Abstract
AIM: To define optimum management of the pyogenic liver abscess and assess new trends in treatment.
METHODS: One hundred and sixty nine patients with pyogenic liver abscess managed at Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir (India) from July 2001 to August 2006 were studied to evaluate and define the optimum treatment.
RESULTS: Mortality in the surgically treated group of patients was 9.4% (12/119), while those treated non-surgically had a fatality rate of 16.66% (7/42). Multiple liver abscesses treated surgically had a surprisingly low mortality of 30%. The biliary tract (64.97%) was the most common cause of liver abscess. Multiple abscesses, mixed organisms and abscess complications are all associated with a significantly increased mortality. However, the lethality of the primary disease process was the most important factor in determining survival.
CONCLUSION: Transperitoneal surgical drainage and antibiotics are the mainstay of treatment. Percutaneous drainage is recommended for high risk patients only.
Keywords: Liver abscess, Mortality, Antibiotics, Surgical drainage, Percutaneous drainage
INTRODUCTION
Abscess of the liver has been recognised since the time of Hippocrates. Even today, it remains a surgical problem with considerable morbidity and mortality, as reported earlier[1]. The introduction of antibiotics and advances in bacteriology and diagnostic techniques have generally improved the outcome. A basic requirement for effective therapy is early diagnosis[2]. This has been made easier by the introduction of high resolution imaging techniques including ultrasound and computed tomography (CT). Current strategies to improve survival include earlier diagnosis, the use of percutaneous drainage under radiographic control, use of antibiotics and open surgical drainage. This study was undertaken with particular care devoted to ascertaining clinical features, the time interval between diagnosis and therapy, imaging studies, microbiology, initial treatment, necessity for retreatment and outcome, thereby defining optimum management of the pyogenic liver abscess and assessing new trends in treatment.
MATERIALS AND METHODS
All 169 patients in our study were subjected to detailed history taking, clinical examination, routine investigations and various specialised investigations. These comprised ultrasonography in all the 169 patients with 96% sensitivity and CT in 12 patients with 100% sensitivity. Final diagnosis of pyogenic liver abscess was confirmed later microbiologically and/or pathologically.
Pathogenesis, signs and symptoms, laboratory data, diagnostic tests, treatment, pathology, bacteriology, complications, and outcome were analysed. The pathogenesis was considered to be extrahepatic biliary disease if obstruction of the common bile duct was present or if cholangitis was documented concurrently with the liver abscess. The portal vein was implicated as the route of bacterial spread in all intra-abdominal infections within the portal system but remote from the liver abscess. The source of the liver abscess was considered to be generalized septicemia with bacterial entry via the hepatic artery, if the primary infection arose outside the portal system. No source of infection could be positively identified in the cryptogenic abscess.
After patients were thoroughly investigated, they underwent various modalities of treatment: (1) Non-surgical treatment: (a) Conservative management with antibiotics alone; and (b) Percutaneous drainage under USG guidance; and (2) Open surgical drainage.
Initially all uncomplicated patients were put on intravenous antibiotics. In those where the response was seen within 48-72 h, antibiotics were continued for 2 wk followed by oral antibiotics for 4 wk. Response was monitored by clinical examination and ultrasonography. Patients with the following criteria were taken for percutaneous drainage[3]: (1) Patients who continued to be febrile even after 48-72 h of adequate medical treatment; (2) Liver abscess more than 6 cm in size; and (3) Clinical or ultrasonographic features suggest impending perforation.
Open drainage was carried out in patients falling the Kapoor criteria[4] which are as follows: (1) Thick ous which could not be aspirated; (2) Patients with multiple liver abscess; (3) Patients with ongoing sepsis even after antibiotic therapy and percutaneous drainage; (4) Multiloculated abscess; (5) Abscess in the left lobe; and (6) Ruptured abscesses.
The length of illness was defined as time from the first symptom attributable to the liver abscess to the time of definitive treatment. The delay in diagnosis was from the first visit to a physician to the time of definitive treatment. Bacterial data was compiled from the initial culture results only. The abscess was considered microscopic if it was less than 2 cm in greatest dimension. Mortality was defined as death within 30 d of treatment or before discharge from the hospital.
RESULTS
Incidence
The incidence of pyogenic liver abscess found in our hospital over a five year period study was 0.03%.
Age and sex
The average age was 42 years and ranged from 22 to 65 years. Majority of patients were in their fourth decade. Out of 169 patients, 102 were females and 67 males (P > 0.01).
Pathogenesis
The pathogenesis of hepatic abscesses with the frequency is shown in Table 1. Biliary tract disease was the most common source, although no source could be determined in 22 patients (13.01%) despite a thorough investigation. In our series biliary ascariasis was found in 17.75%. Cholecystitis and cholangitis accounted for 19.52% of patients. Generalized sepsis with a medical diagnosis of pyrexia of unknown origin accounted for 10.19% of our patients.
Table 1.
Origin of pyogenic liver abscess
| No. | Origin | n (%) |
| 1 | Biliary calculi | 45 (26.62) |
| 2 | Biliary ascariasis | 30 (17.75) |
| 3 | Cholecystitis/cholangitis | 33 (19.52) |
| 4 | General sepsis/hematogenous seeding | 19 (11.24) |
| 5 | Pancreatitis | 11 (7.00) |
| 6 | Portal vein sepsis | 6 (3.82) |
| 7 | Recent gastric or duodenal surgery | 3 (1.91) |
| 8 | Cryptogenic | 22 (13.01) |
| Total | 169 (100) |
Clinical features
The liver abscess was an indolent process in which 70% had the illness longer than 2 wk and 43% had the diagnosis delayed by more than 2 wk.
The symptoms and signs in this patient population are shown in Table 2. Abdominal tenderness and hepatomegaly were the most helpful signs in suggesting a liver abscess. Pulmonary changes were present in 38% of the patients but the changes lateralized to the side of the pathology in only 14%. Twenty eight of the 34 clinically jaundiced patients had extra hepatic biliary diseases.
Table 2.
Symptoms and signs
| No. | Symptoms | % | Signs | % |
| 1 | Malaise | 98 | Abdominal tenderness | 70 |
| 2 | Anorexia | 92 | Hepatomegaly | 65 |
| 3 | Fever | 91 | Right upper quadrant pain | 62 |
| 4 | Weight loss | 75 | Pulmonary changes | 38 |
| 5 | Chills | 35 | Jaundice | 34 |
| 6 | Vomiting | 18 | Epigastric pain | 14 |
| 7 | Chest pain | 15 | Peripheral Edema | 9 |
| 8 | Nigh sweats | 12 | Splenomegaly | 8 |
| 9 | Cough | 12 | Diffuse pain | 7 |
| 10 | Diarrhoea | 10 | Right flank pain | 4 |
Associated diseases
Diabetes mellitus was present in four patients, severe chronic obstructive pulmonary disease in five patients and severe anaemia in thirty five patients. All individuals associated with some type of co-morbidity were considered as high risk patients.
Laboratory data/diagnosis
Hemoglobin level of the less than 10 gm% was found in 59% of patients, total leucocyte count more than 10 000/cumm in 68% of patients, serum transaminases and alkaline phosphatase levels were elevated in 84% of patients. Prothrombin time index was less than 60% in 60% of patients. Blood culture grew Escherichia coli (E. coli) in 35% and Klebsiella in 23% in patients. Pus culture grew E. coli in 43% and Klebsiella in 25% of patients.
Ultrasonography (Figure 1) had a sensitivity of 96% in diagnosing liver abscess while as CT (Figure 2) scans showed a sensitivity of 100%. A single abscess was found in 70% and multiple abscesses in 30% of patients. Right lobe involvement was found in 68% while left lobe involvement was found in 22% and bilobar involvement in 10% of patients.
Figure 1.
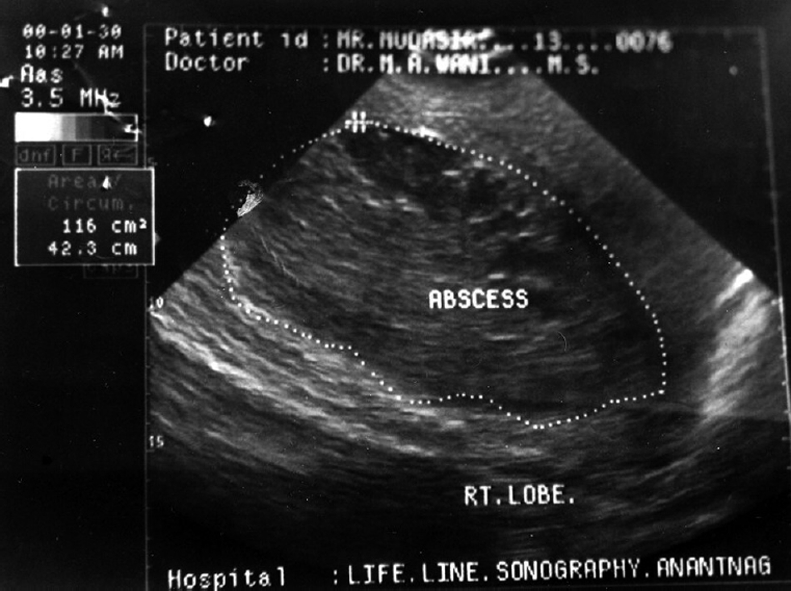
Ultrasonographic picture showing abscess in the right lobe of the liver.
Figure 2.
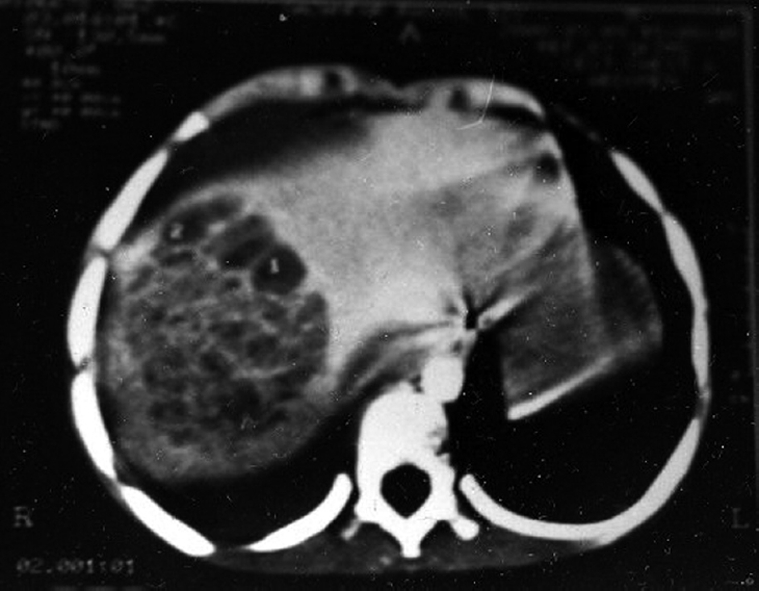
Computed tomography scan picture showing abscess in the right lobe of the liver.
Treatment modalities and outcome
Antibiotics were used in all patients regardless of the mode of management. Open surgical drainage comprising 75.14% of patients had hepatotomy and drainage of the abscess with an accompanying liver biopsy. Drains were placed in all these patients but the type of drain did not appear to affect the outcome. Percutaneous drainage (Figure 3) of the hepatic abscess was performed in 15.38% (n = 26) of patients using ultrasonography as a guide for the procedure (Table 3).
Figure 3.
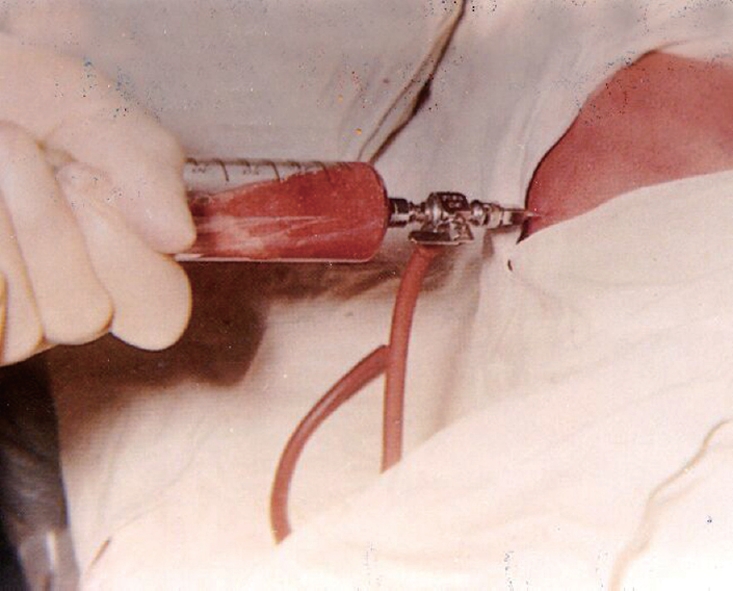
Percutaneous drainage of liver abscess being carried out.
Table 3.
Various modalities of management used in the patients
| No. | Treatment modality | n | Success rate (%) |
| 1 | Open surgical drainage | 127 | 122 (96) |
| 2 | Percutaneous drainage | 26 | 20 (77) |
| 3 | iv antibiotics only | 16 | 6 (37.5) |
| Total | 169 |
Nine patients with open surgical drainage had to undergo re-exploration as they had recurrence of abscess.
Intravenous antibiotic therapy was used as the sole initial modality of treatment in 9.46% (n = 16) of patients in our series. Open surgical drainage was carried out in 62.5% (10 patients) among this group as the patients did not show any response to medical treatment. Among the percutaneous aspiration group 23% (6 patients) needed open surgical drainage after the initial procedure because of inadequate drainage by the percutaneous method. Among patients with open surgical drainage, drainage of abscess cavity alone was carried out in 62 (49%) patients, cholecystectomy, common bile duct exploration and drainage of abscess was carried out in 53 (41.73%) patients, along with T-tube drainage, while as cholecystectomy with drainage of liver abscess was done in 7 (5.51%) patients. Peritoneal lavage was carried in 5 (4%) patients, as they had presented with ruptured liver abscesses (Table 4).
Table 4.
Operative procedures performed on the patients
| No. | Operative procedure | n |
| 1 | Drainage of abscess alone | 62 |
| 2 | Draining of abscess with cholecystectomy with common bile duct exploration | 53 |
| 3 | Drainage of abscess with cholecystectomy | 7 |
| 4 | Drainage of abscess with peritoneal lavage | 5 |
| Total | 127 |
Bacteriology
Positive culture results were obtained in 119 patients and 14 cultures had no growth identified probably because of antibiotic usage over prolonged period. A single organism was present in 44 (58%), while 61 (41.9%) of the cultures had two or more organisms. No culture was obtained in 38 patients. The mortality rate was low (36%) in those patients who were single organism positive and high in patients with mixed organisms in their abscess (64%). Gram negative aerobes were predominantly present in positive cultures (61%). Blood culture results were positive in 55% of the patients with cholangitis.
Complications
Thirty four patients (20.11%) in our series developed complications followed either surgical or nonsurgical therapy (percutaneous aspiration drainage or intravenous antibiotics). Septicemia was the most common complication and carried a mortality rate of 85%. The frequency of major complications in shown in Table 5.
Table 5.
Major complications and their frequency
| No. | Complication | % |
| 1 | Septicemia | 22 |
| 2 | Intra Abdominal abscess | 11 |
| 3 | Recurrent liver abscess | 4 |
| 4 | Renal failure | 4 |
| 5 | Hepatic Failure | 3 |
| 6 | Massive upper gastrointestinal blood | 3 |
| 7 | Free peritonitis | 2 |
| 8 | Prolonged billiary drainage | 2 |
| 9 | Mortality | |
| Non surgical group | 7/42 (16.66) | |
| Surgical group | 12/127 (9.44) |
Death analysis
Death occurred in 19 patients with pyogenic liver abscesses. There were more deaths within the non surgically drainage group (7 out of 42 patients) than the surgically drained group (12 out of 127 patients) (Table 5). Henceforth, open surgical drainage remains the preferred treatment for pyogenic liver abscesses rather than less invasive options i.e percutaneous drainage and/or medical therapy. In 9 patients (7%) re-exploration for recurrence of abscess after initial surgical drainage was performed. The overall mortality in our series was 11.24%. The surgically treated group had a mortality of 9.4% (12 patients), while mortality among the non-surgically treated group was 16.66%. All seven surgically managed patients who died had some associated morbidity (P < 0.05), including diabetes in two, chronic obstructive pulmonary disease in two and anemia in one. In the surgical group, ten out of twelve deaths had an associated morbidity (P < 0.05), including diabetes in one, chronic obstructive pulmonary disease in three and anemia in six (Table 6). Klebsiella pneumonia was the organism most commonly grown from the cultures of patients who died during our study, both surgical and non-surgical (Table 7).
Table 6.
Impact of co-morbidity on mortality
| Comorbidity | No. of deaths in surgical group (n = 12) | No. of deaths in non-surgical group (n = 7) | Total deaths (n = 19) | P-value |
| Diabetes (n = 4) | 1 | 2 | 3 | > 0.05 |
| Chronic obstructive pulmonary disease (n = 5) | 2 | 2 | 4 | < 0.05 |
| Anemia (n = 35) | 4 | 3 | 7 | < 0.05 |
P > 0.05 (insignificant), P < 0.05 (significant).
Table 7.
Impact of organism cultured on the outcome of disease
| Complications [organism(s) cultured] |
Recurrent sepsis |
Acute respiratory distress syndrome |
Wound infection |
Recurrence |
Mortality |
|||||
| S | NS | S | NS | S | NS | S | NS | S | NS | |
| Escherichia coli | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 2 |
| Klebsiella pneumonia | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 6 | 4 |
| Streptococcus pneumonia | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Staphylococcus epidermidis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staphylococcus aureus | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Bacteriodes fragilis | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Pseudomonas aeruginosa | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
S: Significant; NS: Non significant.
DISCUSSION
Pyogenic liver abscess is still a serious illness and a diagnostic challenge[5,6]. This is reflected in significant mortality rates and is a result of the lack of specificity of clinical signs[7] and laboratory results. New imaging techniques such as ultrasound and CT scan have made the differential diagnosis easier but cannot always rule out parasitic abscesses[8] or primary and metastatic hepatic tumor[9]. Diagnosis can be missed even intra-operatively[10]. The results of our series confirm the decreasing incidence of portal pyemia[11] and support the rising incidence of abscesses related to biliary disease generally reported elsewhere (31% to 45 %)[5,6,10,12-14]. Our 9.55 percent incidence of “Cryptogenic” abscess is lower than the 12 to 20 percent incidence reported commonly[9,14,15].
The majority of liver abscesses have an underlying source that must be controlled before successful treatment of the abscess is possible. Most patients with hepatic abscesses can be cured with aggressive surgical and antibiotic therapy if the origin of the abscess is removed. Open surgical drainage has been the traditional treatment, although percutaneous drainage is available because of newer radiologic techniques. Historically, liver abscesses developed in otherwise healthy patients with an intra-abdominal infection. Ochsner reported a peak incidence in the fourth decade, as has been found in our series. The incidence of pyogenic liver abscess found in our series over a five year period was 0.03% which is similar to that reported by others[1,14]. In this study, significantly more females were affected because of the increased incidence of biliary calcular disease in women. Most studies show a male majority[1,15], although recent reports suggest a trend to an equal sex incidence[14,16]. This study contradicts that view.
The source of the liver abscess greatly affects the subsequent mortality. Extra hepatic biliary disease was the largest etiologic group in this series, in agreement with the report by Miedema et al[9] in 1984. Mortality as high as 79% has been reported in this etiologic group[14] while the rate in our series was about 62%. Unfortunately, most of these deaths are not preventable with the present day therapeutic tools. Most had non reconstructable biliary tract disease, and the hepatic abscess reflected an inability to deal adequately with the primary disease. A more aggressive approach to the diagnosis and treatment of biliary disease in general will salvage a small proportion of these patients.
Biliary ascariasis leading to a liver abscess via the common bile duct or the portal vein is of special interest in this series. The association of biliary ascariasis with cholangitis has been well documented[17,18]. The fact that biliary ascariasis is one of the common etiologic factor in our series is probably because of high incidence of ascaris lumbricoides infestation in this region[18]. Worms have been recovered from abscess cavities in the majority of our patients among this group. With the large number of cryptogenic abscess in this and other reports, a better understanding of the underlying pathogenesis is needed. Incidences have been reported ranging from 4% to nearly 60%[1,19], although in recent series a fairly consistent 20% incidence has been reported[14-16]. The incidence in this series of 9.55% is lower than most recent reports but correlates well with the 11% incidence reported by Sherman and Robbins in an autopsy study at the Mallory Institute of Pathology[20].
Many investigators have tried to find a unifying pathogenesis for all cryptogenic hepatic abscesses but multiple causes appear to be involved. Our data is consistent with the observation of Beaver that most abscesses are secondary to an infection within the region of portal drainage[21]. This may be from a healed focus or from a persistent underlying disease that was not identified in the diagnostic evaluation. The culture results also add validity to this thesis, since majority of cultures grew gastrointestinal flora.
Newer radiological techniques such as ultrasound and CT scanning have greatly enhanced our ability to establish the diagnosis of hepatic abscess and have increased our understanding of the natural history of this process. Ultrasonography is the preferred initial tool for the diagnosis of liver abscess with a sensitivity of 85% to 95%. Ultrasound can identify lesions more than 2 cm in diameter. On the other hand, CT offers several advantages over ultrasonography. It has a sensitivity of 95% and can detect abscesses as small as 0.5 cm. CT can also delineate small abscess near the diaphragm and in fatty livers. CT also helps in detecting any associated intra - abdominal pathology including pancreatic masses, colonic cancers, diverticulitis, appendicitis and intraperitoneal abscesses. All our patients were subjected to ultrasound examination and a sensitivity of 96% in diagnosis was achieved. Only 12 patients in our series were subjected to CT scan examination with a sensitivity of 100%. Ultrasound is also cost effective as compared to CT scan. Ultrasound was found to be useful in the percutaneous drainage group and in the group treated with antibiotics alone. It has proved a very useful technique for documentation of the course of the hepatic abscess in our series. On ultrasound scans the pyogenic liver abscess appears as a hypoechoic lesion with irregular margin. Within the lesion these may be irregular areas of increased echogenicity. On the other hand liver abscess on CT appears as a low density lobulated lesion with poorly defined edges[22]. Hepatic scans utilizing radioisotopes are obsolete for definition of hepatic abscess while magnetic resonance imaging does not provide information of greater usefulness than ultrasound or CT scanning[23].
Surgical treatment continues to give the best chance of survival in patients with pyogenic liver abscess. The surgical mortality of 11.24% is a marked improvement over the previous mortality rate of 69% reported by Ochnsner, De Bakey and Murray from 1928-1937. Surgical intervention has the advantage of thorough exploration of the abdomen and extirpation of known or unsuspected primary foci of infection that might not have been detected in imaging[24,25].
We recommend transperitoneal surgical drainage to allow abdominal exploration and thorough exploration of the liver for multiple hepatic abscesses. From our series it is difficult to evaluated which type of drain is best. However, dependent drainage with multiple drains consisting of large tube drains and soft rubber drains is recommended. The tube drains in particular allow for diagnostic contrast studies and even irrigation treatment after surgery.
Medical treatment alone without any drainage procedure has shown poor results in our series. Only 16 patients (9.46%) were subjected to this modality of treatment in our series and 10 of these patients (62.5%) subsequently needed open surgical drainage because of poor response to antibiotic treatment alone. The poor success rate has been documented by others as well[1,14,26]. Although some continue to encourage the use of antibiotics alone to treat the pyogenic liver abscess[27], this approach seems risky and we, therefore, recommend that all liver abscesses of pyogenic origin should be drained to provide optimum treatment.
Percutaneous treatment of abdominal[28] and pyogenic hepatic abscesses[22,29] has been praised for its simplicity and excellent results. Although of considerable benefit, percutaneous drainage is not necessarily the best treatment for all patients and is associated with a significantly higher failure rate than surgical drainage[22,29]. This is also evident from our series where 26 patients (15.38%) were subjected to this mode of treatment and 6 patients (23%) among them subsequently needed open surgical drainage because of inadequate response to percutaneous drainage. It is recommended that several factors be considered in choosing between surgical and percutaneous drainage. These include the anaesthetic risk posed to the patient, the presence or absence of a coexisting primary intra-abdominal pathology requiring surgery, the relatively limited size of the drains that can be introduced percutaneously, the complication and failure rates of the two procedures and also the local expertise. Percutaneous drainage of liver abscesses does hold promise for definitive therapy or to delay surgery in high risk patients who may not tolerate general anaesthesia. However, a prospective randomized trial comparing patients drained surgically or percutaneously is needed to evaluate differences in cost and morbidity. Bari et al[22] compared the results of percutaneous aspiration of liver abscess with open drainage in children. They concluded that open surgical drainage is the best modality of management for liver abscess.
We conclude with the message that although percutaneous drainage is safe and effective the open surgical procedure is the most reliable and effective means of management because we can deal not only with liver abscess, but also with associated intra abdominal pathology.
COMMENTS
Background
Liver abscess is a distinct clinicopathologic entity with systemic manifestations of toxemia and vague clinical signs in the abdomen. Modern non-invasive tests are highly sensitive in diagnosing liver lesions. Difficulty remains in identifying small hepatic abscesses and differentiating large abscesses from tumor.
Research frontiers
Pyogenic liver abscess is a potentially fatal disease. Over the decades, there has been significant improvement in its mortality. This has been attributed to the introduction of antibiotics, advances in imaging studies and critical care. There has also been a paradigm shift in the treatment modality of choice from the traditional open surgery to the minimally-invasive percutaneous drainage. However, whether this has lowered the mortality rate is debatable. The treatment of choice remains controversial. The aim of the study was to define optimum management of the pyogenic liver abscess and to assess new trends in treatment.
Innovations and breakthroughs
While percutaneous drainage is appropriate as first-line surgical treatment in most cases, open surgical drainage is prudent in cases of rupture, multi loculation, associated biliary or intra-abdominal pathology. Percutaneous drainage may help to optimize clinical condition prior to surgery. Laparoscopic drainage show promising results and is a feasible surgical option for the future.
Applications
The final verdict on the outcome of percutaneous versus open surgical drainage of pyogenic liver abscesses requires further studies in a controlled trial setting. Nevertheless, in current good clinical practices the choice of therapy needs to be individualized according to patient’s clinical status and abscess factors. The available therapies are complementary in the management of liver abscesses. This study may represent a future strategy for therapeutic intervention in the treatment of patients with pyogenic liver abscess.
Peer review
The authors undertook an extensive study to assess the clinical presentation, diagnosis and management of patients with pyogenic liver abscess. The aim was to define optimum management of the pyogenic liver abscess and assessing new trends in treatment.
Footnotes
Supported by The Department of Surgery and Medical Records Section Sheri Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India
Peer reviewer: Uwe Klinge, MD, Professor, Institute for Applied Medical Engineering AME, Helmholtz Institute, RWTH Aachen Pauwelsstrabe 30, Aachen 52074, Germany
S- Editor Wang JL L- Editor Hughes D E- Editor Lin YP
References
- 1.Ochsner A, DeBakey M, Murray S. Pyogenic abscess of the liver: II. An analysis of forty-seven cases with review of the literature. Am J Surg. 1938;40:293–319. [Google Scholar]
- 2.Silver S, Weinstein A, Cooperman A. Changes in the pathogenesis and detection of intrahepatic abscess. Am J Surg. 1979;137:608–610. doi: 10.1016/0002-9610(79)90032-1. [DOI] [PubMed] [Google Scholar]
- 3.Porvas RG. Hepatic abscess in children. J Pediatric Surg. 1995;30:5. doi: 10.1016/0022-3468(95)90684-3. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Srinivasan S, Sharma AK. Pyogenic liver abscess in children--South Indian experiences. J Pediatr Surg. 1998;33:417–421. doi: 10.1016/s0022-3468(98)90081-1. [DOI] [PubMed] [Google Scholar]
- 5.Schraibman IG. Non-parasitic liver abscess. Br J Surg. 1974;61:709–712. doi: 10.1002/bjs.1800610908. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Macpherson DS, Holm J, Fossard DP. Pyogenic liver abscess: a study of forty-four cases in two centres. Acta Chir Scand. 1982;148:415–421. [PubMed] [Google Scholar]
- 7.Perera MR, Kirk A, Noone P. Presentation, diagnosis and management of liver abscess. Lancet. 1980;2:629–632. doi: 10.1016/s0140-6736(80)90293-7. [DOI] [PubMed] [Google Scholar]
- 8.Bari S, Sheikh KA, Ashraf M, Hussain Z, Hamid A, Mufti GN. Ascaris liver abscess in children. J Gastroenterol. 2007;42:236–240. doi: 10.1007/s00535-006-1989-5. [DOI] [PubMed] [Google Scholar]
- 9.Miedema BW, Dineen P. The diagnosis and treatment of pyogenic liver abscesses. Ann Surg. 1984;200:328–335. doi: 10.1097/00000658-198409000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyorffy EJ, Frey CF, Silva J Jr, McGahan J. Pyogenic liver abscess. Diagnostic and therapeutic strategies. Ann Surg. 1987;206:699–705. doi: 10.1097/00000658-198712000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sørensen MR, Baekgaard N, Kirkegaard P. Pyogenic liver abscess. A case report with a short review of current concepts of diagnosis and management. Acta Chir Scand. 1983;149:437–439. [PubMed] [Google Scholar]
- 12.Greenstein AJ, Lowenthal D, Hammer GS, Schaffner F, Aufses AH Jr. Continuing changing patterns of disease in pyogenic liver abscess: a study of 38 patients. Am J Gastroenterol. 1984;79:217–226. [PubMed] [Google Scholar]
- 13.Frey CF, Zhu Y, Suzuki M, Isaji S. Liver abscesses. Surg Clin North Am. 1989;69:259–271. doi: 10.1016/s0039-6109(16)44784-5. [DOI] [PubMed] [Google Scholar]
- 14.Greenstein AJ. Abscesses of the liver (other than amoebic) In: Hambrich WS, Schaffner F, Berk JE, Bockus HL, editors. Bockus Gastroenterology. Philadelphia: WB Saunders Co; 1985. pp. 234–239. [Google Scholar]
- 15.Pearce NW, Knight R, Irving H, Menon K, Prasad KR, Pollard SG, Lodge JP, Toogood GJ. Non-operative management of pyogenic liver abscess. HPB (Oxford) 2003;5:91–95. doi: 10.1080/13651820310001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altemeier WA, Schowengerdt CG, Whiteley DH. Abscesses of the liver: surgical considerations. Arch Surg. 1970;101:258–266. doi: 10.1001/archsurg.1970.01340260162025. [DOI] [PubMed] [Google Scholar]
- 17.Lazarachick J, de Souza E Silva NA, Nichls DR, Washington JA II. Pyogenic liver abscess. Mayo Clin Proc. 1973;4:349–355. [PubMed] [Google Scholar]
- 18.Kamath PS, Joseph DC, Chandran R, Rao SR, Prakash ML, D'Cruz AJ. Biliary ascariasis: ultrasonography, endoscopic retrograde cholangiopancreatography, and biliary drainage. Gastroenterology. 1986;91:730–732. doi: 10.1016/0016-5085(86)90646-3. [DOI] [PubMed] [Google Scholar]
- 19.Khuroo MS, Zargar SA. Biliary ascariasis. A common cause of biliary and pancreatic disease in an endemic area. Gastroenterology. 1985;88:418–423. [PubMed] [Google Scholar]
- 20.Kolli A. Management of pyogenic Liver abscess. New York: Kings County Hospital Center, July; 2006. [Google Scholar]
- 21.Strong RW, Fawcett J, Lynch SV, Wall DR. Hepatectomy for pyogenic liver abscess. HPB (Oxford) 2003;5:86–90. doi: 10.1080/13651820310001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bari S, Sheikh KA, Malik AA, Wani RA, Naqash SH. Percutaneous aspiration versus open drainage of liver abscess in children. Pediatr Surg Int. 2007;23:69–74. doi: 10.1007/s00383-006-1812-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Tomlinson C, Temple M, Amaral J, Connolly BL. Imaging-guided percutaneous needle aspiration or catheter drainage of neonatal liver abscesses: 14-year experience. AJR Am J Roentgenol. 2008;190:616–622. doi: 10.2214/AJR.07.2888. [DOI] [PubMed] [Google Scholar]
- 24.Donovan AJ, Yellin AE, Ralls PW. Hepatic abscess. World J Surg. 1991;15:162–169. doi: 10.1007/BF01659049. [DOI] [PubMed] [Google Scholar]
- 25.Soballe PW, Campbell JJ. Perforated appendix masquerading as an hepatic abscess. Mil Med. 1985;150:606–608. [PubMed] [Google Scholar]
- 26.Farges O, Leese T, Bismuth H. Pyogenic liver abscess: an improvement in prognosis. Br J Surg. 1988;75:862–865. doi: 10.1002/bjs.1800750910. [DOI] [PubMed] [Google Scholar]
- 27.Johnson G Jr, Glenn F. Multiple liver abscesses following biliary tract surgery. Ann Surg. 1954;140:227–233. doi: 10.1097/00000658-195408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher JA Jr, Reynolds TB, Yellin AE. Successful medical treatment of pyogenic liver abscess. Gastroenterology. 1979;77:618–622. [PubMed] [Google Scholar]
- 29.McCorkell SJ, Niles NL. Pyogenic liver abscesses: another look at medical management. Lancet. 1985;1:803–806. doi: 10.1016/s0140-6736(85)91457-6. [DOI] [PubMed] [Google Scholar]


