Abstract
One form of cognitive control is the ability to resist temptation in favor of long-term goal-oriented behavior. Historically, the development of cognitive control capacity has been described by a linear function from infancy to adulthood. However, the context in which control is required can impact this capacity, such that emotionally charged or rewarding contexts can diminish control. More recently, studies have begun to examine the development of cognitive control in contexts that vary in motivation. These studies suggest specific windows of development in which cognitive control capacity is more vulnerable to incentive-based modulation. In this review we highlight the most recent work on neurobiological changes supporting motivational and cognitive development, underscoring the importance of functional organization and development of the underlying circuitry implicated in these processes, and provide a theoretical perspective that moves away from discussing singular functional regions toward considering functional circuitry.
Resistance to temptation or delay of immediate gratification has been studied in the context of social, developmental and cognitive psychology. Developmentally, this ability has been measured by assessing how long a toddler can resist an immediate reward (e.g., a cookie) in favor of a larger reward later (e.g., two cookies)[1]. Although we each vary in this ability even as adults, developmental studies suggest windows of development when we are particularly susceptible to temptations. This ability has been described as a form of cognitive control[2] and is operationally defined as the ability to accomplish goal-directed behavior in the face of salient, competing inputs and actions.
Developmental studies have shown a steady improvement in cognitive control capacity from infancy to adulthood[3] using experimental paradigms in controlled laboratory settings. Yet, in less controlled settings within the real world that involve emotionally charged interactions, we often see diminished cognitive control. This reduced control is especially evident during the period of adolescence, when suboptimal choices in sexual and drug related behaviors peak[4-7]. These observations imply that developmental trajectories in cognitive control are complex and can be modulated by heated or emotionally charged contexts, in which cognitive control demands interact with motivational drives or processes.
In the last few years, there has been an explosion of studies examining the developmental neurobiology of adolescence. These studies have focused predominantly on evaluating the hypothesis that during adolescence, unique patterns of brain activity arise that predict stereotypical aspects of adolescent behavior including risk-taking and suboptimal decision making in the face of incentives[8,9]. This work challenges the more traditional view that adolescent risky behavior is due to immature cognitive control capacities and their underlying neural substrates (e.g., prefrontal cortex)[10]. According to recent studies, adolescents show a unique sensitivity to motivational cues that challenges the less mature cognitive control system, resulting in an imbalance between these systems and ultimately patterns of behavior that are unique to adolescents.
Inflections in adolescent behavior, distinct from child and adult behavior represent dynamic maturation of brain circuitry underlying motivational and cognitive processes[11,12]. Two key regions implicated in cognitive and motivational behavior are the prefrontal cortex known to be important for cognitive control[13], and the striatum critical in detecting and learning about novel and rewarding cues in the environment[14]. This review highlights the most recent work on neurobiological changes supporting these motivational and cognitive systems across development. We underscore the importance of examining circuitry rather than regional change, especially within frontostriatal circuits that underlie different forms of goal-oriented behavior. This theoretical perspective moves the field away from examination of how each region matures in isolation to how they may interact in the context of interconnected circuits.
Motivational Modulation of Cognitive Control Across Development
Incentives can modulate cognitive control in numerous ways. Being rewarded for performance on a given task may make people work harder and ultimately perform better than when not rewarded. Alternatively, the capacity to exert control is challenged when required to suppress thoughts and actions toward appetitive cues. Recent studies of adolescent development have begun to compare cognitive control capacity in relatively neutral versus motivational contexts. These studies suggest a change in sensitivity to environmental cues, especially reward-based ones (see [8,15] for discussion of sensitivity to aversive cues), at different points in development, and suggest a unique influence of motivation on cognition during the adolescent years.
In general, cognitive control capacities improve in a linear function from childhood to adulthood. This observation is supported by a wealth of behavioral evidence from tasks including the Go-NoGo, Simon task, and task-switching paradigms requiring participants to override a prepotent response in order to achieve a correct one[3]. However, when it is advantageous to suppress a response to incentive-related cues, adolescents' cognitive control suffers.
Recent studies provide elegant demonstrations of how adolescent behavior is differentially biased in motivational contexts. Using a gambling task in which reward feedback was provided during decision-making (“hot” trials which heightened task-elicited arousal) or held until after the decision (“cold” trials), Figner and colleagues[16] showed that adolescents made disproportionately more risky gambles compared to adults but only in the emotionally-charged “hot” condition. Steinberg and colleagues, using a similar gambling task[17] and a delay discounting task[18], have shown that sensitivity to rewards and incentives actually peaks during adolescence, with a steady increase from late childhood to adolescence and subsequent decline from late adolescence to adulthood. These findings illustrate a ∩-shaped function, peaking between 14 and 16, and then declining. Taken together, these studies suggest that during adolescence, motivational cues of potential reward are particularly salient and can lead to riskier or suboptimal choices that diminish effective goal-oriented behavior.
Developmental Neurobiology of Corticosubcortical Control
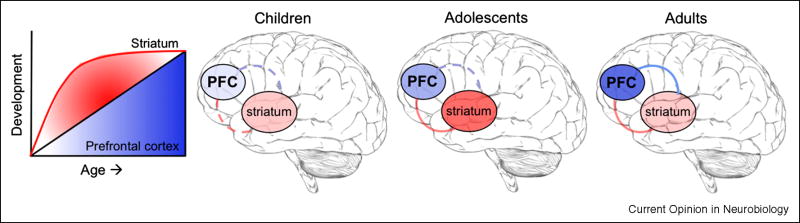
We have recently proposed a simple and testable neurobiological model of cognitive and motivational processes ([7] see Figure 1) to account for real world behavior of adolescents that is supported by recent laboratory evidence. This model suggests linear development of top down prefrontal regions relative to a ∩-shaped function for development of bottom-up striatal regions involved in detecting salient cues in the environment.
Figure 1.
Cartoon model of striatal and prefrontal interactions across development. Deeper color indicates greater regional signaling. Line represents functional connectivity, with solid line indicating mature connection and dotted line indicating immaturity.
Evidence in support of this model comes from animal and human studies of frontostriatal function and development[19-21]. Seminal work has shown how striatal and prefrontal cortical regions shape goal-directed behavior. Using single-unit recordings in monkeys, Pasupathy & Miller[22] demonstrated that when flexibly learning a set of reward contingencies, very early activity in the dorsal striatum lays down reward-based associations, whereas later, more deliberative prefrontal mechanisms are engaged to maintain the behavioral outputs that optimize the greatest gains. A role for the striatum in early temporal coding of reward contingencies prior to onset of prefrontal regions has also been extended in humans[23]. These findings suggest that understanding the interactions between regions (along with their component functions)- particularly within frontostriatal circuitry- is critical to developing a model of cognitive and motivational control.
Recent human imaging studies provide further support for the importance of examining connections between frontostriatal regions in establishing circuit-specific function across development. Using diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI), Casey and colleagues have linked connection strength between these regions with the capacity to effectively engage cognitive control in typically and atypically developing individuals[24,25]. These studies illustrate the importance of signaling within corticostriatal circuitry in supporting the capacity to effectively engage in cognitive control.
In the last few years much attention has been given to how subcortical systems like the striatum and the prefrontal cortex interact to give rise to aberrant behavior observed in adolescents. Developmentally, cortical association areas including the prefrontal and parietal cortex thought to subserve age-related improvement in cognitive control[26-30] undergo delayed maturation[31-33]. These studies provide insights into the role of these regions in cognitive control processes across development, though the delineation of circuit-level development has been less clear. Using network modeling techniques, Fair and colleagues recently mapped the developmental trajectory of cognitive control networks using small-world network modeling[34]. From childhood to adulthood, the functional interactions between regions within a frontoparietal network and cingulate-lateral prefrontal network show lessening of short-range functional connections with neighboring regions and strengthening long-distance connections between distal regions. These novel network-level findings bolster the claim that cognitive maturation occurs not in unitary structures but in the connectivity and interactions between structures(e.g., [35]).
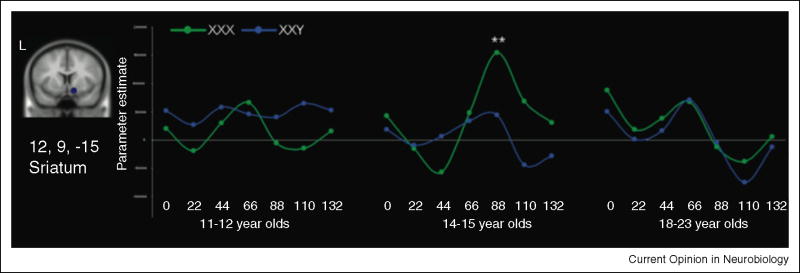
Recent studies have mapped developmental modulation of motivational systems such as the striatum in salient and motivational contexts. Seminal work by Ernst, Galvan, Luna and Crone and others supports the notion that adolescents show an enhanced sensitivity to incentives relative to children and adults within areas of the dorsal and ventral striatum[36-39]. For example, a recent study by van Leijenhorst and colleagues [40] observed exaggerated ventral striatal responses in adolescents during the anticipation and receipt of a monetary reward (Figure 2). The magnitude of ventral striatum response to reward cues has been linked to real-world behavior, with greater ventral striatal activity to rewards being predictive of real-life risk-taking tendencies[41]. These studies together suggest that striatal responses show an inverted U function across development in response to incentives.
Figure 2.
Adolescents show enhanced striatal sensitivity to the receipt of a monetary reward relative to children or adults. XXX=Reward, XXY=No Reward. Adapted with permission from[40].
A scientific area that has received less attention is determining how cognitive control and motivational systems interact over the course of development. Very recent work has suggested that adolescents possess an enhanced ability to flexibly upregulate cognitive performance if an incentive is at stake. Work by Ernst and colleagues[42,43] used an antisaccade task to measure cognitive control behavior and promised a financial reward for accurate performance on some trials but not others. Results showed that promise of a reward facilitated adolescent cognitive control behavior more than for adults, a finding that has recently been extended to social rewards (e.g, happy faces) as well[44].
Geier and colleagues[37] identified the neural substrates of this cognitive upregulation using a variant of this antisaccade task during functional brain imaging. In adolescents and adults, trials for which money was at stake speeded performance and facilitated accuracy, but this effect was larger in adolescents. Following a cue that the next trial would be rewarded, adolescents showed exaggerated activation in the ventral striatum while preparing for and subsequently executing the antisaccade (Figure 3). An exaggerated response was observed in adolescents within prefrontal regions important for controlling eye movements, suggesting a reward-related upregulation in control regions as well. Together, these studies suggest incentive modulation of frontostriatal circuits at the level of the striatum and are consistent with our proposed neurobiological model of striatal and prefrontal interactions across development.
Figure 3.
Striatal and prefrontal responses are upregulated in adolescents while preparing for rewarded versus nonreward trials. Adapted with permission from[37].
Conclusions
This is an exciting time in developmental neurobiology research of cognitive and motivational processes, with a surge in studies focused on the development of these processes in adolescence. Recent findings suggest that an enhanced sensitivity to motivational cues in adolescents, represented at the level of the striatum, modulates cognitive control-related processes differently from children or adults. As such, adolescent cognitive control capacity can be enhanced or impaired, depending on whether task demands require suppression of or attention to these motivational cues. More research is needed to understand the interplay between how subcortical and cortical regions interact to accomplish these dynamic cognitive control processes across development and how motivational cues may vary in salience across development (see Box 1).
Box 1. Issues for Developmental Neurobiology of Cognitive and Motivational Processes.
Measuring neurobiological change across development poses several unique challenges and current areas of debate, outlined below.
1) What constitutes “maturation” in fMRI signal?
Conflicting arguments have been made with regard to what pattern of functional activity represents functional maturity. Some studies report higher-magnitude activity as immature, reflecting neural inefficiency or increased effort. Other studies interpret higher-magnitude activity as more mature, reflecting greater capacity to utilize the functionality of the region. Others have asserted that maturation is reflected in refinement of activation extent, shifting from larger, diffuse activations to more focal activations with increasing age[45]. For a more extensive discussion of this complex issue, see [26].
2) How can age and performance-related activity be deconfounded?
Cognitive task performance often co-varies with age. Some studies assess functional recruitment in performance-matched samples[46] whereas others use differential behavioral performance as a variable of interest[15]. A final approach is to separately characterize the variance accounted for by age and performance[47], though this requires a larger sample than is typically acquired.
3) How can nonlinear developmental changes best be detected?
In this report we describe nonlinear aspects of cognitive development. These patterns are overlooked in many studies using exclusively linear models to assess changes across age and when testing small samples and relatively small discrete age ranges (e.g., comparing 12-13 year olds with 21 year olds) rather than acquiring the full age range. A gold standard in developmental neurobiology is to examine developmental trajectories as individuals transition into and out of developmental periods of interest.
4) How do developmental differences in representation of motivation bias findings?
In experiments involving reward and affect, the assumption is often made that the potency of an experimental manipulation or stimulus is equivalent across ages. For example, is a $1 gain equally “rewarding” to a child, an adolescent, and an adult? Preliminary evidence suggests this is not the case[36]. Future work will need to equate the rewarding and aversive properties of experimental stimuli and manipulations across development.
Acknowledgments
This work was supported by NIDA grants DA018879, DA007274, and NIMH grant MH62196. We thank Erika Ruberry and Rebecca Jones for assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 2.Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, Davidson MC, Lawrence Aber J, Casey BJ. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17:478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- 3.Davidson MC, Amso D, Anderson LA, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Eaton LK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, et al. Youth Risk Behavior Surveillance - United States, 2007, surveillance summaries. Morbidity and Mortality Weekly Report. 2008;57:1–131. [PubMed] [Google Scholar]
- 6.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •7.Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article lays out the theoretical rationale supporting the relationship between dynamic frontostriatal interactions and risky behavior in adolescence.
- 8.Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2009 doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •9.Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: Developmental considerations. Development and Psychopathology. 2008;20:1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]; This article provides new insights into understanding linkages between developmental variations in reward processing and the maturation of corticostriatal circuitry.
- 10.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- •12.Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacology Biochemistry and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]; This article provides a comprehensive overview of the roles of the physical changes of puberty and the maturation of dopamine systems on changes in motivated behavior in adolescence.
- 13.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–247. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 14.Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 15.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •16.Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: Age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]; This article utilizes behavioral and psychophysiological techniques to examine the influence of affective and cognitive processes predictive of adolescents' heightened risk-taking behavior.
- 17.Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham SJ, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychobiology. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- ••18.Steinberg L, Graham SJ, O'Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]; With an impressive sample of participants ranging from 10 to 30 years, this article reports that early adolescents demonstrate weaker future orientation, whereas future planning develops steadily throughout adolescence and young adulthood.
- 19.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer MJ. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haber SN, Knutson B. The reward circuity: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;1:1–23. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 23.Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. Journal of Neuroscience. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 25.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 26.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. doi: 10.1016/j.bandc.2009.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astle DE, Scerif G. Using developmental cognitive neuroscience to study behavioral and attentional control. Developmental Psychobiology. 2009;51:107–118. doi: 10.1002/dev.20350. [DOI] [PubMed] [Google Scholar]
- 28.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 30.Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam MM, Booth JR. Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AL, Rapoport J. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 32.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 33.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- •34.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5:1–14. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article presents findings mapping the dynamic neurodevelopment of multiple functional networks involved in cognitive processing.
- 35.Stevens MC, Skudlarski P, Pearlson GD, Calhoun VD. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. Neuroimage. 2009;48:738–746. doi: 10.1016/j.neuroimage.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- ••37.Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp225. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an antisaccade task, this article demonstrates how reward motivation dynamically influences recruitment of striatal and prefrontal regions in adolescents.
- 38.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- ••40.Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp078. epub ahead of print. [DOI] [PubMed] [Google Scholar]; Using a passive viewing gambling task, this article reports straital and prefrontal regions that more sensitive to the anticipation and receipt of rewards in adolescence.
- •41.Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk. Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]; Here authors report a positive relationship between striatal sensitivity to reward cues and real life risk-taking behavior.
- 42.Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Child Psychology and Psychiatry. 2009;50:1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohls G, Petzler J, Hepertz-Dahlmann B, Konrad K. Differential effects of social and nonsocial reward on response inhibition in children and adolescents. Developmental Science. 2009;12:614–625. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- 45.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 46.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluff E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 47.Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]