancer Network guidance document (United Kingdom)20
The Society of Hospital Pharmacist of Australia Committee of Specialty Practice in Cancer Services. Standards of Practice for the Provision of Pharmaceutical Care of Patients Receiving Oral Chemotherapy for the Treatment of Cancer30
Guidelines for the Safe Prescribing, Dispensing and Administration of Cancer Chemotherapy; a consultative report prepared by Clinical Oncological Society of Australia, November 200826
The Grampians Integrated Cancer Service guidelines (Australia)18
The Management and Awareness of Risks of Cytotoxic Handling (MARCH) guidelines16
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan Goodin, Merck (C); Niesha Griffith, Merck (C); Karen Chuk, Merck (C); Mikael Daouphars, Merck (C); Christian G. Doreau, Merck, sanofi aventis (C), GlaxoSmithKline (C); Robert Terkola, Merck (C); Barbara Vadnais, Merck (C); Debbie Wright, Merck (C) Stock Ownership: None Honoraria: Beth Chen, Merck; Rinku A. Patel, Merck; Maria José Tamés, Merck; Debbie Wright, Merck Research Funding: None Expert Testimony: None Other Remuneration: Mikael Daouphars, Roche; Christian G. Doreau, LFB, Jannsen-Cilag
Author Contributions
Conception and design: Susan Goodin, Niesha Griffith, Mikael Daouphars, Christian G. Doreau, Rinku A. Patel, Rowena Schwartz, María José Tamés, Robert Terkola, Barbara Vadnais, Debbie Wright, Klaus H. Meier
Collection and assembly of data: Susan Goodin, Niesha Griffith, Beth Chen, Karen Chuk, Maria José Tamés, Robert Terkola, Barbara Vadnais
Data analysis and interpretation: Susan Goodin, Beth Chen, Karen Chuk, Christian G. Doreau, Rinku A. Patel, Maria José Tamés, Robert Terkola, Barbara Vadnais
Manuscript writing: Susan Goodin, Niesha Griffith, Karen Chuk, Mikael Daouphars, Rinku A. Patel, María José Tamés, Barbara Vadnais
Final approval of manuscript: Susan Goodin, Niesha Griffith, Beth Chen, Karen Chuk, Mikael Daouphars, Christian G. Doreau, Rinku A. Patel, Rowena Schwartz, María José Tamés, Robert Terkola, Barbara Vadnais, Debbie Wright, and Klaus Meier
References
- 1.Aisner J. Overview of the changing paradigm in cancer treatment: Oral chemotherapy. Am J Health Syst Pharm. 2007;64(suppl 5):4–7. doi: 10.2146/ajhp070035. [DOI] [PubMed] [Google Scholar]
- 2.Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):1–14. [PubMed] [Google Scholar]
- 3.Greco FA. Evolving role of oral chemotherapy for the treatment of patients with neoplasms. Oncology (Williston Park) 1998;12(suppl 4):43–50. [PubMed] [Google Scholar]
- 4.Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 5.Schulmeister L. Chemotherapy medication errors: Descriptions, severity, and contributing factors. Oncol Nurs Forum. 1999;26:1033–1042. [PubMed] [Google Scholar]
- 6.Cohen MR, Anderson RW, Attilio RM, et al. Preventing medication errors in cancer chemotherapy. Am J Health Syst Pharm. 1996;53:737–746. doi: 10.1093/ajhp/53.7.737. [DOI] [PubMed] [Google Scholar]
- 7.National Patient Safety Agency. Oral anti-cancer medicines: Risks of incorrect dosing. http://www.nrls.npsa.nhs.uk/resources/?entryid45=59880.
- 8.Blecher C, Barefoot J, Davis D, et al. A team approach toward promoting patient adherence to oral chemotherapy protocols. Oncol Nurs Forum. 2008;35:537. abstr 2985. [Google Scholar]
- 9.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 10.Escalada P, Griffiths P. Do people with cancer comply with oral chemotherapy treatments? Br J Community Nurs. 2006;11:532–536. doi: 10.12968/bjcn.2006.11.12.22424. [DOI] [PubMed] [Google Scholar]
- 11.Birner A. Pharmacology of oral chemotherapy agents. Clin J Oncol Nurs. 2003;7(suppl 6):11–19. doi: 10.1188/03.CJON.S6.11-19. [DOI] [PubMed] [Google Scholar]
- 12.Association paritaire pour la santé et la sécurité du travail du secteur affaires sociales (AASTSAS): Prevention guide: Safe handling of hazardous drugs. http://www.irsst.qc.ca/files/documents/PubIRSST/CG-002.pdf.
- 13.Sessink PJ, Bos RP. Drugs hazardous to healthcare workers. Evaluation of methods for monitoring occupational exposure to cytostatic drugs. Drug Saf. 1999;20:347–359. doi: 10.2165/00002018-199920040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Valanis B, Vollmer W, Labuhn K, et al. Occupational exposure to antineoplastic agents and self-reported infertility among nurses and pharmacists. J Occup Environ Med. 1997;39:574–580. doi: 10.1097/00043764-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Labor, Occupational Safety and Health Administration. OSHA Technical Manual, Section VI, Chapter 2. Washington, DC: 1999. Controlling occupational exposure to hazardous drugs. [Google Scholar]
- 16.TEVA Hospitals. Management and Awareness of Risks of Cytotoxic Handling (MARCH) guidelines: Safe handling of oral chemotherapy. http://marchguidelines.com/members/guidelines/PNF1_OralChemotherapy.aspx.
- 17.National Institute for Occupational Safety and Health. Atlanta, GA: 2004. Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH publication 2004-165. [Google Scholar]
- 18.Grampians Integrated Cancer Service, Grampians Regional Palliative Care Team. Clinical guidelines for the administration of oral chemotherapy agents in the community setting. http://www.gics.com.au/resources/ClinGuidelinesForOralChemoInCommunity.pdf.
- 19.Gallagher TH, Lucas MH. Perry MC, editor. Patients' and physicians' attitudes regarding disclosure of harmful medical errors. Am Soc Clin Oncol Ed Book. 2005:254–258. [Google Scholar]
- 20.Merseyside & Cheshire Cancer Network. Network guidance for safe prescribing, handling and administration of cytotoxic drugs. http://www.mccn.nhs.uk/userfiles/ documents/MCCN%20Safe%20Prescribing%20handling%20%20administration%20 of%20Cytotoxic%20Drugs_April06_revDec07_oral%20August%2008_vincOct08.pdf.
- 21.American Society of Health-Systems Pharmacists. ASHP guidelines on handling hazardous drugs. www.ashp.org/s_ashp/docs/files/BP07/Prep_Gdl_HazDrugs.pdf. [DOI] [PubMed]
- 22.Barbería E, Hernandez C, Miralles V, et al. Paediatric patients receiving oncology therapy: Review of the literature and oral management guidelines. Eur J Paediatr Dent. 2008;9:188–194. [PubMed] [Google Scholar]
- 23.Birner A. Safe administration of oral chemotherapy. Clin J Oncol Nurs. 2003;7:158–162. doi: 10.1188/03.CJON.158-162. [DOI] [PubMed] [Google Scholar]
- 24.Birner AM, Bedell MK, Avery JT, et al. Program to support safe administration of oral chemotherapy. J Oncol Pract. 2006;2:5–6. doi: 10.1200/jop.2006.2.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canadian Association of Pharmacy in Oncology. Standards of practice for oncology pharmacy in Canada, version 2. http://www.capho.org/docs/StandardsofPractice/StandardsofPracticeFORWEBV2Dprintable.pdf.
- 26.Carrington C, Stone L, Koczwara B, et al. The Clinical Oncological Society of Australia (COSA) guidelines for the safe prescribing, dispensing and administration of cancer chemotherapy. Asia Pac J Clin Oncol. 2010;6:220–237. doi: 10.1111/j.1743-7563.2010.01321.x. [DOI] [PubMed] [Google Scholar]
- 27.Griffin E. Safety considerations and safe handling of oral chemotherapy agents. Clin J Oncol Nurs. 2003;7(suppl 6):25–29. doi: 10.1188/03.CJON.S6.25-29. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson JO, Polovich M, McNiff KK, et al. American Society Of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol. 2009;27:5469–5475. doi: 10.1200/JCO.2009.25.1264. [DOI] [PubMed] [Google Scholar]
- 29.Pratt S. Washington, DC: The Advisory Board Company; 2002. The Oncology Roundtable: Oral Anticancer Agents. Implications for Patient Management and Program Economics (Practice Brief No. 31) [Google Scholar]
- 30.SHPA Committee of Specialty Practice in Cancer Services. SHPA standards of practice for the provision of oral chemotherapy for the treatment of cancer. J Pharm Pract Res. 2007;37:149–152. [Google Scholar]
- 31.Viele CS. Managing oral chemotherapy: The healthcare practitioner's role. Am J Health Syst Pharm. 2007;64(suppl 5):S25–S32. doi: 10.2146/ajhp070037. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PE, Chambers CR, Vaida AJ. Oncology medication safety: A 3D status report 2008. J Oncol Pharm Pract. 2008;14:169–180. doi: 10.1177/1078155208097634. [DOI] [PubMed] [Google Scholar]
- 33.Chan A, Leow YC, Sim MH. Patients' perspectives and safe handling of oral anticancer drugs at an Asian cancer center. J Oncol Pharm Pract. 2009;15:161–165. doi: 10.1177/1078155208100584. [DOI] [PubMed] [Google Scholar]
- 34.Weingart SN, Flug J, Brouillard D, et al. Oral chemotherapy safety practices at US cancer centres: Questionnaire survey. BMJ. 2007;334:407. doi: 10.1136/bmj.39069.489757.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anon. Advancing the safe and appropriate use of oral chemotherapy agents. Am J Health Syst Pharm. 2007;64(suppl 5):36–40. [Google Scholar]
- 36.Birner A, Rezendes M. Oral chemotherapy. Clin J Oncol Nurs. 2005;9:107–109. doi: 10.1188/05.CJON.107-109. [DOI] [PubMed] [Google Scholar]
- 37.Hartigan K. Patient education: The cornerstone of successful oral chemotherapy treatment. Clin J Oncol Nurs. 2003;7(suppl 6):21–24. doi: 10.1188/03.CJON.S6.21-24. [DOI] [PubMed] [Google Scholar]
- 38.Kiffmeyer TK, Niemöller M, Schirpenbach R, et al. [External contamination of drug packaging. Suggestions for a cleaning procedure] Krankenhauspharmazie. 2001;22:207–212. [Google Scholar]
- 39.Elliott RA, Marriott JL. Standardised assessment of patients' capacity to manage medications: A systematic review of published instruments. BMC Geriatr. 2009;9:27. doi: 10.1186/1471-2318-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health. 2005;78:403–412. doi: 10.1007/s00420-004-0595-1. [DOI] [PubMed] [Google Scholar]
- 41.Wick C, Slawson MH, Jorgenson JA, et al. Using a closed-system protective device to reduce personnel exposure to antineoplastic agents. Am J Health Syst Pharm. 2003;60:2314–2320. doi: 10.1093/ajhp/60.22.2314. [DOI] [PubMed] [Google Scholar]
- 42.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: Underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43:1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Goodin S. Oral chemotherapeutic agents: Understanding mechanisms of action and drug interactions. Am J Health Syst Pharm. 2007;64(suppl 5):15–24. doi: 10.2146/ajhp070034. [DOI] [PubMed] [Google Scholar]
- 44.Bartel SB. Safe practices and financial considerations in using oral chemotherapeutic agents. Am J Health Syst Pharm. 2007;64(suppl 5):8–14. doi: 10.2146/ajhp070036. [DOI] [PubMed] [Google Scholar]
- 45.Wong CM, Ko Y, Chan A. Clinically significant drug-drug interactions between oral anticancer agents and nonanticancer agents: Profiling and comparison of two drug compendia. Ann Pharmacother. 2008;42:1737–1748. doi: 10.1345/aph.1L255. [DOI] [PubMed] [Google Scholar]