Abstract
Potassium (K+) is a major osmoticum of plant cells, and the vacuolar accumulation of this element is an especially crucial feature for plants under high-salt conditions. Emerging evidence indicates that cation/proton transporters of the NHX family are instrumental in the H+-linked K+ transport that mediate active K+ uptake at the tonoplast for the unequal partitioning of K+ between vacuole and cytosol. However, and in spite of tenuous supporting evidence, NHX proteins are widely regarded as key players in the sequestration of sodium (Na+) into vacuoles to avert ion toxicity in the cytosol of plants under salinity stress. Here, we propose an updated model positing that NHX proteins fulfill a protective function to minimize salt-related stress mainly through the vacuolar compartmentalization of K+ and, in some cases, of Na+ as well thereby preventing toxic Na+-K+ ratios in the cytosol while accruing solutes for osmotic balance.
Key words: NHX exchangers, vacuole, sodium, potassium, salinity, nutrition
All living cells routinely expel Na+ ions, maintaining a lower concentration in the cytosol than in the surrounding environment. In plants, this export usually occurs at the expense of the H+-motive force created by proton pumps and is driven by Na+/H+ exchangers localized in the plasmalemma and endosomal membranes.1 Given the inside negative electrical membrane potential at the plasma membrane (−120 to −200 mV), a rise in extracellular Na+ concentrations will establish a large Na+ electrochemical gradient that will drive the passive transport of Na+ across the plasma membrane and the potential accumulation of Na+ in the cytosol to 100- to 1,000-fold greater concentrations than those of the extracellular space (either apoplast or soil solution).2 Most of the cytosolic Na+ taken up by roots is extruded back to the soil solution by an energetically costly process. Comparisons of unidirectional Na+ fluxes and rates of net accumulation of Na+ in roots of several plant species indicate that 70–95% of the Na+ permeating into the root symplast is extruded back.1 To date, the electroneutral exchange of Na+ for H+ by plasma membrane Na+/H+ antiport activity is the only mode of transport that has been measured for Na+ efflux in vascular plants.1,3 The SOS1 exchanger, a highly conserved transport protein that seems ubiquitous in plant species, has been shown to mediate Na+ efflux.4,5 Plasma membrane vesicles from the Arabidopsis sos1 mutant retained some Na+/H+ exchange activity, suggesting than additional exchangers might be present, although genetic evidence indicates that SOS1 plays a major role in salt tolerance.4,6
In spite of the large efflux component in Na+ metabolism, the ratio of influx vs. efflux rates dictates that, over time, Na+ will inevitably accumulate in the cytosol of root cells, and eventually in all plant tissues. Hence, the compartmentation of Na+ ions into vacuoles is regarded as a critical mechanism to avert the toxic effects of Na+ in the cytosol while providing additional osmoticum for water uptake and turgor maintenance.3 This function has been attributed to tonoplast localized NHX-like antiporters.3,7 The founding member of this family of cation exchangers,8,9 protein AtNHX1 of Arabidopsis, was initially described as a selective Na+/H+ antiporter because K+ ions in the assay medium did not affect the ion exchange driven by AtNHX1.7 However, subsequent research demonstrated that AtNHX1 mediated both Na+/H+ and K+/H+ exchange in tonoplast vesicles from transgenic tomato plants,10 in artificial proteoliposomes in which AtNHX1 was the only transport protein present,11 and in vacuoles of a yeast mutant strain lacking the endogenous Na+/H+ and K+/H+ antiport activities at the tonoplast.12 Determination of the relative Na+-K+ selectivity of AtNHX1 has produced conflicting results, ranging from preferred Na+ transport over K+,10 to lack of significant Na+-K+ discrimination.11–13 To complicate matters further, the activity of AtNHX1 is regulated by the binding of the calmodulin-like protein AtCaM15 to its C-terminus.14 This interaction, which has been suggested to occur upon stress-induced rises in intracellular Ca2+, modified the Na+-K+ selectivity of the antiporter, lowering the Vmax of the Na+/H+ exchange activity without affectation of the K+/H+ exchange activity, thereby decreasing the Na+-K+ transport ratio. Moreover, mutants of AtNHX1 that conferred improved halotolerance to yeast cells showed greater substrate discrimination, favoring K+ transport over that of Na+.12
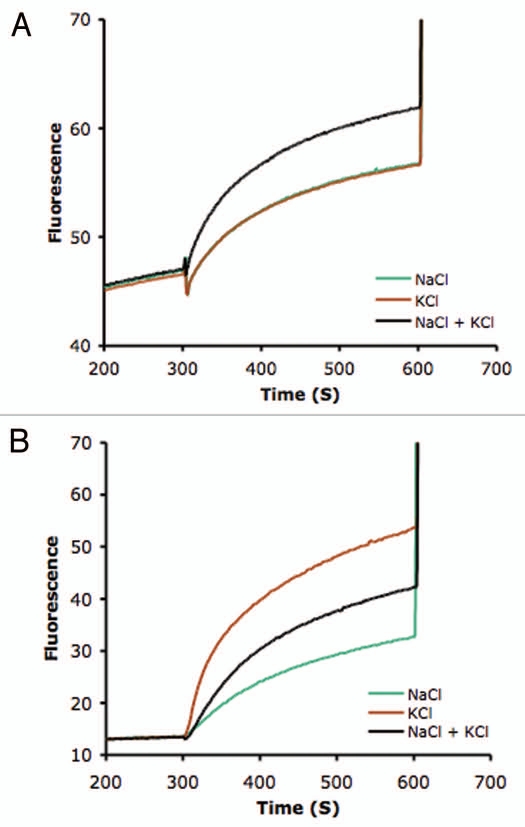
Plant and fungal NHX antiporters are phylogenetically related to NHE family of mammalian Na+/H+ exchangers. On the basis of protein sequence similarity, the NHE/NHX family can be classified into two major groups that have been termed Plasma Membrane (PM) and Intra-Cellular (IC) according to their subcellular localization.15 The PM group is exclusively present in animal cells whereas members of the IC group can be found in animals, plants and fungi. All plant NHXs characterized to date can be assigned to the IC group and be further subdivided into two subgroups, class-I and class-II.15,16 Class-I proteins are localized in the vacuolar membrane (reviewed in ref. 16). Although the subcellular localization of only one class-II NHX protein has been reported to date, namely the tomato LeNHX2 protein that is targeted to the prevacuolar compartment (PVC),17,18 other members of class-II group are also found in non-vacuolar endosomal compartments (Andres Z, Cubero B, Pardo JM, unpublished results). To begin addressing the issue of how the ion selectivity of NHX proteins fits with their suggested cellular function, we have compared the Na+-K+ selectivity of representative members of the two types of plant NHX proteins. Histidine-tagged AtNHX1 (class-I, tonoplast) and LeNHX2 (class-II, PVC) proteins were purified by affinity chromatography, reconstituted into lipid vesicles, and the cation-dependent H+ exchange was determined with the fluorescent pH indicator pyranine as described.11 As depicted in Figure 1A, the tonoplast localized AtNHX1 showed equal transport rates for Na+/H+ and K+/H+ exchange, in agreement with our previous reports.11,12 Furthermore, mixing Na+ and K+ salts produced an additive effect, indicating the either ion serves as substrate for transport. By contrast, the non-vacuolar LeNHX2 protein demonstrated a strong preference for K+/H+ exchange over Na+/H+, and Na+ salts reduced the net rate of transport when mixed with K+, an indication of competitive inhibition of K+ transport by Na+ ions (Fig. 1B; Jiang X, Rodriguez-Rosales MP, Venema K, unpublished results). In this regard, it is worth noting that AtNHX1 complemented the lack of endogenous ScNHX1 in the yeast Saccharomyces cerevisiae12,19 and that the ScNHX1/VPS44 protein plays an important role in endosomal trafficking and protein sorting of the late endosome/prevacuolar compartment, presumably by regulating the lumenal pH of endosomes.20,21 Remarkably, mutants of AtNHX1 that conferred greater halotolerance to yeast cells demonstrated improved K+-Na+ selectivity, presumably by averting sodic poisoning of the endomembrane network and preserving endosome/prevacuolar functions. Together, these results strongly suggest that the endosome/prevacuolar compartment of yeast, and likely in plant cells too, is a target for Na+ toxicity, which can be prevented by improved K+-Na+ discrimination of the resident class-II NHX antiporters, in agreement with previous reports17,18 and biochemical data shown in Figure 1B.
Figure 1.
Cation transport activity in proteoliposomes. AtNHX1 and LeNHX2 proteins were purified and reconstituted into liposomes to measure their cation/H+ exchange activity as described.11,17 An acid-inside pH gradient was created by dilution of proteoliposomes containing (NH4)2SO4 into ammonium-free assay buffer at pH 7.5. Fluorescence recovery, indicative of vesicle alkalinization, was measured upon addition of NaCl and/or KCl salts to the assay medium. Addition of 25 mM (NH4)2SO4 disrupted the pH gradient and ended the assay. Transport activities are given as percent of maximal fluorescence. (A) Proton exchange rates of AtNHX1 were determined in the presence of 50 mM NaCl (green trace), 50 mM KCl (red trace), and 50 mM of each salt (black trace). (B) Transport activity of LeNHX2 with 125 mM NaCl, 125 mM KCl and 125 mM of each salt; trace colors as in (A).
At face value, insufficient Na+-K+ discrimination of vacuolar (class-I) NHX exchangers creates a conceptual problem with the prevailing model positing that these proteins are major players in the vacuolar compartmentation of Na+. Cytosolic Na+ concentrations of 10–30 mM have been estimated in root cells of salt-treated glycophytes by X-ray microanalysis and ion-sensitive electrodes, while cytosolic K+ will often remain above 60 mM.1,22 Since cytosolic K+ concentration will most likely exceed that of Na+ under salinity stress, the relevant question is how a non-selective Na+,K+/H+ antiporter could mediate efficient Na+ compartmentation in vacuoles at high cytosolic K+-Na+ ratios. One possibility is that K+/H+ exchange by NHX1-like proteins would predominate over, but not suppress, Na+/H+ exchange (see Fig. 1A), and that the backflow of K+ from the vacuole to the cytosol were greater than that of Na+. Over time, the net balance of this counteracting, bidirectional transport would result in the accumulation of Na+ into the vacuolar lumen, albeit at the expense of a seemingly futile cycle of vacuolar K+ import and export. To investigate this issue, we constructed transgenic tomato plants overexpressing AtNHX1. These plants showed improved salt tolerance, although only if the stress was imposed as salt-shock.13 Importantly, the transgenics had larger K+ vacuolar pools in all growth regimes tested, but no consistent enhancement of Na+ accumulation was observed under salt stress. Plants overexpressing AtNHX1 had a greater capacity to retain intracellular K+ under various salinity regimes, an especially crucial feature for plants under high-salt conditions. They also showed elevated concentrations of soluble sugars before and after salinity stress. Proline concentrations, which were not different among plant lines prior transfer to salt, were also significantly greater in the transgenic plants under stress. These are all well known features that correlate with salinity tolerance in many plant species that may contribute additively to the halotolerance of plants expressing AtNHX1. Similar findings have been reported by others.23–26
Strikingly, when transgenic tomato overexpressing AtNHX1 were subjected to K+-limiting conditions, the enhanced K+ compartmentation into the vacuole continued at the expense of the cytosolic K+ pool, which was 2-fold lower in transgenic plants relative to control plants.13 The K+cyt measured with double-barreled K+-selective microelectrodes in impaled root epidermal cells was 98 ± 1.3 mM in wild-type seedlings and 55 ± 2.2 mM in the transgenics. The drop in K+cyt caused the early induction of the K+-starvation responsive gene HAK5, triggered the high-affinity mode of K+ uptake, enhanced net K+ uptake by roots, and increased the K+ content in plant tissues and the xylem sap. Transformed plants became much more sensitive to low-K+ than wild-type plants, presumably as a consequence of compromised K+cyt. Likewise, in tomato plants that overexpress the K+/H+ exchanger LeNHX2, the induction of HAK5 upon K+ starvation is much stronger than in untransformed control plants (Huertas R, Venema K, Rodriguez-Rosales MP, unpublished results). These findings do no lend support to the above proposition that K+ ions transported by AtNHX1 are readily fluxed back to the cytosol. Instead, they strongly suggest that NHX proteins are likely candidates for the H+-linked K+ transport that is thought to facilitate active K+ uptake at the tonoplast, and that K+ remains partitioned between vacuole and cytosol. They also represent a fundamental challenge to the underlying assumptions about the mechanism by which vacuolar NHX exchanger enhance the salt tolerance of plants, namely that the prevailing function of NHX proteins is to drive the efficient compartmentation of Na+ into the vacuolar lumen. We posit that the primary function of NHX1-like transporter is to mediate K+ compartmentation, with or without concurrent sequestration of Na+ depending on the selectivity of the individual NHX protein and the ionic environment in the cytosol of salinized plants.
To be able to extend this new model to other NHX-like proteins, we must determine whether our findings arise from a bizarre behavior of AtNHX1 expressed ectopically in transgenic tomato plants or there is indeed additional evidence supporting this paradigm shift. Numerous reports have shown improved salt tolerance of a variety of plant species expressing vacuolar NHX-like proteins from various sources (reviewed in refs. 3 and 16). Higher Na+ contents in tissues of transgenic Arabidopsis and tomato overexpressing AtNHX1 have been reported.7,10 However, Zhang et al.10 did not determine the extent to which the ectopic expression of AtNHX1 increased Na+ concentration in tissues of transgenic plants relative to non-transformed plants, and neither of these early reports measured K+ contents in the transgenics. Subsequent studies found greater accumulation of both Na+ and K+ in shoots of transgenic plants overexpressing NHX-like proteins.23,27 By contrast, several reports failed to find a significant correlation between increased salt tolerance and enhanced accumulation of Na+,26,28–30 or described greater K+ contents, rather than of Na+, in tissues of the transgenic plants.18,24,25 It should be noted that these reports relied on determinations of total Na+ and K+ contents in lieu of direct measurements of vacuolar content as in Leidi et al.13 Therefore, although Na+/H+ exchange at the tonoplast is generally agreed to play a major role in the salt tolerance of plants, no conclusive evidence has been presented to substantiate that NHX-like antiporters are crucial in this process.
Independent lines of research have also lead to the conclusion that vacuolar NHX protein mediate K+/H+ exchange for turgor regulation and pH control. This is best illustrated by the involvement of NHX exchangers in petal expansion and flower color development. The vacuolar pH (pHv) greatly affects coloration of lumenal anthocyanin pigments. The sole known structural genes that regulate pHv with relevance to flower coloration are antiporters of the NHX family. In Japanese morning glory (Ipomeas sp.) NHX1-like proteins are specifically expressed before flower opening and mediate the pHv shift from ca. 6.6 to 7.7 that renders blue flowers.31–33 The purple (pr) mutation of I. nil abolishes the activity of InNHX1, abrogates vacuolar alkalinization and impedes the color shift from red to blue in opening flowers.32 Petunia hybrida flowers normally have a lower pH than Ipomoea flowers, and the color of wild-type flowers stays on the reddish (low pH) side of the color spectrum. Petunia loci (named PH1 to PH7) that regulate pHv have been identified.34 Gene PH4, which activates vacuolar acidification from pH 6 to 5.5, encodes an R2R3 Myb transcription factor whose target genes remain to be identified.35 These finding indicate that the regulation of pHv is widely used by flowering plants to define the ultimate petal color, and that NHX proteins are instrumental in this process. Modulation of pHv has been linked to K+/H+ exchange but not to Na+/H+ exchange.33
In summary, a significant and growing body of evidence indicates that NHX antiporters play a fundamental, basic role in the plant cell. At regular growth conditions they contribute to K+ uptake, capturing K+ into vacuoles for cellular storage, turgor generation and pH regulation. Under salt or osmotic stress NHX proteins fulfill a protective function through the vacuolar compartmentalization of K+ and, in some cases, of Na+ thereby preventing toxic Na+-K+ ratios in the cytosol while accruing solutes both osmotic balance.
Acknowledgements
We are indebted to Pilar Rodriguez-Rosales and Kees Venema for helpful insights and for sharing unpublished data. This work was supported by the Spanish Ministry of Science and Technology grants BIO2009-08641 and CSD2007-00057 to J.M.P. X.J. was supported by a JAE-Doc grant from C.S.I.C.
Abbreviations
- NHX and NHE
sodium-proton exchanger
- SOS1
salt overly sensitive 1
- CaM15
calmodulin 15
- Vmax
maximum velocity
- K+cyt
cytosolic potassium activity
- R2R3 Myb
transcription factor with structural homology to the R2 and R3 repeats of the Myb oncogene derived from the avian myeloblastosis virus
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11767
References
- 1.Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niu XM, Bressan RA, Hasegawa PM, Pardo JM. Ion Homeostasis in Nacl Stress Environments. Plant Physiol. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apse MP, Blumwald E. Na+ transport in plants. FEBS Letts. 2007;581:2247–2254. doi: 10.1016/j.febslet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Atienza J, Jiang XY, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi HZ, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 8.Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, et al. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;30:529–539. doi: 10.1046/j.1365-313x.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- 11.Venema K, Quintero FJ, Pardo JM, Donaire JP. The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem. 2002;277:2413–2418. doi: 10.1074/jbc.M105043200. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez A, Jiang X, Cubero B, Nieto PM, Bressan RA, Hasegawa PM, et al. Mutants of the Arabidopsis thaliana cation/H+ antiporter AtNHX1 conferring increased salt tolerance in yeast: the endosome/prevacuolar compartment is a target for salt toxicity. J Biol Chem. 2009;284:14276–14285. doi: 10.1074/jbc.M806203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz T, Cubero B, et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 2010;61:495–506. doi: 10.1111/j.1365-313X.2009.04073.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci USA. 2005;102:16107–16112. doi: 10.1073/pnas.0504437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol-Cell Ph. 2005;288:223–239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 16.Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57:1181–1199. doi: 10.1093/jxb/erj114. [DOI] [PubMed] [Google Scholar]
- 17.Venema K, Belver A, Marin-Manzano MC, Rodriguez-Rosales MP, Donaire JP. A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J Biol Chem. 2003;278:22453–22459. doi: 10.1074/jbc.M210794200. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Rosales MP, Jiang X, Galvez FJ, Aranda MN, Cubero B, Venema K. Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol. 2008;179:366–377. doi: 10.1111/j.1469-8137.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 19.Quintero FJ, Blatt MR, Pardo JM. Functional conservation between yeast and plant endosomal Na+/H+ antiporters. FEBS Lett. 2000;471:224–228. doi: 10.1016/s0014-5793(00)01412-5. [DOI] [PubMed] [Google Scholar]
- 20.Brett CL, Tukaye DN, Mukherjee S, Rao RJ. The yeast endosomal Na+(K+)/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav J, Muend S, Zhang Y, Rao R. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol Biol Cell. 2007;18:1480–1489. doi: 10.1091/mbc.E06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carden DE, Walker DJ, Flowers TJ, Miller AJ. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol. 2003;131:676–683. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YY, Chen QJ, Chen M, Chen J, Wang XC. Salt-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained by Agrobacterium tumefaciens-mediated transformation of the vacuolar Na+/H+ antiporter gene. Plant Sci. 2005;169:65–73. [Google Scholar]
- 24.Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breed. 2006;17:341–353. [Google Scholar]
- 25.Liu H, Wang Q, Yu M, Zhang Y, Wu Y, Zhang H. Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ. 2008;31:1325–1334. doi: 10.1111/j.1365-3040.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Gao X, Kong X, Zhao Y, Zhang H. Molecular cloning and functional analysis of a Na+/H+ antiporter gene ThNHX1 from a halophytic plant Thellungiella halophila. Plant Molec Biol Repr. 2009;27:1–12. [Google Scholar]
- 27.Lu S-Y, Jing Y-X, Shen S-H, Zhao H-Y, Ma L-Q, Zhou X-J, et al. Antiporter gene from Hordum brevisubulatum (Trin.) link and its overexpression in transgenic tobaccos. J Integr Plant Biol. 2005;47:343–349. [Google Scholar]
- 28.Ohta M, Hayashi Y, Nakashima A, Hamada A, Tanaka A, Nakamura T, et al. Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett. 2002;532:279–282. doi: 10.1016/s0014-5793(02)03679-7. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, An R, Tang J-H, Cui X-H, Hao F-S, Chen J, et al. Overexpression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed. 2007;19:215–225. [Google Scholar]
- 31.Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N, Iida S. Colour-enhancing protein in blue petals. Nature. 2000;407:581. doi: 10.1038/35036683. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Fukada-Tanaka S, Inagaki Y, Saito N, Yonekura-Sakakibara K, Tanaka Y, et al. Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol. 2001;42:451–461. doi: 10.1093/pcp/pce080. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, Miki N, Momonoi K, Kawachi M, Katou K, Okazaki Y, et al. Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc Japan Acad Ser B. 2009;85:187–197. doi: 10.2183/pjab.85.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Quattrocchio F, Verweij W, Kroon A, Spelt C, Mol J, Koes R. PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell. 2006;18:1274–1291. doi: 10.1105/tpc.105.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]