Abstract
Two sperm cells are required to achieve double fertilization in flowering plants (angiosperms). In contrast to animals and lower plants such as mosses and ferns, sperm cells of flowering plants (angiosperms) are immobile and are transported to the female gametes (egg and central cell) via the pollen tube. The two sperm cells arise from the generative pollen cell either within the pollen grain or after germination inside the pollen tube. While pollen tube growth and sperm behavior has been intensively investigated in model plant species such as tobacco and lily, little is know about sperm dynamics and behavior during pollen germination, tube growth and sperm release in grasses. In the March issue of Journal of Experimental Botany, we have reported about the sporophytic and gametophytic control of pollen tube germination, growth and guidance in maize.1 Five progamic phases were distinguished involving various prezygotic crossing barriers before sperm cell delivery inside the female gametophyte takes place. Using live cell imaging and a generative cell-specific promoter driving α-tubulin-YFP expression in the male germline, we report here the formation of the male germline inside the pollen grain and the sperm behaviour during pollen germination and their movement dynamics during tube growth in maize.
Key words: male gametophyte, generative cell, sperm, pollen tube, tubulin, fertilization, maize
Spermiogenesis in Maize
With the exception of ginkgo and cycads belonging to the gymnosperms,2,3 a major characteristic of seed plants is the lack of motile sperm and thus the occurrence of a male gamete transport and delivery system, the pollen tube, which is considered as key innovation in the evolutionary success of flowering plants.4 In contrast to animals, where germline cells are established early during embryogenesis, the male germline of angiosperms arise late during development after vegetative meristems have been converted into flowering meristems to form the reproductive organs of the flower. The male germline is established in the anther after diploid sporogenous cells called microspore mother cells undergo meiotic divisions to generate tetrads of haploid microspores.5 Unicellular microspores are released from tetrads to undergo asymmetric cell divisions known as Pollen Mitosis I (PMI).4 The small generative or germ cell (GC), which represents the initial of male germline, becomes covered in the cytoplasm of the large vegetative cell (VC) forming a cell-within-a-cell structure. The GC then forms a spindle-like shape in many plant species that is maintained by a cage-like organization of cytoplasmic bundled microtubules.6,7 A second round of mitosis, known as Pollen Mitosis II (PMII), is required to generate two sperm cells for double fertilization, the major characteristic of flowering plants. In tricellular pollen types, such as Arabidopsis and grasses including maize, PMII takes place inside the developing pollen grain, while PMII in bicellular pollen, such as tobacco or lily, occurs in the growing pollen tube.4
In order to find cellular markers labelling sperm cells of maize, we have analysed various maize fluorescent protein tagged lines available via the maize Cell Genomics DB.8 We identified an α-tubulin-YFP line expressing the fusion protein under control of the endogenous promoter in many tissues, but within the microspores and developing pollen grain exclusively in the male germline (Fig. 1). Labeling tubulin and thus microtubules (MTs) is especially interesting as these structures are highly dynamic and are involved in many cellular processes such as cell division, cell shape formation and elongation, cell motility and intracellular transport.9 Tubulins are encoded by multigene families, of which single members display a pollen-specific or preferential expression pattern. One of the six α-tubulin and one of the nine β-tubulin genes of Arabidopsis, for example, are expressed preferentially in pollen.13,14 Similar findings have been observed in other plant species15,16 indicating that pollen isoforms might possess different functions and/or physicochemical properties. In maize the male germline-specific α-tubulin-YFP fusion protein was first visible at the bicellular stage after PMI was completed and the GC was engulfed by the plasma membrane of the vegetative cell (Fig. 1A–C). While YFP signals were visible throughout the GC, the large vegetative tube did not show signals. Occasionally signal reflections were visible at the wall of the microspore (Fig. 1B). At the late bicellular stage MTs formed a basket-like structure around the nucleus of the GC continued into tail-like extensions at both poles involved in PMII and separation of the twin sperm nuclei (Fig. 1D and E). The length of tail-like bundled microtubules further increased during pollen maturation (Fig. 1F and G) both between connected sperm cells and at opposite poles. A tubuli-knot becomes visible at half distance between sperm cells. We are not aware that such a structure has been described before. Studies with other plant species reported that the GC contains it own cytoskeleton10 and its MTs have been shown to be more stable towards depolymerization treatments than the cortical microtubules of the pollen tube cell.7 The existence of a male germline-specific α-tubulin reported here provides an explanation for these observations.
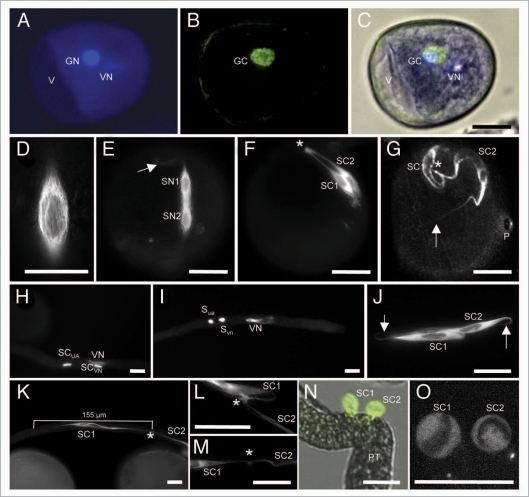
Figure 1.
Establishment and properties of the male germline in maize. Germline cells are labeled with α-tubulin-YFP. (A–D) Microspore at late bicellular stage: (A) the vegetative cell still contains a vacuole (V) and the generative cell nucleus (GN) already underwent DNA-syntheses as indicated by its bright signals compared with the vegetative nucleus (VN) after DAPI staining. (B) α-tubulin-YFP expression is restricted to the generative cell (GC). (C) Merged image of (A and B). (D) α-tubulin-YFP forms a cage around the GC nucleus during Pollen Mitosis II . (E) Transition towards tricellular stage: microtubuli bundles have been formed around and between sperm nuclei (SN1/2). Note that first microtubular tail-like extensions are visible (arrow). (F) At early tricellular stage, twin sperm cells (SC1/2) are arranged in parallel. A microtubuli knot (asterisk) becomes visible at half distance between sperm nuclei. (G) Late and mature tricellular pollen stage showing twin sperm cells with long microtubular extensions (arrow), a microtubuli knot (asterisk) connecting both cells and the germination pore (P). (H and I) DAPI staining to show that sperm cells and vegetative nucleus travel as male germ unit (MGU). Note that initially the nucleus of the leading sperm cell (SCVN) seems closely associated with the vegetative nucleus (VN). (I and J) At later stages leading and trailing sperm cells (SCVN and SCUA) are hardly distinguishable as they change positions inside the growing pollen tube. Arrows mark tail-like microtubuli extensions. (K) Stretched sperm cells measure up to 155 µm in length. An asterisk labels the position of a microtubuli knot between both sperm cells. (L and M) Examples of enlarged microtubuli knots. (N) A manipulated pollen tube (PT) releases twin sperm cells that become spherical within seconds. (O) Spherical sperm cells have completely lost their microtubular structure. Dark areas inside sperm cells are sperm nuclei. Scale bars: 20 µm.
Serial ultrathin sectioning analysis of sperm cells inside mature maize pollen grains revealed a long and narrow appearance with elongating extensions at each end. The flattened cells were reported to be about 35 µm in length, 5 µm wide, but only 1 µm in thickness.11 Our measurement of sperm cell length is almost doubled and we think that the fine extensions visible, for example, in Figure 1G have been overlooked in previous reports. Within pollen tubes we even measured stretched sperm cells of up to 155 µm in length (Fig. 1K). For comparison, tobacco sperm cells are smaller ranging from 10 to 20 µ in length, 2 to 3 µm in width and 0.3 to 0.5 µm in thickness.12
Sperm Cells Travel as Male Germ Unit
It has been reported for many angiosperm species including maize that the sperm cells and vegetative pollen tube cell nucleus travel together within the pollen tube. This unit was therefore termed male germ unit (MGU)18 and has successfully been isolated from plant species such as tobacco.19 We found the movement of the maize sperm cells into and within the pollen tube extremely dynamic (see below). During pollen tube germination the leading sperm cell seemed associated with the vegetative nucleus (Fig. 1H), while at later stages usually the vegetative nucleus took the leading position often more than 30 µm apart from the leading sperm nucleus (Fig. 1I). However, sperm cells inside the tube have very long extensions (Fig. 1J) probably still connected to the vegetative nucleus. In previous reports using fluorescent microscopy with DNA fluorochromes it was concluded that sperm cells of cereals are not connected17 due to the large distance measured between nuclei. Here we could show that this conclusion was wrong. Although the nature of the physical association between the vegetative nucleus and the two sperm cells is still unclear, we have reported here that α-tubulin is involved in the connection between both sperm cells and stays intact during sperm movement in the pollen tube (Figs. 1K–M and 2). The maize sperm cells are likely also bounded by an envelope comprising paired sperm and vegetative membranes that have been reported in other plant species. Cell wall material was not detected in the periplasm between both membranes.11 In tobacco the paired membranes are shared by both sperm cells generating a small cytoplasmic bridge between both cells.12 A connection via tubulin as indicated here was not observed in tobacco.
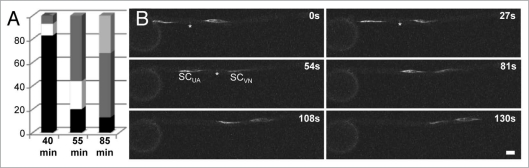
Figure 2.
Sperm cell movement into and within the pollen tube of maize. (A) The position of the twin sperm cells was determined at different time points after germination in vitro. Time points of 40 (n = 29), 55 (n = 45) and 85 min (n = 31) are given. Position of sperm cells in percentage is indicated inside the the pollen grain (black bars), while leaving the grain (white bars), in the middle of the pollen tube (dark grey bars) and at the tip of the tube (light grey bar). (B) Time series showing stretched sperm cells (leading and trailing sperm cell SCVN and SCUA, respectively) leaving the pollen grain (left). Movement is shown within a period of 130 seconds (s) as indicated. Please note that movement is very fast after pollen grain exit (top left image) and slows down in the middle of the tube (lower right image). Sperm cells are labelled with α-tubulin-YFP. The tubular knot connecting twin sperm cells is visible in some images (asterisks). Scale bar: 20 µm.
Sperm Movement Significantly Exceeds Pollen Tube Growth Rate in vitro
The maize pollen tube cell belongs to the fastest growing plant cells with a linear growth rate of up to 12 mm h−1 in vivo (3,300 nm s−1).24 In vitro, we observed a strong variation in growth rate of up to 900 nm s−1 in liquid medium. On average we measured about 100 nm s−1 (data not shown), which is more than ten times slower compared with in vivo measurements. However, in vitro, growth rates of tobacco pollen tubes range between 20 and 70 nm s–1 (average of about 30 nm s−1)22 and lily pollen tubes, which are almost twice the width of tobacco pollen tubes, grow at rates of 100–300 nm s−1 (average about 180 nm s−1),22 which is slightly faster than maize pollen tube growth in vitro.
Sperm cells enter the pollen tube around 40–60 min after germination in vitro (Fig. 2A), when pollen tubes are already 300–500 µm in length. 55 min after germination the MGU is already observed in the middle part of the pollen tube, but not in the tube tip and about 85 min after germination one-third of the MGUs are visible in the tube tip indicating that sperm movement is significantly faster than pollen tube growth rates. 180 min after germination all sperm pairs are visible within the tube tip of functional pollen grains/tubes (data not shown). In summary sperm movement in the pollen tube is highly dynamic as shown using live time imaging (Fig. 2B). Movement is very fast within the first two minutes after pollen grain exit (Suppl. Movie 1) and slows down during further movement within the tube cell. Leading and trailing sperm cells can be distinguished only initially. During further growth sperm cell movement is very dynamic, sperm cells change their directions (Suppl. Movies 2 and 3), become stretched, turn direction again and again (Fig. 1J), but finally accumulating in the tube tip. On average we determined sperm cell movement at a speed of 650 nm s−1 thus exceeding pollen tube tip growth rates in vitro by five to six times. Tobacco sperm cells have been reported to move at an average of 20,000 nm s−1 in the style.12 Comparing with our measurements reported above this seems to represent a miscalculation.
The role of actin filaments and corresponding myosin motor based movement of organelle motility during pollen tube growth as well as actin dependent polar pollen tube tip growth is already well understand.21,22 The role of microtubules is less clear. While microtubules are involved to shape the GC as described above, they also seem to be involved in the transport of the tube cell nucleus and GC cell in tobacco pollen tubes.23 In tobacco colchicine treatment lead to depolymerization of the cortical pollen tube microtubules, while microtubule bundles and mitosis of the GC was not affected.7 Tip growth and cytoplasmic streaming was also not affected in other angiosperms as indicated by similar microtubule disassembling experiments.22 We assume that in maize sperm cell movement dynamics are also mainly dependent on cytoplasmic streaming and the activity of myosin-based motor activity.
Breakdown of the Microtubular Cytoskeleton after Sperm Release
Interestingly, when we manipulated pollen tubes to release sperm cells, cells became immediately spherical as shown in Figure 1N. The bundled microtubules were completely disintegrated and no longer detectable (Fig. 1O). YFP signals were evenly distributed in the cytoplasm around the nucleus of both sperm cells indicating that α-tubulin existed as monomer or short oligomers after sperm release. Compared with stretched sperm cells inside the pollen tube measuring up to 155 µm in length, released spherical sperm cells measured only between 8 to 10 µm in diameter. This value is similar to reports on isolated sperm cells of maize that measured approximately 7.2 µm on average.25 Moreover, in isolated sperm cells it was further reported that microtubules could also not be detected in scanning electron micrographs25 and that the vegetative plasma membrane and periplasmatic material was lost.20 The microtubular cytoskeleton has also been studied in isolated GCs and it appeared that they are lost or form a mesh-like pattern.20 It was further observed that the connection between the two sperm cells generally disappears after release from the pollen grain or pollen tube in most species.20 Our report supports this finding.
Finally, we think that it is noteworthy that from 3-dimensional reconstructions it was concluded that maize sperm cells are dimorphic in shape.11 This conclusion mainly based on investigations about the shape of the sperm nuclei. However, we also found that twin sperm cells are often different in size (Fig. 1O). The germline specific tubulin marker line reported here now provides the first appropriate tool to study whether maize sperm cells are also functionally different. Replacing, for example, the YFP marker in the construct used against a photo-switchable fluorescent protein allows labelling leading and trailing sperm cells after pollen grain exit separately to follow their individual behaviour during pollen tube growth and during the fertilization process.
In summary, we suggest that cytoplasmic sperm MTs are very labile in maize pollen tubes and may also disintegrate immediately upon sperm delivery inside the egg apparatus. This event might be a prerequisite to separate twin sperm cells to fuse with either of the two female gametes. Moreover, we hope that our report about the establishment of the male germline and movement dynamics of twin sperm cells during pollen tube germination and growth using a novel marker line will help to address the exciting question whether preferential fertilization occurs in angiosperms.
Acknowledgements
The German Research Council DFG (Grant DR 334/2-6) is acknowledged for financial support and Dave Jackson from the Maize Cell Genomics project for providing the α-tubulin-YFP marker line.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12033
Supplementary Material
References
- 1.Lausser A, Kliwer I, Srilunchang KO, Dresselhaus T. Sporophytic control of pollen tube growth and guidance in maize. J Exp Bot. 2010;61:673–682. doi: 10.1093/jxb/erp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renzaglia KS, Garbary DJ. Motile gametes of land plants: Diversity, development and evolution. Crit Rev Plant Sci. 2001;20:107–213. [Google Scholar]
- 3.Southworth D, Cresti M. Comparison of flagellated and nonflagellated sperm in plants. Am J Bot. 1997;84:1301–1311. [PubMed] [Google Scholar]
- 4.Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. J Exp Bot. 2009;60:1465–1478. doi: 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- 5.Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol. 2005;56:393–434. doi: 10.1146/annurev.arplant.55.031903.141717. [DOI] [PubMed] [Google Scholar]
- 6.Yu HS, Russell SD. Three-dimensional ultrastructure of generative cell mitosis in the pollen tube of Nicotiana tabacum. Eur J Cell Biol. 1993;61:338–348. [PubMed] [Google Scholar]
- 7.Joos U, Vanaken J, Kristen U. Microtubules are involved in maintaining the cellular polarity in pollen tubes of Nicotiana sylvestris. Protoplasma. 1994;179:5–15. [Google Scholar]
- 8.Mohanty A, Luo A, DeBlasio S, Ling X, Yang Y, et al. Advancing cell biology and functional genomics in maize using fluorescent protein-tagged lines. Plant Physiol. 2009;149:601–605. doi: 10.1104/pp.108.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Curr Opin Cell Biol. 2010;22:104–111. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Palevitz BA, Tiezzi A. Organization, composition and function of the generative cell and sperm cytoskeleton. Int Rev Cytol. 1992;140:149–185. [Google Scholar]
- 11.Mcconchie CA, Hough T, Knox RB. Ultrastructural analysis of the sperm cells of mature pollen of maize (Zea mays) Protoplasma. 1987;139:9–19. [Google Scholar]
- 12.Yu HS, Hu SY, Russell SD. Sperm cells in pollen tubes of Nicotiana tabacum L. 3-dimensional reconstruction, cytoplasmic diminution and quantitative cytology. Protoplasma. 1992;168:172–183. [Google Scholar]
- 13.Carpenter JL, Ploense SE, Snustad DP, Silflow CD. Preferential expression of an alpha-tubulin gene of Arabidopsis in pollen. Plant Cell. 1992;4:557–571. doi: 10.1105/tpc.4.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng ZG, Snustad DP, Carter JV. Temporal and spatial expression patterns of TUB9, a beta-tubulin gene of Arabidopsis thaliana. Plant Mol Biol. 2001;47:389–398. doi: 10.1023/a:1011628024798. [DOI] [PubMed] [Google Scholar]
- 15.Yu YL, Li YZ, Li LL, Lin JX, Zheng CC, et al. Overexpression of PwTUA1, a pollen-specific tubulin gene, increases pollen tube elongation by altering the distribution of alpha-tubulin and promoting vesicle transport. J Exp Bot. 2009;60:2737–2749. doi: 10.1093/jxb/erp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakley RV, Wang YS, Ramakrishna W, Harding SA, Tsai CJ. Differential expansion and expression of alpha- and beta-tubulin gene families in Populus. Plant Physiol. 2007;145:961–973. doi: 10.1104/pp.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heslop-Harrison J, Heslop-Harrison Y. The disposition of gamete and vegetative cell nuclei in the extending pollen tubes of a grass species, Alopecurus pratensis L. Acta Bot Neerl. 1984;33:131–134. [Google Scholar]
- 18.Dumas C, Knox RB, Gaude T. The spatial association of the sperm cells and vegetative nucleus in the pollen grain of Brassica. Protoplasma. 1985;124:168–174. [Google Scholar]
- 19.Tian HQ, Zhang Z, Russell SD. Isolation of the male germ unit: organization and function in tobacco (Nicotiana tabacum L.) Plant Cell Reports. 1998;18:143–147. [Google Scholar]
- 20.Theunis CH, Pierson ES, Cresti M. Isolation of male and female gametes in higher plants. Sex Plant Repr. 1991;4:145–154. [Google Scholar]
- 21.Cai G, Cresti M. Organelle motility in the pollen tube: a tale of 20 years. J Exp Bot. 2009;60:495–508. doi: 10.1093/jxb/ern321. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AY, Duan QH, Costa SS, de Graaf BHJ, Di Stilio VS, et al. The dynamic pollen tube cytoskeleton: Live cell studies using actin-binding and microtubule-binding reporter proteins. Mol Plant. 2008;1:686–702. doi: 10.1093/mp/ssn026. [DOI] [PubMed] [Google Scholar]
- 23.Astrom H, Sorri O, Raudaskoski M. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex Plant Repr. 1995;8:61–69. [Google Scholar]
- 24.House LR, Nelson OE. Tracer study of pollen-tube growth in cross-sterile maize. Heredity. 1958;49:18–21. [Google Scholar]
- 25.Cass DD, Fabi GC. Structure and properties of sperm cells isolated from the pollen of Zea mays. Can J Bot. 1988;66:819–825. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.