Abstract
Objective
The goal of this study was to test the contributing role of increasing glucose uptake in vascular smooth muscle cells (VSMC) in vascular complications and disease.
Methods and Results
A murine genetic model was established in which GLUT1, the non-insulin dependent glucose transporter protein, was overexpressed in smooth muscle using the sm22α promoter. Overexpression of GLUT1 in smooth muscle led to significant increases in glucose uptake (n=3, p<0.0001) as measured using radiolabeled 2-deoxyglucose. Fasting blood glucose, insulin and non-esterified fatty acids (NEFA) were unchanged. Contractility in aortic ring segments was decreased in sm22α-GLUT1 mice (n=10, p<0.04). In response to vascular injury, sm22α-GLUT1 mice exhibited a pro-inflammatory phenotype including a significant increase in the percentage of neutrophils in the lesion (n=4, p <0.04) and an increase in MCP-1 immunofluorescence. Circulating haptoglobin and GSH/total GSH were significantly higher in the sm22α-GLUT1 mice post injury compared to controls (n=4, p<0.05), suggesting increased flux through the pentose phosphate pathway. sm22α-GLUT1 mice exhibited significant medial hypertrophy following injury that was associated with a significant increase in the percent of VSMC in the media staining positive for nuclear phosphoSMAD2/3 (n=4, p <0.003).
Conclusions
In summary, these finding suggest that increased glucose uptake in VSMCs impairs vascular contractility, and accelerates a pro-inflammatory, neutrophil-rich lesion in response to injury as well as medial hypertrophy that is associated with enhanced TGFβ activity.
Keywords: GLUT1, Vascular smooth muscle, haptoglobin, hypertrophy, phosphoSMAD2/3
INTRODUCTION
Cardiovascular complications remain the number one cause of death from individuals with diabetes. Epidemiological studies to date have reported conflicting results over the role of glucose as a contributing risk factor to coronary artery disease in individuals with type 1 diabetes1–9. A recent report found that glycated hemoglobin in non-diabetic adults was strongly associated with coronary artery disease; further suggesting that increasing cellular glucose uptake promotes vascular complications and coronary artery disease10. However, the mechanisms through which glucose increases risk of coronary artery disease are not well understood. Specifically, the contributing role of increasing glucose uptake in different cell types to coronary artery disease is not well understood.
We tested the hypothesis that increasing glucose uptake in vascular smooth muscle cells would alter the contractility properties of the vessel. In addition, we tested the hypothesis that in response to vascular injury, increased glucose uptake would exacerbate vascular intima formation.
We used a genetic approach to increase expression of the non-insulin dependent glucose transporter protein GLUT1 in VSMCs using the sm22α promoter. This led to a significant increase in glucose uptake in smooth muscle without altering circulating levels of glucose, insulin or NEFA. The advantage of this model is the ability to specifically isolate the effects of glucose in the absence of alterations in free fatty acids and insulin specifically in VSMCs in the vessel wall. This model allows us to study how enhanced glucose uptake in VSMCs over the life span of the mouse alters the phenotype of the vasculature. These results represent novel findings as well as verification of recent work in other new genetic models that are leading to a better understanding of how glucose increases the risk of coronary artery disease.
MATERIALS AND METHODS
Genetic Model
sm22α-GLUT1 mice were generated by utilizing the well-characterized 441bp region (exon 1 to +41) of the sm22α promoter11 and the full length human GLUT1 cDNA (gift from Dr. Mueckler). The fragment for injection containing the 441bp sm22a promoter (including the exon1 to +41), human glut1, and SV40 polyA region was removed by digestion with NotI and XhoI and the fragment was run on a 0.8% gel, and excised. Injection concentration was 4ng/ul. Standard microinjection into C57BL/6 fertilized eggs and implantation into foster females was completed at the Mouse Genetics Laboratory at the University of Minnesota. Mice remained in a C57BL/6 background. Primers used for genotyping were (sm22α-forward) 5’-TAAACCCCTCACCCAGCCGGCGCCCC-3’ and (HuGLUT-reverse) 5’-CCGGGATGAAGATGATGCTCAGCAGCAGGGG-3’. Mice were euthanized according to our approved IACUC protocol with a compressed air carbon dioxide chamber.
Quantitative Real-Time PCR (RTQPCR)
RNA was isolated from murine aorta with Qiagen’s RNeasy kit and reverse transcribed to cDNA performed according to manufacturer’s directions (Clontech). Transcript abundance was performed using the Applied Biosystems (ABI) primer probe sets and the ABI 7900HT (see Supplemental Table 1). Target amplification and detection were performed in replicated fashion, allowing for minimization of experimental variability and calculation of ΔCt based on the corresponding reference control, hypoxanthine phosphoribosyltransferase1 (HPRT1) (i.e., Target ΔCtHPRT1)12.
Western blotting
was performed on murine aorta by standard methods as previously described13 utilizing the following primary antibodies: GLUT1 (gift Frank Brosius, U of Michigan, Chemicon), Myosin Light Chain (Cat# 3672, Cell Signaling), Calponin (Cat# sc-28545, Santa Cruz Biotechnology, Inc.), SM Alpha Actin (Cat# A2547, Sigma-Aldrich), Osteopontin (Cat# AB10910, Millipore), Caldesmon (Cat# 2980, Cell Signaling), Rock I (Cat # 4035, Cell Signaling), Rock II (Cat# sc-5561, Santa Cruz Biotechnology, Inc.) and Fibronection (Cat# ab23750, Abcam), Tubulin (Cat# 2148, Cell Signaling) and Vinculin (Cat# V4139, Sigma-Aldrich). Secondary antibodies included goat Anti-Rabbit IgG-HRP (Cat# sc-2004, Santa Cruz Biotechnology, Inc.), goat anti-Rabbit IgG-HRP (Cat# sc-2030, Santa Cruz Biotechnology, Inc.), and goat anti- Mouse HRP (Cat# 1858413, Pierce). Substrates used for developing the blot included SuperSignal West Dura Extended Duration Substrate (Cat# 34075, Thermo Scientific), SuperSignal West Femto Maximum Sensitivity Substrate (Cat# 34095, Thermo Scientific), SuperSignal West Pico Chemiluminescent Substate (Cat# 34080, Thermo Scientific), and Pierce ECL Western Blotting Substrate (Cat# 32106, Thermo Scientific).
Metabolic measurements
were performed as previously described14–17. Briefly, fasting blood glucose was monitored with a hand-held clinical glucometer (Roche), fasting NEFA were measured spectrophotometrically utilizing a kit (Wako Chemicals), and insulin was measured with an ultrasensitive mouse insulin ELISA kit from ALPCO Diagnostics. Glucose uptake was measured with radiolabeled 2-deoxyglucose in primary VSMC in the presence and absence of cytochalasin B as we have described14. Samples were incubated for 30 min in glucose free MEM. 2-3H-deoxyglucose (0.33 µCi) was added for an additional 5–15 min (we confirmed that transport was linear during this interval), washed with ice-cold buffer containing phloretin (0.1mM) to terminate transport, solubilized in 1N NaOH, neutralized, and counted in a beta scintillator. Glucose uptake was normalized to protein content.
Isolation of VSMCs
Aortic smooth muscle cells were isolated from 16 week old mice according to the protocol by Ray et al18. Briefly, aortas were harvested and digested in collagenase Type 2. Cell were plated and maintained in high glucose DMEM supplemented with 1% penicillin and streptomycin and 10% fetal calf serum.
Vascular Contractility
Aortic ring segments (non-denuded) were mounted on stirrups in ring baths in Earle’s balanced salts solution as previously described19 and allowed to equilibrate at a resting tension of 400 mg for at least 60 minutes. Aortic segments were exposed three times to 60 mM KCl at 30-minute intervals. The contractile responses to 1 µM phenylephrine (PE) and 10 µM serotonin (5-HT) were then assessed in random order with washout and re-equilibration periods of 30 minutes. Vascular relaxation measurements were also performed in non-denuded aortic rings in response to 10−6 M Acetylcholine. No differences were identified in vascular relaxation properties between groups (data not shown).
Surgical Intervention to induce Vascular Lesion
All protocols were in accordance with institutional IACUC guidelines. Briefly, a straight guide wire (0.38 mm in diameter) was inserted into the left femoral artery of anaesthetized mice via a small muscular branch as described20–21. The wire was left in the lumen for 1 min to denude and dilate the artery. After removing the wire the small branch was tied off and blood flow was restored in the injured vessel.
Morphometric measurements of
femoral arteries collected at 7, 14 and 28 days post wire injury. Mice were perfused with PBS followed by 10% neutral-buffered formalin (In Vivo Rodent Perfusion System, Automate Scientific). Morphometric evaluation of femoral arteries was performed by staining serial 5 µm thick paraffin embedded sections with hematoxylin-eosin (H&E) and examination under an Olympus BX41 microscope. Sections (8–10 per mouse), covering 500 µm of femoral artery length were measured for internal elastic lamina (IEL), external elastic lamina (EEL) length and intimal and medial areas. All the measurements were performed using the ImageJ software (NIH ImageJ)20.
Immunostaining
5 µm sections of paraffin embedded femoral arteries from injured and uninjured animals was stained for GLUT1 (Gift from Dr. Brosius). Macrophages (anti-Mac3, BD Biosciences; anti-F4/80, Serotec) and neutrophils (anti-Ly6G antibody, Novus Biologicals) were identified by using the respective IgG ABC Vectastain kit according to the manufacturer’s protocol (Vector Laboratories). Elastin was assessed by Veerhoff Van Giesons’s stain. VSMC were identified by immunostaining with smooth muscle alpha actin (SM Alpha Actin (clone 1A4, Sigma-Aldrich). Proliferating VSMC were identified by co-staining with Ki-67 (Cat# RM-9106, Thermo Scientific). Apoptotic cells were detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) (Cat# 11 684 795 910, Roche Diagnostics). Neutrophils, macrophages and VSMCs (TUNEL and Ki67 positive) in the sections were imaged and quantified at 60X on a Zeiss AxioVision Upright microscope (n=4 animals, 3–4 sections per animal, per cohort) and are represented as total cells in the lesion at 7 days post injury. PhosphoSMAD stained nuclei were identified by using an anti-pSMAD2/3 antibody (ab65847, Abcam), imaged and quantified at 100X on a Zeiss AxioVision Upright microscope (n=3 animals, 3–4 sections per animal, per cohort) and are represented as percent of total cells in the media.
Confocal microscopy
of MCP-1 staining was performed using an anti-MCP-1 antibody (Santa Cruz Biotechnology) followed by secondary incubation was in Alexa Fluor 488 (Invitrogen) in sections of vessels 7 day post injury (Olympus Fluoview Confocal microscope, 60X magnification, n=3 animals, 3–4 sections per animal, per cohort).
Measurement of circulating factors
Measurements of circulating factors in serum 16 week old male mice were performed by utilizing the following kits: Haptoglobin (ELISA, Alpco Diagnostics, 41-HAPMS-E01), MCP-1 (ELISA, R&D Systems) and Glutathione/Total Glutathione (GSH) (Abcam). All the assays were run according to the manufacturers’ instructions.
Statistical analysis
Values are represented as mean ± SE. An analysis of variance (ANOVA) with a Tukey’s post hoc test was used for multiple comparisons. A Student’s t test was used for comparisons between two groups.
RESULTS
The goals of this study were to determine if increasing glucose uptake in smooth muscle specifically contributed to changes in vascular phenotype at baseline and in response to injury. We established a novel murine model in which the non-insulin dependent glucose transporter protein, GLUT1, was up-regulated in smooth muscle. Increased immunostaining of GLUT1 was seen in the medial wall of sm22α-GLUT1 femoral arteries compared to controls (Figure 1a and b). GLUT1 protein levels in aorta of sm22α-GLUT1 mice were also increased (Figure 1c). The increase in GLUT1 resulted in increased glucose uptake as measured by radiolabeled 2-deoxyglucose in VSMCs isolated from male adult mice (p< 0.0001) (Figure 1d). Fasting blood glucose, insulin, and NEFA concentrations were not significantly different in sm22α-GLUT1 mice compared to controls (see Table 1). Medial thickness (data not shown) and smooth muscle cell number in the medial wall of the femoral artery were unaffected (54 ± 9 VSMC vs. 52 ± 5 VSMC in control and sm22α-GLUT1 respectively, ns). Neutrophils and macrophages in the vessel wall were also not different (data not shown).
Figure 1.
Representative microphotographs of GLUT1 immunostaining in smooth muscle layer of femoral artery in control (a) and sm22α-GLUT1 (b). Representative Western blotting of GLUT1 in aorta from control and sm22α-Glut1 mice (c). Glucose uptake in VSMCs is significantly higher in isolated aortic VSMCs from sm22α-GLUT1 mice vs. control (c.) (n=3, p < 0.0001).
Table 1.
Fasting blood glucose, insulin and NEFA levels in serum from 8–12 week old control and sm22α-GLUT1 mice.
| Genotype | Fasting Blood Glucose (mg/dL) |
Insulin (mIU/ml) |
NEFA (mEq/L) |
|---|---|---|---|
| Control | 135 ± 7 | 3.6 ± 0.3 | 1.3 ± 0.8 |
| sm22α-GLUT1 | 128 ± 7 | 3.7 ± 0.6 | 1.1 ± 0.03 |
Data represented as mean ± SE, n=3-16 per group per test, p=ns.
Next, we assessed vascular contractility in aortic rings. Overexpression of GLUT1 was associated with a significant decrease in vascular contractility in response to phenylephrine (PE) (p < 0.03) and serotonin (5-HT) (p < 0.04) (Figure 2). To examine the potential mechanisms whereby vascular contractility was decreased in response to increased glucose uptake, we assessed expression of extracellular matrix proteins, contractile proteins, matrix, and calcium handling targets via Western blotting (Figure 3a) and gene expressions via RTQPCR (Supplemental Table I). Fibronectin, a component of the basal lamina of the smooth muscle layer was decreased in the sm22α-GLUT1 aorta as measured by densitometry (Figure 3a and 3b) (n=4, p < 0.003). The matrix gene, decorin harboring a glucose response element in the promoter region, exhibited a minor yet statistically significant increase in sm22α-GLUT1 aorta (Supplemental Table I) (n = 8–9/group, p <0.04). No significant differences were detected in decorin at the protein level (data not shown). Thus, increased glucose uptake resulted in decreased expression protein expression of fibronectin and an increase in the RNA expression of the matrix gene, decorin, which contains a glucose response element in the promoter region.
Figure 2.
Contractility of aortic ring segments from sm22α-GLUT1 mice was significantly reduced in the presence of phenylephrine (PE) (p<0.03) or serotonin (5-HT) (p<0.04). Aortic ring segments (control n=10 and sm22α-GLUT1 n=10) were allowed to equilibrate at a resting tension of 400 mg for at least 60 min prior to exposure to 60 mM potassium chloride (KCl) at 30 min intervals, 3x. The contractile responses to 1 µM PE and 10 µM 5-HT were then assessed in random order with washout and re-equilibration periods of 30 minutes.
Figure 3.
Western analysis of proteins in aorta from control and sm22α-GLUT1 mice (a). Fibronectin expression in sm22α-GLUT1 (n=4) is significantly decreased as compared to control aorta (n=4), p<0.003 (b).
We utilized a well-established femoral artery wire injury model to determine if increased glucose uptake in smooth muscle affected the process of vascular remodeling. Femoral arteries were harvested 7, 14 and 28 days post injury. Seven days following injury, a significant increase in the number of inflammatory cells was seen in the lesion of sm22α-GLUT1 mice compared to controls (n = 4 per group, p < 0.05) (Figure 4a–c). A specific increase in neutrophils was detected in the sm22α-GLUT1 mice by staining with anti-Ly6G antibody (Figure 4d–f). The number of macrophages in the lesion 7 days post injury was not different between the groups (Figure 4g–i). The number of VSMCs in the lesion from sm22α-GLUT1 mice was significantly lower as compared to controls 7 days post injury (control 53 ± 4 and sm22α-GLUT1 24 ± 1*, p < 0.002). Despite the reduced total number of VSMC in the lesions of sm22α-GLUT1, a greater percentage of these VSMCs were proliferating, as assessed by Ki67 staining, as compared to control mice (15 ± 3 and 6 ± 1* respectively, p<0.02). Apoptosis measurements of VSMC in the intima were not different between the sm22α-GLUT1 and control mice. The increase in neutrophils in the sm22α-GLUT1 mice was associated with an increase in MCP-1 staining in the vessel (Figure 5a–c). In addition, circulating MCP-1 was significantly higher in sm22α-GLUT1 in response to injury (Figure 5d).
Figure 4.
Inflammation induced by increased glucose uptake. Representative H & E stained photomicrographs showing lesion at 7 days post injury in control (a) and sm22α-GLUT1 (b) mice and total number of inflammatory cells in lesion (n=4, p < 0.04) (c). Representative photomicrographs showing neutrophils, as assessed by staining with anti-Ly6G antibody, in lesion at 7 days post injury in control (d) and sm22α-GLUT1 (e). Increased neutrophils in sm22α-GLUT1 lesion (n=4, p < 0.04) (f). Representative photomicrographs showing macrophages (highlighted with red arrowhead), as assessed by staining with anti-Mac3 antibody, in lesion at 7 days post injury in control (g) and sm22α-GLUT1 (h). The total number of macrophages was not significantly different between the groups (n=4, p = ns) (i).
Figure 5.
MCP-1 immunofluorescence in lesion at 7 days post injury in control (a) and sm22α-GLUT1 mice (b). Isotype IgG stained section from control with DAPI positive nuclei (c). Circulating MCP-1 is higher in sm22α-GLUT1 as compared to control in response to vascular injury (d). *p<0.002 control (n=4) vs sm22α-GLUT1 (n=5) values at 7 days post injury. #p<0.003 sm22α-GLUTI value at 0 day (n=9) vs sm22α-GLUT1 (n=5) values at 7 days post injury. There was no significant difference in MCP-1 between controls at 0 day (n=16) and controls at 7 days post injury (n=4).
In response to vascular injury, the sm22α-GLUT1 mice also exhibited an increase in circulating haptoglobin and glutathione (Table 2). The significant increase in these two factors suggests increased flux through the pentose phosphate shuttle in the sm22α-GLUT1 mice in response to injury.
Table 2.
Circulating Haptoglobin and Glutathione (GSH) levels in serum from control and sm22α-GLUT1 mice.
| Days | Haptoglobin (µg/mL) | GSH/Total GSH (µg/uL) | ||
|---|---|---|---|---|
| Control | sm22α-GLUT1 | Control | sm22α-GLUT1 | |
| 0 | 85 ± 1 (n=6) |
92 ± 19 (n=6) |
0.072 ± 0.004 (n=8) |
0.067 ± 0.006 (n=8) |
| 7 | 302 ± 194 (n=4) |
1322 ± 596* (n=4) p<0.05 |
0.095 ± 0.020 (n=3) |
0.144 ± 0.027* (n=4) p<0.003 |
Serum was collected from control and sm22α-GLUT1 mice at 7 days post injury. Haptoglobin was assessed by ELISA (Alpco Diagnostics). GSH was detected by a fluorometric assay performed according to the manufacturer’s instruction (Abcam). GSH levels are represented as a ratio of GSH to total GSH. Values are mean ± SE, statistical significance as compared to corresponding sm22α-GLUT1 values at day 0.
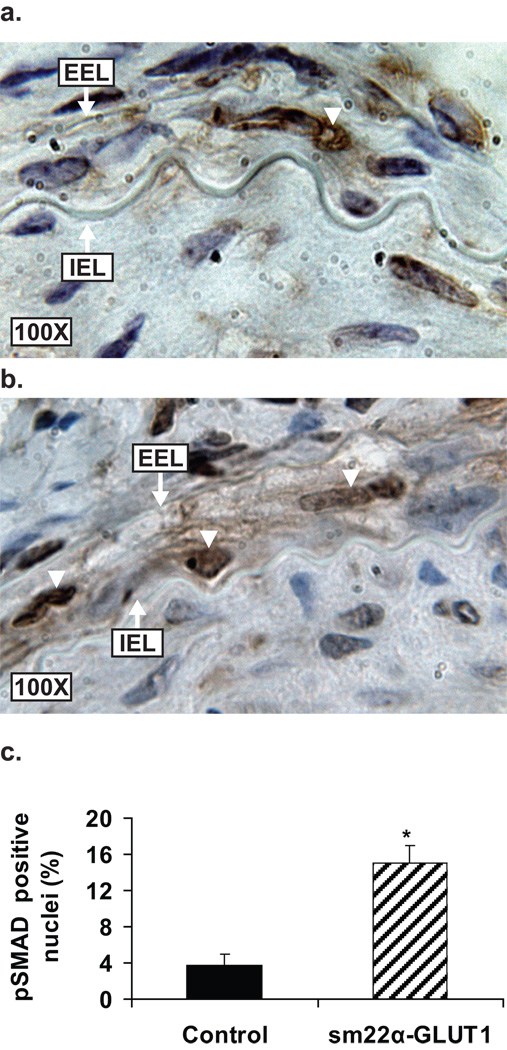
No change in the intimal area was detected between the control and sm22α-GLUT1 mice at 14 or 28 days post injury (Table 3). Sm22α-GLUT1 mice exhibited a significant increase in medial area at 28 days (medial hypertrophy) (Table 3). We hypothesized that the increase in medial area in the sm22α-GLUT1 mice was due to activation of the TGF-β signaling pathway in the nucleus of medial VSMCs. To test this hypothesis we stained injured vessels from sm22α-GLUT1 and control mice for phosphoSMAD2/3 – a molecular marker of activated TGFβ signaling. Figure 6a–c shows a significant increase in the percentage of VSMC nuclei staining positive for phosphoSMAD2/3 in the media of sm22α-GLUT1 compared to control mice at 28 days post injury. The number of medial VSMCs at 28 days was not significantly different between the groups (control 42 ± 3 and sm22α-GLUT1 48 ± 6, n=4, p=ns). Thus, the increase in medial area combined with the increase in phosphoSMAD2/3 staining in the media supports a hypertrophic signal in the vessel wall in response to injury.
Table 3.
Morphometric analysis of femoral arteries in response to wire injury at 14 and 28 days.
| Measurements | 14 days | 28 days | ||
|---|---|---|---|---|
| Control (n=9) |
sm22α-GLUT1 (n=9) |
Control (n=9) |
sm22α-GLUT1 (n=6) |
|
| Intimal area (µm2) | 23,222 ± 2,598 | 19,901 ± 383 | 47,599 ± 4,733 | 51,470 ± 4,979 |
| Medial area (µm2) | 20,531 ± 1,458 | 16,936 ± 928 | 16,861 ± 1,839 | 27,129 ± 3,091* |
| I/M Ratio | 1.17 ± 0.11 | 1.32 ± 0.33 | 2.99 ± 0.12 | 2.06 ± 0.24* |
Control and sm22α-GLUT1 mice underwent wire injuries and were sacrificed and perfusion fixed 14 and 28 days post injury. Intimal and medial areas were calculated following image capture with NIH imageJ. Data are represented as mean ± SE (n=6-9, *p < 0.05).
Figure 6.
Increased phosphoSMAD2/3 in the nuclei of medial VSMC post injury in sm22αα-GLUT1 mice (a) and control mice (b). Percent of phosphoSMAD2/3 positive nuclei (white arrowheads) is increased in sm22α-GLUT1 as compared to controls at 28 days post injury (n=4, p<0.003).
DISCUSSION
The major findings from this study are that increasing glucose uptake in vascular smooth muscle via overexpression of GLUT1 impairs contractility of the vessel wall, and potentiates inflammation and medial hypertrophy in response to vascular injury. The decreased contractility was accompanied by a significant loss of fibronectin in the sm22α-GLUT1 mice. In response to vascular injury, sm22α-GLUT1 mice exhibited a significant increase in neutrophils in the lesion that was associated with elevated circulating MCP-1 as well as increased localized MCP-1 staining in the vessel wall. Finally, increased expression of GLUT1 in smooth muscle resulted in a significant increase in medial hypertrophy that was associated with an increase in phospho-SMAD2/3 staining, indicative of enhanced TGFβ activity.
The decrease in vascular contractility in a mouse model with significant increases in glucose uptake has been reported in previous models of diabetic animal models22–24. This impaired vascular contractility was associated with a decrease in fibronectin. Recent work has identified a role for multiple extracellular matrix proteins and arterial stiffness and contractility including fibronectin25–28. Fibronectin binds to many different integrin binding proteins that regulate both contraction and relaxation. A limitation of this study is that we did not perform this experiment on vessel with denuded endothelium. We did however perform a small number of experiments in which we assessed vascular relaxation in response to acetylcholine and did not detect differences between the sm22α-GLUT1 and control vessels (data not shown). Finally, we found no loss of elastin by Veerhoff Van Gieson staining (data not shown) that has been reported in previous models of diabetes29–30. More work will be needed to determine the mechanisms governing the decrease in contractility in response to increased glucose metabolism.
We identified a significant increase in circulating haptoglobin in response to injury in sm22α-GLUT1 mice. Haptoglobin is a serum protein that binds to extracorpuscular hemoglobin. Released from red blood cells within a hemorrhaging plaque, extracorpuscular hemoglobin induces a potent stimulus for inflammation31. Haptoglobin binding to extracorpuscular hemoglobin attenuates the inflammatory and oxidative potential31. Haptoglobin is significantly increased in both type 1 and type 2 diabetic individuals32–33 and also in response to acute coronary syndrome, inflammation, tissue destruction and neoplasia34–36. The increase in circulating haptoglobin in diabetic individuals and in mice with increased GLUT1 suggests a common mechanism of increased glucose metabolism. A landmark genetic finding in 1963 identified an association between deficiency in the enzyme glucose-6-phosphate dehydrogenase and decreased serum haptoglobin37. Glucose-6-phosphate dehydrogenase is a rate-determining enzyme in the pentose phosphate shuttle. The link between glucose metabolism and haptoglobin appears to be through the pentose phosphate pathway. Suzuki and colleagues showed that flux through the pentose phosphate pathway is minimal under resting conditions in vascular smooth muscle cells (4% under low glucose conditions and 8% under high glucose conditions)38. In response to vascular injury, haptoglobin is significantly increased in the sm22α-GLUT1 mice, suggesting that flux through the pentose phosphate pathway is significantly increased post injury. Glutathione levels are also increased in response to injury in the sm22α-GLUT1 mice – a second indication that flux through the pentose phopsphate pathway may be enhanced. Irregardless, this data suggests that increased glucose flux in VSMC promotes two anti-oxidative signaling pathways – suggesting two protective mechanisms of glucose flux.
We postulated that MCP-1 was significantly elevated in sm22α-GLUT1 mice. This hypothesis was based on previous work showing increased MCP-1 in diabetic animal models and in response to high glucose39–40. In line with this hypothesis, MCP-1 immunostaining in the vessel wall and circulating MCP-1 levels were significantly elevated in sm22α-GLUT1 mice. Burke et al reported that the inflammatory cell composition of lesions in humans with type 1 and type 2 diabetes is increased compared with non-diabetics41. Booth and colleagues demonstrated increased leukocyte rolling and adherence in response to glucose infusion in the rat42. A novel model to study the effect of glucose on vascular inflammation has also been developed in which diabetes is induced through a virus in an LDL-receptor deficient background. In this model, inflammation is enhanced through increased macrophage accumulation43–44. Olive et al demonstrated the involvement of inflammatory cells including neutrophils and macrophages in neointimal burden following vascular injury in C57BL/6 mice maintained on a normal diet45. Similarly, in our model of overexpressing GLUT1, in a C57BL/6 background we identified macrophages as well as neutrophils in the lesion at 7 days post injury. We did not detect an increase in macrophage accumulation in injured vessels but instead identified an increase in neutrophils in the vessel wall that was associated with an increase in MCP-1 staining in the vessel and circulating in the blood. These findings support and extend earlier work by Torreggiani in which a transgenic mouse model with decreased circulating levels of advanced glycosylated end products exhibited decreased inflammation46. Few studies have assessed the role of glucose in vascular inflammation in the absence of alterations in insulin or lipids. The work presented in this study adds new information to the field and suggests that increased glucose flux in smooth muscle promotes pro-inflammatory signaling pathways mediated in part through MCP-1 that recruit neutrophils to the vessel wall.
Finally, the medial wall of the GLUT1 overexpressing mice underwent significant hypertrophy, but only in response to injury. Previous work has shown an essential role for TGFβ signaling in vascular smooth muscle cell hypertrophy47–48. We hypothesized that the increase in medial area seen in the sm22α-GLUT1 mice was mediated in part through activation of the TGFβ – Smad3 pathway. We identified an increase in phospho-SMAD2/3 immunostaining specific for VSMC within the media of sm22α-GLUT1 mice compared to control mice in response to injury. This correlated with the increased medial thickness in the sm22α-GLUT1 mice. Previous studies have shown a role of TGFβ – Smad3 pathway in glucose induced hypertrophy49–50. The increase in number of phospho-SMAD2/3 stained nuclei together with the absence of any significant difference in the number of medial VSMCs in sm22α-GLUT1 supports a role of TGFβ – Smad3 pathway in glucose dependent hypertrophic signal in the vessel wall in response to injury.
We did not detect any differences in intimal hyperplasia in response to vascular injury. Landmark work by Kunjathoor and colleagues showed that the effect of diabetes on lesion formation (atherosclerosis) was strain-dependent (BALB/c vs. C57BL/6) – suggesting that the role of glucose, fat and cholesterol on lesion development was dependent on the underlying genetic structure of these mouse strains. C57BL/6 mice showed extensive lesion development in response to fat feeding alone51. Earlier work by Nishina using the C57BL6 strain showed that mutations in genes that predisposed these mice to diabetes or obesity did not alter lesion formation when fed a high fat diet52. In children with type 1 diabetes, aortic/intima 53 media thickness (IMT), but not carotid IMT was found to correlate with glycosylated hemoglobin53. A study by Larsen et al also found a significant correlation between HbA1c and carotid IMT54. Jensen-Urstad and colleagues found that intensified conventional insulin treatment in patients with type 1 diabetes decreased carotid intima media thickness and arterial wall stiffness compared to standard insulin therapy55. Thus, our data would suggest that increased glucose uptake in VSMC in the absence of vascular intervention does not induce hypertrophy or proliferation of VSMC. These findings are in agreement with our previous in vitro studies supporting a role for increased glucose metabolism and increased cell cycle progression56. In large animal diabetic models, previous work has shown that high glucose does not directly stimulate proliferation in the absence of injury38. Our work in the uninjured vessels supports this.
In summary, we have utilized a genetic approach to provide new insights into the role of glucose in vascular complications. This study specifically identifies a role for increased glucose uptake into VSMC in contributing to altered vascular contractility. In addition, the increased glucose uptake in VSMC potentiated inflammatory signaling pathways in the vessel wall. However, increased glucose uptake restricted to VSMC did not alter the size of the lesion in response to vascular intervention but did result in medial hypertrophy at 28 days.
Supplementary Material
ACKNOWLEDGEMENTS
GLUT1 antibody was a gift from Dr. Frank Chip Brosius, University of Michigan, Ann Arbor.
Sources of funding
J.L.H. is supported by the JDRF 1-2007-819 and E.K.W. is supported by NIH RO1 HL-65322.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION
N.A. hypothesis, experimental design, performed experiments, interpreted data, wrote manuscript, reviewed manuscript, edited manuscript
D.L.B. performed experiments
M.C. performed experiments
A.M. performed experiments
Z.H. performed experiments, experimental design, interpreted data, wrote manuscript
U.L. performed experiments
S.M. performed experiments
E.K.W. performed experiments, experimental design, interpreted data, wrote, reviewed manuscript
J.L.H. hypothesis, experimental design, interpreted data, wrote, reviewed and edited manuscript
Disclosures
None.
REFERENCES
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33). Uk prospective diabetes study (ukpds) group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Forrest KY, Becker DJ, Kuller LH, Wolfson SK, Orchard TJ. Are predictors of coronary heart disease and lower-extremity arterial disease in type 1 diabetes the same? A prospective study. Atherosclerosis. 2000;148:159–169. doi: 10.1016/s0021-9150(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 3.Hashim S, Li Y, Anand-Srivastava MB. G protein-linked cell signaling and cardiovascular functions in diabetes/hyperglycemia. Cell Biochem Biophys. 2006;44:51–64. doi: 10.1385/CBB:44:1:051. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in iddm by sex and duration. Pittsburgh epidemiology of diabetes complications study ii. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 6.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779–784. doi: 10.1136/bmj.313.7060.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, Manes C, Fuller JH. Risk factors for coronary heart disease in type 1 diabetic patients in europe: The eurodiab prospective complications study. Diabetes Care. 2004;27:530–537. doi: 10.2337/diacare.27.2.530. [DOI] [PubMed] [Google Scholar]
- 9.Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, Krahenbuhl S, Diem P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006;152:27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Miano JM, Cserjesi P, Olson EN. Sm22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 12.Hall JL, Grindle S, Han X, Fermin D, Park S, Chen Y, Bache RJ, Mariash A, Guan Z, Ormaza S, Thompson J, Graziano J, de Sam Lazaro SE, Pan S, Simari RD, Miller LW. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004;17:283–291. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the beta-catenin/t-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90:340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- 14.Hall JL, Henderson J, Hernandez LA, Kellerman LA, Stanley WC. Hyperglycemia results in an increase in myocardial interstitial glucose and glucose uptake during ischemia. Metabolism. 1996;45:542–549. doi: 10.1016/s0026-0495(96)90022-0. [DOI] [PubMed] [Google Scholar]
- 15.Hall JL, Lopaschuk GD, Barr A, Bringas J, Pizzurro RD, Stanley WC. Increased cardiac fatty acid uptake with dobutamine infusion in swine is accompanied by a decrease in malonyl coa levels. Cardiovasc Res. 1996;32:879–885. [PubMed] [Google Scholar]
- 16.Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase c-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.res.87.7.574. [DOI] [PubMed] [Google Scholar]
- 17.Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD, McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. Am J Physiol. 1996;271:H2320–H2329. doi: 10.1152/ajpheart.1996.271.6.H2320. [DOI] [PubMed] [Google Scholar]
- 18.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 19.Hong Z, Smith AJ, Archer SL, Wu XC, Nelson DP, Peterson D, Johnson G, Weir EK. Pergolide is an inhibitor of voltage-gated potassium channels, including kv1.5, and causes pulmonary vasoconstriction. Circulation. 2005;112:1494–1499. doi: 10.1161/CIRCULATIONAHA.105.556704. [DOI] [PubMed] [Google Scholar]
- 20.Basi DL, Adhikari N, Mariash A, Li Q, Kao E, Mullegama SV, Hall JL. Femoral artery neointimal hyperplasia is reduced after wire injury in ref-1 +/− mice. Am J Physiol Heart Circ Physiol. 2007;292:H516–H521. doi: 10.1152/ajpheart.00246.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol. Cell Cardiol. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 22.Abboud K, Bassila JC, Ghali-Ghoul R, Sabra R. Temporal changes in vascular reactivity in early diabetes mellitus in rats: Role of changes in endothelial factors and in phosphodiesterase activity. Am J Physiol Heart Circ Physiol. 2009;297:H836–H845. doi: 10.1152/ajpheart.00102.2009. [DOI] [PubMed] [Google Scholar]
- 23.Ajay M, Achike FI, Mustafa MR. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol Res. 2007;55:385–391. doi: 10.1016/j.phrs.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Zeydanli EN, Turan B. Omega-3e treatment regulates matrix metalloproteinases and prevents vascular reactivity alterations in diabetic rat aorta. Can J Physiol Pharmacol. 2009;87:1063–1073. doi: 10.1139/Y09-112. [DOI] [PubMed] [Google Scholar]
- 25.Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48:433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, James JF, Li DY, Narmoneva DA, Mecham RP, Benson DW. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res. 2010;107:549–557. doi: 10.1161/CIRCRESAHA.110.221358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hocking DC, Titus PA, Sumagin R, Sarelius IH. Extracellular matrix fibronectin mechanically couples skeletal muscle contraction with local vasodilation. Circ Res. 2008;102:372–379. doi: 10.1161/CIRCRESAHA.107.158501. [DOI] [PubMed] [Google Scholar]
- 28.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan CY, Wang RR, Beazley JS, Lee RM. Alterations of elastin and elastase-like activities in aortae of diabetic rats. Biochim Biophys Acta. 1988;967:322–325. doi: 10.1016/0304-4165(88)90027-x. [DOI] [PubMed] [Google Scholar]
- 30.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, Chait A, O'Brien KD. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149–1157. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, Moreno PR. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2007;27:134–140. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- 32.McMillan DE. Increased levels of acute-phase serum proteins in diabetes. Metabolism. 1989;38:1042–1046. doi: 10.1016/0026-0495(89)90038-3. [DOI] [PubMed] [Google Scholar]
- 33.Van Campenhout A, Van Campenhout C, Lagrou AR, Abrams P, Moorkens G, Van Gaal L, Manuel-y-Keenoy B. Impact of diabetes mellitus on the relationships between iron-,inflammatory- and oxidative stress status. Diabetes Metab Res Rev. 2006;22:444–454. doi: 10.1002/dmrr.635. [DOI] [PubMed] [Google Scholar]
- 34.Nosslin BF, Nyman M. Haptoglobin determination in diagnosis of haemolytic diseases. Lancet. 1958;1:1000–1001. doi: 10.1016/s0140-6736(58)91804-x. [DOI] [PubMed] [Google Scholar]
- 35.Nyman M. Serum hatoglobin; methodological and clinical studies. Scand J Clin Lab Invest. 1959;11:1–169. [PubMed] [Google Scholar]
- 36.Brunetti ND, Correale M, Pellegrino PL, Cuculo A, Biase MD. Acute phase proteins in patients with acute coronary syndrome: Correlations with diagnosis, clinical features, and angiographic findings. Eur J Intern Med. 2007;18:109–117. doi: 10.1016/j.ejim.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb A, Wisch N, Ross J. Familial hypohaptoglobinemia. A genetically determined trait segragating from glucose-6-phosphate dehydrogenase deficiency. Blood. 1963;21:129–140. [PubMed] [Google Scholar]
- 38.Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: Lack of direct growth-promoting effects of high glucose levels. Diabetes. 2001;50:851–860. doi: 10.2337/diabetes.50.4.851. [DOI] [PubMed] [Google Scholar]
- 39.Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappab activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol. 2002;13:894–902. doi: 10.1681/ASN.V134894. [DOI] [PubMed] [Google Scholar]
- 40.Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17:1362–1372. doi: 10.1681/ASN.2005111196. [DOI] [PubMed] [Google Scholar]
- 41.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: A postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 42.Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: Effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–E856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 43.Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. Journal of Clinical Investigation. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in ldl receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olive M, Mellad JA, Beltran LE, Ma M, Cimato T, Noguchi AC, San H, Childs R, Kovacic JC, Boehm M. P21cip1 modulates arterial wound repair through the stromal cell-derived factor-1/cxcr4 axis in mice. The Journal of Clinical Investigation. 2008;118:2050–2061. doi: 10.1172/JCI31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torreggiani M, Liu H, Wu J, Zheng F, Cai W, Striker G, Vlassara H. Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. Am J Pathol. 2009;175:1722–1732. doi: 10.2353/ajpath.2009.090138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owens GK, Geisterfer AA, Yang YW, Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. The Journal of Cell Biology. 1988;107:771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. Hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin ii. The Journal of Clinical Investigation. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LD. R. Essential role of tgf-beta signaling in glucose-induced cell hypertrophy. Dev Cell. 2009;17:35–48. doi: 10.1016/j.devcel.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor pten. Diabetes. 2006;55:2115–2125. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- 51.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishina PM, Naggert JK, Verstuyft J, Paigen B. Atherosclerosis in genetically obese mice: The mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994;43:554–558. doi: 10.1016/0026-0495(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 53.Harrington J, Pena AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr. 156:237–241. doi: 10.1016/j.jpeds.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 54.Larsen JR, Brekke M, Bergengen L, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Mean hba1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia. 2005;48:776–779. doi: 10.1007/s00125-005-1700-z. [DOI] [PubMed] [Google Scholar]
- 55.Jensen-Urstad KJ, Reichard PG, Rosfors JS, Lindblad LE, Jensen-Urstad MT. Early atherosclerosis is retarded by improved long-term blood glucose control in patients with iddm. Diabetes. 1996;45:1253–1258. doi: 10.2337/diab.45.9.1253. [DOI] [PubMed] [Google Scholar]
- 56.Hall JL, Chatham JC, Eldar-Finkelman H, Gibbons GH. Upregulation of glucose metabolism during intimal lesion formation is coupled to the inhibition of vascular smooth muscle cell apoptosis. Role of gsk3beta. Diabetes. 2001;50:1171–1179. doi: 10.2337/diabetes.50.5.1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.