Abstract
The present study uses two independent genetic strategies to explore the requirement for Phosphoinositide dependent kinase-1 (PDK1) in the development of mature T cell populations from CD4/CD8 double positive thymocytes. The data show that CD4/CD8 double positive thymocytes that do not express PDK1 or express a catalytically inactive PDK1 mutant fail to produce mature invariant Vα14 NKT cells but can differentiate to conventional CD4, CD8 or regulatory T cell subsets in the thymus. The PDK1 requirement for Vα14 NKT cell development reflects that these cells require the PDK1 substrate Protein Kinase B (PKB, also called Akt) to meet the metabolic demands for proliferative expansion in response to Interleukin 15 or antigen receptor stimulation. There is also constitutive PDK1 signaling in conventional α/β T cells that is not required for lineage commitment of these cells but fine-tunes the expression of coreceptors and adhesion molecules. Also, while PDK1 is dispensable for thymic development of conventional α/β T cells, peripheral cells are substantially reduced. This reflects a PDK1 requirement for lymphopenia-induced proliferation, a process necessary for initial population of the peripheral T cell niche in neonatal mice. PDK1 is thus indispensable for T cell developmental programs but the timing of the PDK1 requirement is unique to different T cell subpopulations.
Introduction
T cell development in the thymus is a process that aims to populate the peripheral immune system with non-autoreactive T lymphocytes that can control adaptive immune responses to pathogens. T cells produced by the thymus include conventional α/β T cells expressing T cell antigen receptor complexes comprised of a highly variable TCRα and β subunits that recognise peptide/MHC complexes. The different developmental stages of thymocytes can be indentified by the expression of CD8 and CD4, the receptors for MHC class I and class II molecules respectively. T cell progenitors are CD4/CD8 double negative (DN) and progress to a CD4/CD8 double positive (DP) stage if they successfully rearrange their TCR β locus and express a pre-TCR complex. In the CD4/CD8 double positive compartment cells undergo TCRα chain rearrangements and if they express a functional α/β TCR complex they are then subjected to selection processes that generate CD4 or CD8 single positive (SP) cells that can populate the periphery. CD4 positive T cells can be further subdivided into conventional CD4 T cells, regulatory T cells (Tregs) (1) and Natural Killer T (NKT) cells (2). A significant proportion of NKT cells express an invariant Vα14 T cell receptor that recognizes glycolipid/CD1d antigen complexes (iNKTs) and play a role in immune surveillance and immune homeostasis (2, 3).
The balanced production of peripheral T cell subpopulations is essential to ensure the function and the homeostasis of the peripheral immune system. Accordingly, one key issue for T cell developmental biology is the nature of the signals that determine why CD4/CD8 DP thymocytes can produce different T cell subpopulations each with unique functions. One insight is that the strength and/or duration of signaling plays a key role in lineage commitment. For example, it has been suggested that regulatory T cells may derive from thymocytes selected to express α/β TCRs with relatively high affinity for self peptide/MHC complexes (4). The commitment of DPs to either the CD4 or CD8 lineage is also linked to signal strength in the sense that persistent TCR signaling drives cells to the CD4 lineage (5). Other important insights come from the differential requirements for transcriptional factors for the different peripheral T cell lineages. For example, ThPOK and Runx transcription factors cross regulate each other in a balancing act that controls T cell commitment to either CD4 helper or CD8 cytotoxic T cell lineages (6, 7) while the transcription factor Foxp3 is critical for Treg differentiation (1). It is also evident that there are distinct signaling requirements for the differentiation of iNKT cells. For example, DP thymocytes lacking expression of Fyn, SAP, T-bet, NF-kB and c-myc fail to produce iNKT cells despite normal development of conventional α/β T cells (8-15).
One explanation for the apparently unique molecular requirements for iNKT cell development could be that these cells undergo a large proliferative burst as they differentiate from DPs (14). This is in contrast to conventional α/β T cells where a major proliferative burst occurs as TCR β chain selected cells transit from DNs to DPs (16). In DN T cell progenitors a key signalling pathway that supports the biosynthetic demands of rapid cell division is mediated by phosphoinositide-dependent kinase l (PDK1) (16, 17). This serine/threonine kinase phosphorylates and activates the PI3K controlled serine/threonine kinase protein kinase B (PKB), also called Akt (18). PDK1 also phosphorylates and activates the 70-kilodalton ribosomal protein S6 kinase-1 (S6K1) and the 90-kilodalton ribosomal protein S6 kinase (RSK) and members of the protein kinase C family (18). T cell progenitors lacking PDK1 or PKB arrest at the CD4/CD8 double negative stage of thymocyte development because they cannot increase their metabolism to support the pre-TCR induced burst of proliferation required for the DN to DP transition of conventional T cells (16, 17, 19-21). Is PDK1 similarly required for the proliferative burst required for iNKT cell development at the DP to SP stage? In this context it has been reported that deletion of PDK1 in CD4/CD8 double positive thymocytes did not prevent the formation of CD4 SP thymocytes (22). The caveat was that the CD4 SP cells that developed in the absence of PDK1 did not activate in response to antigen receptor/CD28 co-stimulation (22). It was also reported that the loss of PDK1 in DPs caused fewer CD8 single positive thymocytes to develop (22). However, the impact of PDK1 loss on the production of different subsets of α/β T cells such as regulatory T cells or iNKT cells has not been explored. This is pertinent because it has been described recently that PKB signaling pathways control the differentiation of peripheral effector T cells following immune activation (23-25).
Accordingly, the object of the present study was to analyze the role of PDK1 in the DP to SP transition of thymocytes and to compare the PDK1 requirements for the development of T cell sub-populations. The data show that PDK1 is indispensable for T cell developmental programs but the timing of the PDK1 requirement, either thymic or peripheral, is unique to different T cell subpopulations.
Materials and Methods
Mice
PDK1fl/fl (26), CD4-Cre (27) and PDK1K111A/WT mice were bred and maintained in the University of Dundee in compliance with UK Home Office Animals (Scientific Procedures) Act 1986 guidelines. PDK1fl/fl CD4-Cre mice were generated crossing mice with floxed PDK1 alleles and mice expressing Cre recombinase under the control of the proximal CD4 promoter (CD4-Cre) (27). PDK1K111A/fl CD4-Cre mice were generated by backcrossing PDK1fl/fl CD4-Cre mice with PDK1K111A/WT mice.
Generation of catalytically inactive PDK1 knockin mice
A targeting construct was generated as described (28), to replace the wild-type exon 4 of the gene encoding PDK1 with a mutant form of exon 4 encoding for Alanine in the position of Lysine111 (K111A). A neomycin resistance ‘cassette’ flanked with Cre recombinase-removable loxP sites was present in the targeting construct to permit selection of targeted embryonic stem cells. The targeting vector was introduced into E14 mouse embryonic stem cells by electroporation and embryonic stem cell clones generated. Targeted embryonic stem cells were identified by Southern analysis using probes external to the targeting vector. The neomycin cassette was removed from the targeted embryonic stem cells by transient expression of Cre recombinase. Heterozygous PDK1K111A/WT knockin embryonic stem cell clones were microinjected into C57/Bl6 x Balb/c blastocysts, which were then re-implanted into recipient female mice. Chimeric mice that had a high degree of embryonic stem cell contribution were identified by coat colour and were then crossed to Balb/c mice. This allowed germline transmission of the knockin allele to be identified by the grey coat of the resulting pups. Genotyping for the PDK1K111A knockin allele was carried out by PCR of genomic DNA isolated from ear clips using the same methodology and primers employed to genotype PDK1155E/155E knockin mice described previously (28).
Flow cytometric analysis
Antibodies conjugated to FITC, PE, allophycocyanin (APC) and biotin were obtained from either BD Pharmingen or eBioscience. TriColour, PE-Cy5.5 and APC Alexa Fluor 750 conjugated antibodies were obtained from Caltag. Cells were stained for surface expression of the following markers using the antibodies in parentheses: CD4 (RM4-5), CD8 (53-6.7), CD24 (M1/69), CD25 (7D4), CD44 (IM7), CD62L (MEL-14), Thy1.2 (53-2.1), TCRβ (H57-597), CD122 (TM-β1), CD16/CD32 (2.4G2) (Fc Block™). Intracellular Foxp3 staining was performed using a Foxp3 intracellular staining kit (eBiosciences). Cells were stained with saturating concentrations of antibody in accordance with the manufacturer’s instructions. For CCR7 staining, cells were labeled with mouse recombinant CCL19-Fcγ and detected using PE conjugated anti-human Fcγ (both from eBiosciences). For Vα14 TCR staining CD1d-DimerX (BD Pharmingen) was loaded with the α-galactoceromide (αGalCer) synthetic analogue, KRN7000 (Enzo Life Sciences): 0.14μg αGalCer per μg CD1d-DimerX at 37°C overnight (CD1d-αGalCer). Cells were labeled with this CD1d-αGalCer and detected with APC conjugated rat anti-mouse IgG1 (BD Pharmingen). For NKT cell MACS enrichment, cells were labeled with CD1d-αGalCer and IgG1-APC, as described above, then bound to anti-APC magnetic beads, and enriched on an autoMACS cell separator (Miltenyi Biotech). For intracellular flow cytometry of PLZF, thymocytes were fixed and permeabilised using the fixation/permeabilisation set from eBioscience. Intracellular PLZF was detected using 4 μg/ml mouse monoclonal antibody D-9 (Santa Cruz) and PE or APC conjugated rat anti-mouse IgG1 (BD Pharmingen).
Western Blotting
Thymocytes were lysed on ice in lysis buffer (10 mM Tris (pH 7.05), 50 mM NaCl, 0.5% Triton X-100, 50 mM sodium fluoride, 30mM sodium pyrophosphate, 1mM DTT, 50nM Calyculin A, 5μM ZnCl2, 10% Glycerol, complete protease inhibitors (Roche)). Protein was separated by SDS-PAGE, transferred to nitrocellulose membrane and detected by western blot analysis using standard techniques. Phospho-RSK (Ser227) and total RSK antibodies were purchased from Santa Cruz, total PDK1 from Millipore and all other antibodies from Cell Signalling Technologies.
PDK1 kinase Assay
E11.5-12.5 embryo cell lysates (500μg) were used to immunoprecipitate and assay PDK1 kinase activity. The lysates were incubated at 4°C for 1 hour on a shaking platform with 10μg of antibody coupled to 10μl of protein G-Sepharose (Amersham). The immunoprecipitates were washed twice with 1 ml of lysis buffer and once with 1 ml of buffer A (50mM Tris (pH7.5), 0.1% 2-ME, 0.1mM EGTA). The standard assay (50μl) contained: washed Protein G-Sepharose immunoprecipitate, 50 mM Tris/HCl pH 7.7, 0.1 mM EGTA, 0.1% 2-ME, 2.5 μM PKI (TTYADFIASGRTGRRNAIHD, peptide inhibitor of cAMP-dependent protein kinase), 10 mM magnesium acetate, 0.1 mM [γ32P]ATP (~200 cpm/pmol) and 1mM peptide substrate (KTFCGTPEYLAPEVRR). 1 mUnit of activity was that amount of enzyme that catalysed the phosphorylation of 1 pmol of substrate in 1 minute.
Calcium flux analysis
Thymocytes from wild type and Pdkfl/fl CD4-Cre mice were isolated and loaded with 4.5μM indo-1 in the presence of 0.045% (v/v) pluronic F-127 (Molecular Probes) for 45min at 37°C. Cells labelled with antibodies (CD3-biotin, CD4-PE, CD8-FITC) on ice for 30 minutes. After staining cells were place on ice (1×106cells/ml). Cells were warmed to 37°C for 5 min immediately before analysis, stimulated with avidin (20μg/ml), then subjected to flow cytometric analysis.
Adoptive Transfers
Thymocytes from wild type (CD45.1) and Pdkfl/fl CD4-Cre (CD45.2) mice were mixed at a 1:1 ratio and injected into the tail vein of Rag2−/− mice. After 4 weeks mice were sacrificed and analysed for the presence of wild type and Pdkfl/fl CD4-Cre T cells.
Proliferation Assays
Thymocytes were depleted of CD8+ cells by magnetic cell separation (Miltenyi Biotec). Cells were stained with 10μM CFSE at 37°C for 20 min in RPMI-1640 containing L-glutamine (Invitrogen), 50μM 2-ME (Sigma) and penicillin-streptomycin (Gibco). Cells were then cultured in RPMI-1640 containing L-glutamine, 10% (vol/vol) heat-inactivated FBS (Gibco), 50μM 2-ME, 10mM Hepes buffer and penicillin-streptomycin for 4 days. Cell were cultured; +/− IL15 (10-100ng/ml), with CD1d (1μg/106 cells) +/− αGalCer, and +/− AKT-1/2i (1μM) (Calbiochem). Cell proliferation was assessed by flow cytometric analysis of CD1d-αGalCer+/TCRβmid NKT cells or CD1d-αGalCer−/TCRβhigh CD4SP T cells as dilution of CFSE staining.
iNKT cell culture
iNKT cells were purified from wild type thymocytes by magnetic seperation using streptavidin coated magnetic beads (Miltenyl Biotech) following labelling with αGalCer loaded CD1d-DiamerX (BD Biosciences) bound to biotinylated rat anti-mouse IgG1 (BD Biosciences). iNKT cells were cultured in RPMI-1640 containing L-glutamine, 10% (vol/vol) heat-inactivated FBS (Gibco), 50μM 2-ME, 10mM Hepes buffer and penicillin-streptomycin for up to 3 weeks with IL2 (0.25μg/ml). Cells were starved of IL2 for 24 hours before stimulation with IL15 or CD1d-αGalCer.
Results
PDK1 is dispensable for the DP/SP transition of conventional α /β T cells
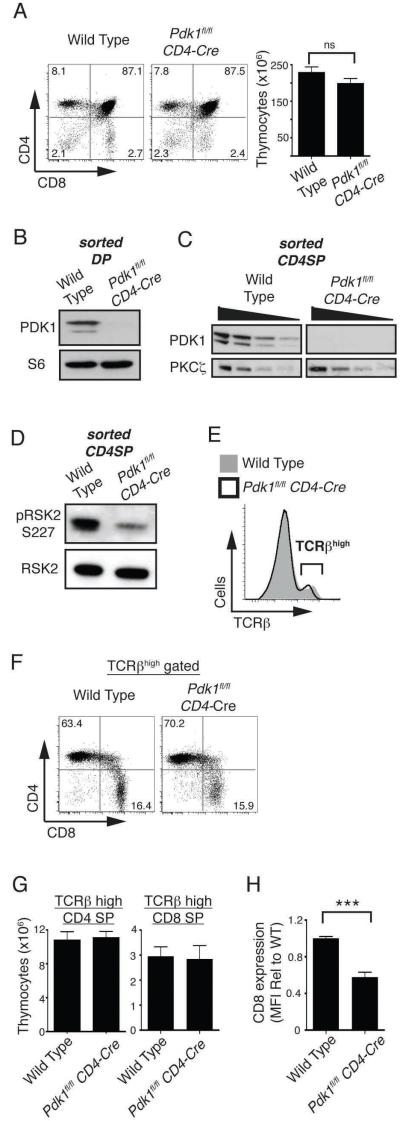
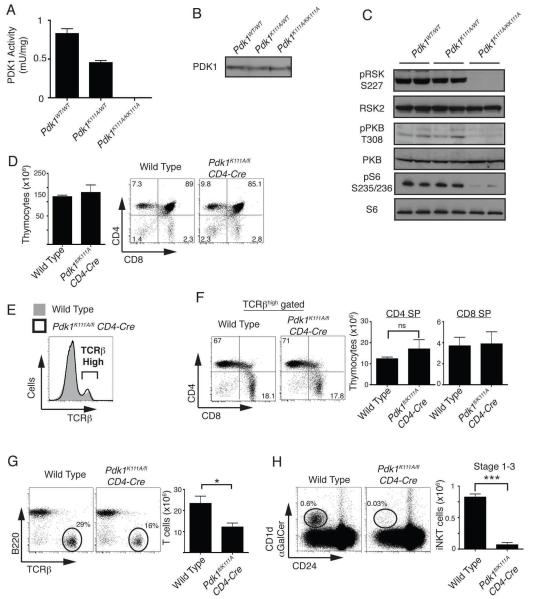
To explore the role of PDK1 as thymocytes transit the DP/SP stage of thymocyte development we backcrossed mice with floxed alleles of PDK1 to a mouse expressing cre recombinase under the control of the CD4 promoter, CD4-cre. Thymocytes were present at normal numbers in Pdk1fl/fl CD4-cre mice and comprised both CD4/CD8 DP, and CD4 or CD8 SP subpopulations (Fig. 1A). The deletion of PDK1 alleles in Pdk1fl/fl CD4-cre DPs was confirmed by PCR analysis (data not shown) and the loss of PDK1 protein was confirmed by western blot analysis of purified Pdk1fl/fl CD4-cre DPs (Fig. 1B). Analysis of purified SP thymocytes also revealed that Pdk1fl/fl CD4-cre SPs cells have deleted PDK1 (Fig. 1C). There is a basal level of PDK1 signalling in wild type SP thymocytes evident through the high basal phosphorylation of RSK on the PDK1 substrate sequence (Ser227) (Fig. 1D). The phosphorylation of RSK Ser227 is lost in Pdk1fl/fl CD4-cre SP thymocytes (Fig. 1D).
Figure 1. CD4 and CD8 SP thymocyte numbers are normal in the Pdk1fl/fl CD4-Cre thymus.
A. CD4/CD8 analysis of total thymocytes (left, n=11) and total thymocyte numbers (right, n=19). B. Western blot analysis of PDK1 and S6 ribosomal protein expression in sorted DP thymocytes. Data is representative of two such experiments. C. Western blot analysis of PDK1 and PKCζ expression in sorted CD4 SP thymocytes. Data shows serial twofold dilutions of each protein lysate and is representative of 2 such experiments. D. Western blot analysis of the PDK1 substrate RSK2 (serine 227) in sorted CD4 SP thymocytes. Data is representative of two such experiments. E. Expression of TCRβ on total thyomcytes. Data is representative of 11 experiments. F. CD4/CD8 analysis of TCRβhigh gated thymocytes. Data is representative of 11 experiments. G. Cell numbers of CD4/CD8 SP thymocytes. Data is mean +/− S.E.M of 11 experiments. H. Expression of CD8 on TCRβhigh CD8 SP thymocytes. Data is mean +/− S.E.M of 9 experiments (*** p<0.001).
Mature SP thymocytes express high surface levels of α/β TCR complexes and figure 1E shows that the frequency of TCRβhigh SP thymocytes is normal in Pdk1fl/fl CD4-cre mice. It was however evident from the CD4/CD8 staining profiles that the CD8 SPs in the Pdk1fl/fl CD4-cre thymus have reduced surface expression of CD8 compared to wild type CD8 SP thymocytes (Fig. 1A). This phenotype of CD4/CD8 expression has been described previously and interpreted to mean that Pdk1fl/fl CD4-cre mice have reduced number of CD8 SP thymocytes (22). However, the level of CD8 expression on SPs is upregulated following positive selection during a process termed ‘coreceptor tuning’ (29). Accordingly, it is conceivable that Pdk1fl/fl CD4-cre thymi do contain normal numbers of CD8 SP cells but these cells express low levels of CD8. Hence, a more accurate way to quantify CD8 SP thymocytes and in particular to distinguish these cells from the CD8 positive thymocytes that are intermediate between DNs and DPs is to first identify cells expressing high levels of TCRβ subunits and then to quantify expression of either CD4 or CD8 on the TCRβhigh cells. Using this analysis the data show that CD4 and CD8 mature SP populations are present in Pdk1fl/fl CD4-cre thymi at equivalent frequency to wild type thymi (Fig. 1F-G). However, while the frequency of CD8 SP thymocytes was normal in Pdk1fl/fl CD4-cre mice, the level of CD8 expressed on the PDK1 null TCRβhigh cells was reduced approximately two fold (Fig. 1H). Analysis of the expression of CD122, CD24 (also called Heat Stable Antigen) and CD8αα confirmed that these cells are conventional CD8 SP thymocytes rather than unconventional innate-like CD8+ T cells that normally develop at very low frequency in the thymus (data not shown) (30, 31). Therefore, CD4 and CD8 SP thymocyte numbers are normal in the Pdk1fl/fl CD4-cre mouse but PDK1 regulates the quantity of CD8 expressed on SPs.
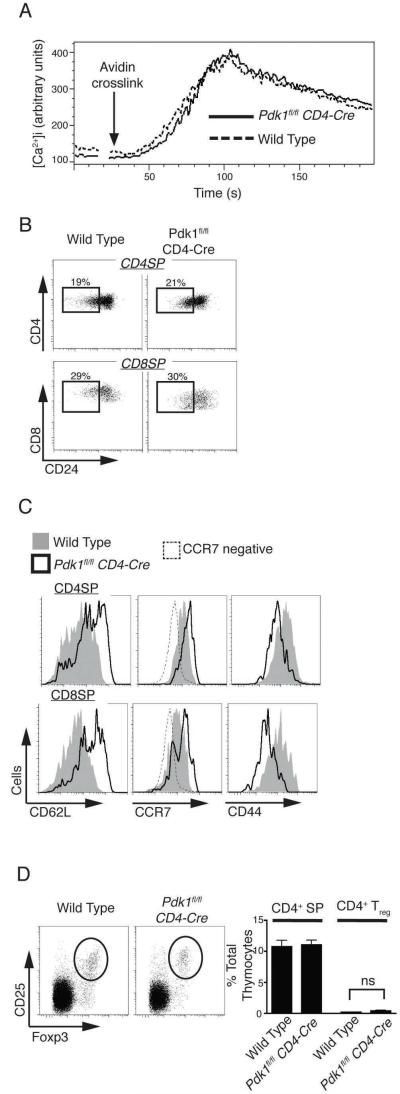
Do PDK1 null SP thymocytes mature normally? First, we assessed if mature PDK1 null SP thymocytes expressed a functional α/β T cell receptor complex by determining whether the cells could respond normally to TCR triggering and initiate downstream signaling. In this context, figure 2A shows that Pdk1fl/fl CD4-cre SP thymocytes can normally increase intracellular Ca2+ concentrations in response to crosslinking TCR complexes. We also examined PDK1 null SP thymocytes for the expression of CD24, a cell surface molecule that is downregulated as SP thymocytes mature in the thymus. In this respect, the frequency of CD24 low mature CD4 and CD8 SP thymocytes in Pdk1fl/fl CD4-cre mice was normal (Fig. 2B).
Figure 2. Pdk1fl/fl CD4-Cre α /β SP thymocytes mature normally but express an altered repertoire of adhesion receptors.
A. Calcium flux in CD4 SP thymocytes following activation of the T cell receptor via crosslinking of pre-bound anti-CD3. Data is representative of two such experiments performed in triplicate. B. CD24 expression on TCRβhigh/CD4 SP and TCRβhigh/CD8 SP thyomcytes. Data is representative of 8 experiments. C. Expression of CD62L, CCR7 and CD44 on TCRβhigh/CD4 or TCRβhigh/CD8 SP thymocytes. Data is representative of 2-4 experiments (CCR7 n=2; CD62L n=4; CD44 n=4)). D. Analysis of the frequency of CD25+/Foxp3+ regulatory T cells within the TCRβhigh CD4 SP thymocyte population. Data shows a representative frequency plot (left) and collated data of total TCRβhigh CD4 SP and CD4+ Treg frequency (right) and is mean +/− S.E.M of 8 experiments.
One other defining phenotype of mature SP thymocytes is that they express high levels of the adhesion molecule CD62L (also called L-Selectin) and the chemokine receptor CCR7. We have shown previously that strong activation of PDK1 signaling in T cells downregulates expression of CD62L and CCR7 and upregulates the expression of the adhesion molecule CD44 (32). PDK1 is a constitutively active kinase but it is not known if the basal levels of PDK1 activity found in SPs (Fig. 1D) play any role in determining the basal levels of CD62L, CCR7 and CD44 expressed by T cells. Figure 2C compares the CD62L, CCR7 and CD44 staining profiles of wild type and Pdk1fl/fl CD4-cre SP thymocytes. The data show that loss of PDK1 causes increased expression of CD62L and CCR7 in SP thymocytes but causes loss of CD44 expression. Collectively these data argue that PDK1 is not required for the DP to SP transition of conventional α/β T cells. However, the basal levels of PDK1 activity control the repertoire of adhesion molecules expressed by mature SP thymocytes.
CD4+ SP thymocytes include populations of regulatory T cells that are characterized by the expression of the transcription factor Foxp3 and the expression of the IL2 receptor α subunit, CD25. Figure 2D shows CD25 and Foxp3 staining of CD4 SP thymocytes and reveals that Treg frequency in the thymus of Pdk1fl/fl CD4-cre mice is similar to that of wild type mice.
PDK1 is required for the development of iNKT cells
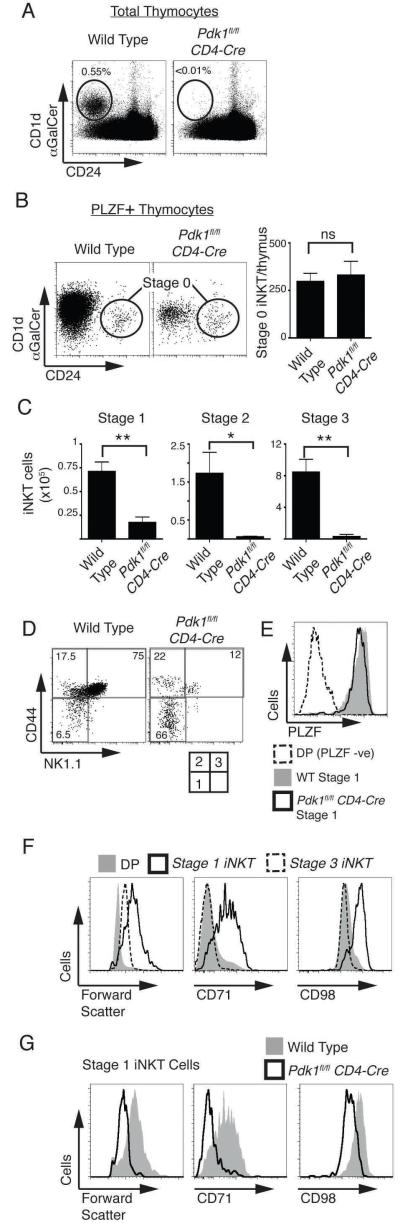
Another important CD4+ thymocyte subpopulation is the NKT cell subset that has an invariant Vα14 T cell receptor that recognizes glycolipid antigens presented by the MHC-like molecule CD1d. iNKT cells are usually present at low frequency in the thymus (about 0.4-0.6% of total thymocytes) but can be distinguished from conventional thymocytes as they can bind CD1d molecules loaded with the iNKT cell antigen α-galactoceramide (αGalCer) (33). Other surface markers can be used to distinguish the different stages of iNKT cell development such as CD24, which is expressed on the earliest iNKT cells progenitors (Stage 0) but not thereafter (Stage 1-3) (34). Accordingly, wild type and Pdk1fl/fl CD4-cre thymocytes were stained with CD1d-αGalCer and CD24 . An initial analysis of total thymocytes (Fig. 3A) indicated a substantial defect in the frequency of Stage 1-3 iNKT cells (CD1d-αGalCer+/CD24−) in Pdk1fl/fl CD4-cre thymocytes. The earliest iNKT cell subset, (Stage 0 iNKT cells) are present at very low frequency in the thymus and arise following positive selection of Vα14 TCR expressing DP iNKT progenitors. Rearrangement of the TCRα chain locus to generate the Vα14-Jα18 TCRα chain gene segment is crucial to the generation of iNKT cells and this process is successfully completed in Pdk1fl/fl CD4-cre CD69−DP (pre-selection) thymocytes (Supplementary Fig. 1). Stage 0 iNKT progenitors; express the Vα14 TCR complex that can bind CD1d-αGalCer complexes, can be characterised as NK1.1−/CD44−/CD24+, and unlike conventional α/β thymocytes they express the Promyelocytic leukaemia zinc finger protein (PLZF) transcription factor (35, 36). Detailed analysis using these key markers revealed normal numbers of stage 0 iNKT cells in the Pdk1fl/fl CD4-cre thymus (Fig. 3B) indicating that initial positive selection of iNKT progenitors proceeds normally in the absence of PDK1. Further analysis quantified the numbers and frequencies of iNKT cells at each of the developmental stages 1-3 (Fig. 3C, 3D). These data show that NK1.1−/CD44− stage 1 cells were reduced 4 fold in numbers with a more severe reduction in numbers of stage 2 and stage 3 iNKT cells (Fig. 3C). Figure 3E shows that PDK1-null stage 1 iNKT express high levels of PLZF, confirming that these are indeed stage 1 iNKT cells.
Figure 3. Selective defect in iNKT cell development in Pdk1fl/fl CD4-Cre mice.
A. Analysis of the frequency of CD1d-αGalCer+/CD24− (Stage 1-3) iNKT cells in total thymocytes. Data is representative of 8 experiments. B. Using magnetic bead purification, CD1d-αGalCer binding iNKT cells were enriched from thymocytes pooled from three thymi. Cells were fixed and stained for intracellular expression of PLZF, then CD1d-αGalCer and CD24 staining analysed on NK1.1−/CD44−/PLZF+ gated cells. CD1d-αGalCer+/CD24+ Stage 0 iNKT cells were identified (left; representative of 3 experiments) and Stage 0 iNKT cell number/thymus determined (right; mean +/− S.E.M of 3 experiments). C. Total numbers of iNKT cells in stage 1-3 subsets, mean +/− S.E.M of 5 experiments. D. Analysis of NK1.1 and CD44 expression on CD1d-αGalCer+/CD24− gated iNKT cells to differentiate the individual stages of iNKT development. Data is representative of 5 experiments. E. Analysis of PLZF expression in NK1.1−/CD44− stage 1 iNKT cells. Data is representative of 3 experiments. F. Analysis of forward scatter and the expression of CD71 and CD98 on wild type DP thymocytes, stage 1 and stage 3 iNKT cells. Data is representative of 4 experiments. G. Analysis of forward scatter and the expression of CD71 and CD98 on stage 1 iNKT cells. Data is representative of 4 experiments.
During stage 1, iNKT progenitors undergo rapid growth and proliferation thus implicating PDK1 in regulating these processes. Analysis of wild type iNKT progenitors confirms that as they transit through stage 1 they increase in size (Fig. 3F) and reveals that during stage 1 iNKT cells upregulate the expression of important nutrient receptors; the iron transporting transferin receptor (CD71) and CD98, a key component of the L-amino acid transporter (Fig. 3F). However, in the absence of PDK1 stage 1 iNKT cells fail to upregulate the expression of CD71 and CD98 and remain small cells (Fig. 3G). Collectively, the data show that Pdk1fl/fl CD4-cre thymoccytes have a block in iNKT cell development as the cells transit stage 1. The results argue that PDK1-null cells cannot meet the metabolic demands required for growth and proliferation.
Analysis of peripheral lymphocyte subpopulations in Pdk1fl/fl CD4-cre mice
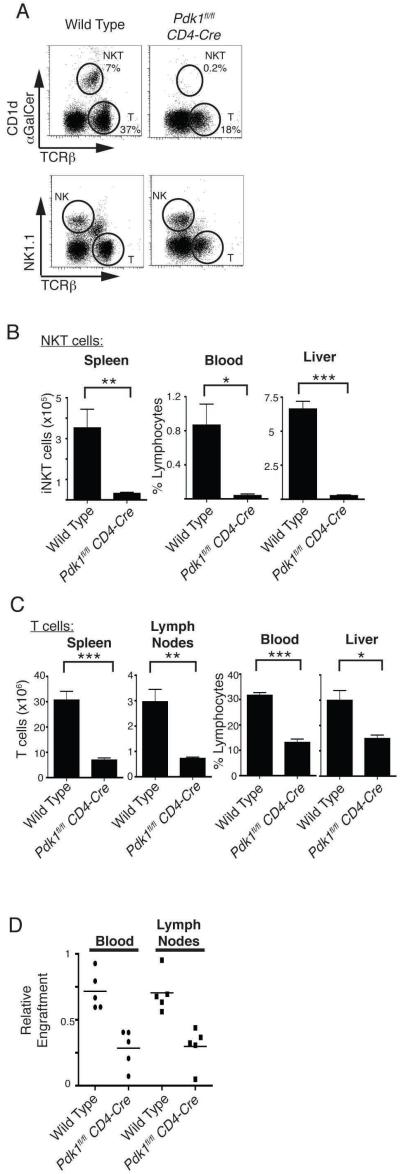
iNKT cells exit the thymus and in the periphery they are found at high frequency in the liver. Figure 4A (top) shows that there was a distinct population of iNKT cells in the liver from wild type mice but no clear population was discernable in the Pdk1fl/fl CD4-cre liver. The livers of Pdk1fl/fl CD4-cre mice did however contain conventional NK cells at normal frequencies (Fig. 4A, bottom). In fact, iNKT cells were largely absent from all Pdk1fl/fl CD4-cre peripheral tissues (liver, spleen and blood) compared to wild type (Fig. 4B). These data confirm the block in the development of iNKT cells in Pdk1fl/fl CD4-cre mice. The data presented in figure 1 show that Pdk1fl/fl CD4-cre thymi contain normal numbers of conventional α/β SP thymocytes. However, the livers of Pdk1fl/fl CD4-cre mice had not only lost iNKT cells but also had reduced levels of conventional α/β T cells (Fig. 4A, 4C). There were similarly reduced numbers of conventional α/β T cells in the spleen, blood and lymph nodes of Pdk1fl/fl CD4-cre mice (Fig. 4C). Hence, although the intra-thymic development of conventional α/β T cells is normal in Pdk1fl/fl CD4-cre mice these PDK1 null α/β cells fail to effectively populate peripheral lymphoid tissues. A defect in T cell egress from the thymus, peripheral survival, or homeostatic proliferation induced in the lymphopenic state in neonates might all account for the observed decrease in peripheral α/β T cell numbers. Here it is relevant that it has been previously demonstrated that PDK1-null T cells display normal IL7-dependant peripheral survival (22), and we confirmed this observation using the present mouse model (data not shown). In a thymus where T cells are unable to egress to the periphery an accumulation of CD24low mature TCRβhigh CD4/CD8 SP T cells occurs (37-39). There was no such accumulation of mature T cells in Pdk1fl/fl CD4-cre thymi (Fig. 2B). We therefore considered that possibility that the peripheral defect in Pdk1fl/fl CD4-cre T cells occurred because these cells failed to undergo lymphopenic induced proliferation. In this context, in neonatal mice there is a physiological process of lymphopenia-induced T cell proliferation that populates the peripheral lymphoid tissues (50). To simulate this process we mixed congenically marked wild type and Pdk1fl/fl CD4-cre thymocytes 1:1 and injected them into the tail vein of a lymphogenic host (Rag2−/− mice) and determined the relative engraftment after 4 weeks. Figure 4D demonstrates deficient engraftment of Rag2−/− mice by Pdk1fl/fl CD4-cre T cells relative to wild type T cells. PDK1 null T cells are thus defective in their ability to undergo homeostatic proliferation in a lymphopenic host.
Figure 4. Analysis of peripheral lymphocyte subpopulations in Pdk1fl/fl CD4-Cre mice.
A. Analysis of the frequency of CD1d-αGalCer+/TCRβmid iNKT cells (top) and NK cells (bottom) in the liver. Also shown are conventional α/β T cells. Data is representative of 4 experiments. B. Total numbers/frequencies of iNKT cells in peripheral tissues. Data is mean +/− S.E.M of 3 experiments. C. T cell numbers/frequencies in peripheral tissues. Data shows the mean +/− S.E.M of 5-13 experiments (Spleen n=13; Lymph nodes n=5; Blood n=7; Liver n=6; * p<0.05, ** p<0.01, *** p<0.001). D. Congenially marked wild type (CD45.1) and Pdk1fl/fl CD4-Cre (CD45.2) thymocytes were mixed at a 1:1 ratio and injected into lymphopenic Rag2−/− mice. After 4 weeks the relative engraftment of T cells was assessed (n=5).
PDK1 catalytic function in DPs is required for iNKT cell development but not for the thymic development of conventional α /β T cells
PDK1 is a kinase with multiple interaction domains, both protein-protein and protein-lipid binding domains and it has been proposed that PDK1 may have a role as a scaffolding protein that is independent of its catalytic activity (40). PDK1 also has a reported role in the assembly of a multi-protein complex that controls NF-κB activation at the membrane following TCR/CD28 triggering of mature T cells (41). To assess whether iNKT cell development required PDK1 catalytic function, we analysed iNKT cell development in mice where wild type PDK1 alleles were substituted with a kinase dead mutant of PDK1. For these experiments, mice with wild type PDK1 alleles substituted with a PDK1 allele with a point mutation at the key lysine residue required for ATP binding (K111A) in the PDK1 catalytic domain were generated. Mice homozygous for catalytically inactive PDK1 did not complete embryogenesis and of 254 pups born from matings between Pdk1WT/K111A mice, none were homozygous for the PDK1K111A mutation. At E10.5 and E11.5 Pdk1K111A/K111A embryos were found at normal mendelian frequency but no embryos homozygous for the PDK1K111A mutation were found after E12.5 (data not shown).
To confirm that the K111A mutation renders PDK1 catalytically inactive, protein lysates were generated from Pdk1WT/WT, Pdk1WT/K111A or Pdk1K111A/K111A E11.5-12.5 embryos. In vitro kinase assays demonstrate that PDK1 kinase activity is reduced by ~50% in Pdk1WT/K111A embryos while no PDK1 catalytic activity is detected in Pdk1K111A/K111A embryo lysates (Fig. 5A). PDK1 phosphorylates RSK on Ser227 and PKB on Thr308. Western blot analysis demonstrates that Pdk1K111A/K111A embryos express PDK1 (Fig. 5B) but these cells fail to phosphorylate RSK or PKB on their PDK1 substrate sequences (Fig. 5C). We also assessed PDK1 activity in Pdk1K111A/K111A cells by examining the phosphorylation of the S6 ribosomal protein by S6K1. S6 ribosomal protein phosphorylation is exquisitely sensitive to PDK1 activity, as it requires dual PDK1 inputs. S6K1 is itself phosphorylated and activated by PDK1 but only following phosphorylation by mTOR which itself is activated by PDK1/PKB signalling. The data (Fig. 5C) show that the phosphorylation of the S6 ribosomal protein was absent in Pdk1K111A/K111A cell lysates. Collectively these data show that Pdk1K111A/K111A cells express a catalytically inactive PDK1.
Figure 5. PDK1 catalytic activity is required for iNKT cell, but not conventional α /β T cell development.
A. Analysis of PDK1 kinase activity. Data show PDK1 kinase activity (mean +/− S.E.M) in cell lysates made from E11.5-12.5 embryos. Data is from 3 experiments. B. Western blot analysis of PDK1 expression in cell lysates made from E11.5-12.5 embryos. Data is representative 3 experiments. C. Western blot analysis of PKB, RSK and S6 ribosomal protein in lysates made from E11.5-12.5 embryos. Data shown is representative of 4 experiments. D. CD4/CD8 analysis of total thymocytes number (left, mean +/− S.E.M of 4 experiments) and CD4/CD8 expression (right, representative of 5 experiments). E. Expression of TCRβ on total thymocytes. Data is representative of 5 experiments. F. Analysis of the frequency (left, representative of 4 experiments) and total numbers (right, mean +/− S.E.M of 4 experiments) of TCRβhigh/CD4 and TCRβhigh/CD8 SP thymocytes. G. Analysis of the frequency (left) and total numbers (right; mean +/− S.E.M of 4 experiments, * p<0.05) of T cells in the spleen. H. Analysis of the frequency (left) and total numbers (right, mean +/− S.E.M of 4 experiments, *** P<0.001) of CD1d-αGalCer+/CD24− Stage 1-3 iNKT cells in total thymocytes.
As mice that are homozygous for the PDK1 K111A mutation do not survive beyond developmental stage E12.5, a conditional strategy was needed to produce thymocytes that selectively express Pdk1K111A. This was achieved by backcrossing mice that express a single Pdk1K111A allele and a single Pdk1fl allele (Pdk1K111A/fl) with mice expressing cre recombinase under the control of the CD4 promoter. The presence of the single PDK1 floxed allele in all tissues allows normal mouse development. However, as Pdk1K111A/fl CD4-cre T cells develop to DP thymocytes and express cre recombinase the floxed PDK1 allele is deleted thereby generating DP that express a single Pdk1K111A allele. Figure 5D shows that thymocyte numbers were normal in Pdk1K111A/fl CD4-cre thymi and the thymocytes comprised both CD4/CD8 DP and CD4 or CD8 SP subpopulations. The frequency and total numbers of TCRβhigh CD4/CD8 SP cells was normal in Pdk1K111A/fl CD4-cre thymi (Fig. 5E, 5F) indicating that the catalytic activity of PDK1 in DP thymocytes is not required for the development of conventional α/β T cells in the thymus. However, the data show that in the absence of PDK1 catalytic activity the surface expression of the CD8 coreceptor on CD8 SP thymocytes is reduced (Fig. 5D, 5F, Supplementary Fig. 2A). Additionally, the expression of CD62L is elevated while CD44 expression is decreased in SP thymocytes in Pdk1K111A/fl CD4-cre mice (Supplementary Fig. 2B). Therefore, this data confirms a role for basal PDK1 signaling in regulating the expression of CD8, CD44 and CD62L on mature thymocytes. Also consistent with the Pdk1fl/fl CD4-cre mouse phenotype; Pdk1K111A/fl CD4-cre mice have a profound defect in peripheral T cells (Fig. 5G, data not shown), there is a stage 1 block in iNKT cells development with small stage 1 iNKT cells lacking the expression of CD71 and CD98 (Fig. 5H, Supplementary Fig. 2C), and iNKT numbers in peripheral tissues are severely decreased (Supplementary Fig. 2D). Together this data confirms that the observed T cell and iNKT cell phenotype of the Pdk1fl/fl CD4-cre mouse is due to the loss of PDK1 kinase activity rather than any scaffold function of PDK1.
PKB is required for iNKT cell proliferation
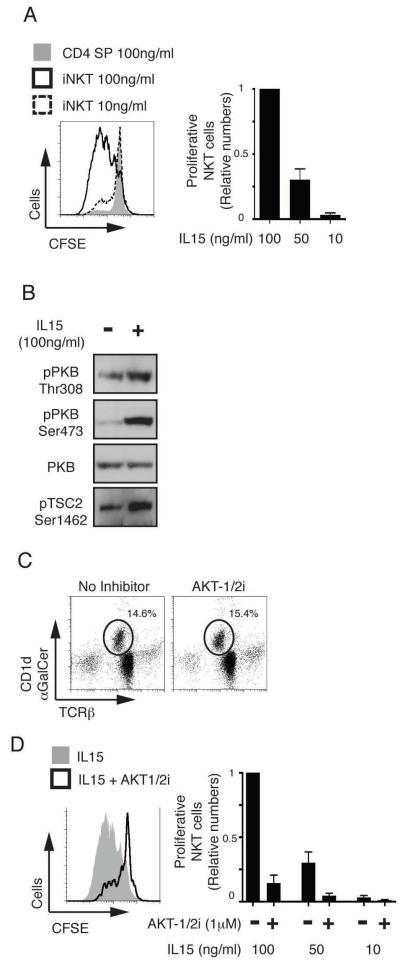
In DN T cell progenitors PDK1 is essential because it is required to couple activation of PI3K to the PKB controlled signaling pathways that support the energy demands of proliferating TCRβ selected thymocytes (16, 17, 19-21). In this respect, the loss of the p110δ isoform of PI3K results in decreased iNKT cell but not conventional T cell numbers (42). Therefore, there is a requirement for both PI3K and PDK1 signaling for iNKT cell versus conventional thymocyte development. In this context, PKB needs to bind PI(3,4,5)P3, the lipid product of PI3K, to be phosphorylated and activated by PDK1 (43, 44). We therefore considered whether the PDK1 dependency for iNKT cell development was explained by a PKB requirement for the robust proliferative burst of antigen receptor primed cells that accompanies iNKT cell differentiation. The greatly reduced numbers of iNKT cells in the Pdk1fl/fl CD4-cre thymus made it technically very difficult to examine whether there was a failed proliferative burst or a failure of iNKT cell survival. Accordingly, we used a pharmacological strategy in vitro to test the hypothesis that PKB is required to support the proliferation of iNKT cells. In initial experiments, we examined the PKB requirement for IL15 mediated iNKT cell proliferation. The rationale for these experiments is that IL15 is a key cytokine for iNKT cells and in its absence there is a block in normal iNKT cell development in the thymus whereas the development of conventional α/β T cells is normal (45, 46). To assess IL15 driven iNKT cell proliferation, wild type thymic iNKT cells were stained with CFSE, cultured in various doses of IL15 and then iNKT cell proliferation assessed by CFSE dilution. Figure 6A shows that wild type iNKT cells, but not CD4 SP thymocytes proliferate in a dose-dependent manner in response to IL15. However, it is not known whether IL15 stimulates PKB activity in iNKT cells. Therefore, we examined whether iNKT cells activate PKB in response to exposure to IL15. The data show that IL15 stimulation of iNKT cells increased the phosphorylation of PKB on the two key residues required for activity, serine 473 and the PDK1 substrate site threonine 308 (Fig. 6B). Analysis of the phosphorylation of the PKB substrate Tuberous sclerosis protein (TSC) 2 (serine 1462) confirmed increased PKB kinase activity following IL15 stimulation (Fig. 6B). We next examined the role of PDK1/PKB in IL15 mediated iNKT cell proliferation by using a selective allosteric inhibitor of PKB, AKT-1/2i (47). This compound prevents the conformational change that occurs when the PKB Pleckstrin Homology (PH) domain binds PI(3,4,5)P3, thus inhibiting the PDK1 mediated phosphorylation essential for PKB activation (48). Inhibition of PKB does not compromise iNKT cells survival (Fig.6C) but strongly inhibits in vitro proliferation of iNKT cells in response to IL15 (Fig. 6D).
Figure 6. PKB signalling is required for IL15 stimulated iNKT cell proliferation.
A. iNKT cells were enriched from total wild type thymocyes by depletion all CD8+ cells, stained with CFSE and cultured in different concentrations of IL15 for 4 days. Proliferative CD1d-αGalCer+/TCRmid iNKT cells and CD1d-αGalCer−/TCRβhigh CD4SP (cells that had divided at least once) were assessed by dilution of CFSE staining. Data shows a representative plot (left) and collated data (right, mean +/− S.E.M of 5 experiments). B. Cultured iNKT cells were stimulated +/− IL15 (100ng/ml) for 1 hour. Data show western blot analysis of PKB and TSC2 and is representative of 2 such experiments. C. iNKT cells were cultured (as in A) in a sub-mitogenic concentration of IL15 (10ng/ml) +/− the PKB inhibitor, AKT-1/2i (1μM). Data is representative of 4 experiments. D. iNKT cell proliferation was analysed (as in A) +/− the PKB inhibitor, AKT-1/2i (1μM). Data shows a representative plot with 100ng/ml IL15 (left) and collated data of IL15 dose response (right, as mean +/− S.E.M of 5 experiments).
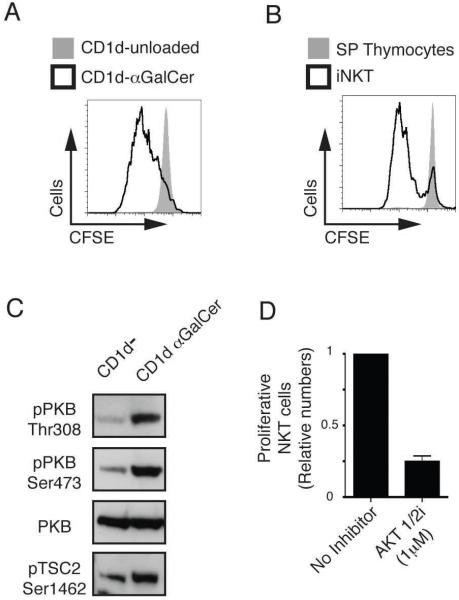
Would failure of IL15 mediated proliferation explain the deficient iNKT cell development in PDK1 null mice? Partially, although the defect in iNKT cell development seen in the absence of the IL15 receptor is less severe to that observed in the Pdk1fl/fl CD4-cre mice (45, 46). This discrepancy indicates that PDK1 must mediate the response of iNKT cells to additional exogenous signals. One possibility is that PDK1 is required for the antigen receptor induced priming of iNKT cells that is required to initiate their proliferative burst in the thymus. To test this hypothesis we examined the PKB requirement for Vα14 T cell antigen receptor induced proliferation of iNKT cells. Figure 7A shows that wild type iNKT cells proliferate following in vitro Vα14 TCR stimulation with αGalCer loaded CD1d. CD1d-αGalCer specifically stimulates the invariant Vα14 T cell receptor as there was no proliferation of conventional α/β CD4 SP T cells in this assay (Fig. 7B). We then assessed whether triggering of the Vα14 TCR in iNKT cells activates PKB. Figure 7C shows that CD1d-αGalCer stimulation of in vitro cultured iNKT cells causes increased phosphorylation of PKB and increased PKB activity as judged by increased phosphorylation of the PKB substrate TSC2. To determine whether PKB is required for TCR stimulated proliferation of iNKT cells experiments with the specific inhibitor AKT-1/2i were performed. The data show that AKT-1/2i treatment abrogates CD1d-αGalCer stimulated iNKT cell proliferation (Fig. 7D). Therefore, TCR induced proliferation of iNKT cells requires PKB activity.
Figure 7. PKB is required for Vα 14 TCR stimulated iNKT cell proliferation.
A. iNKT cells were enriched from total wild type thymocyes by depletion all CD8+ cells, stained with CFSE and cultured for 4 days following stimulation with either unloaded CD1d or αGalCer loaded CD1d. Proliferative CD1d-αGalCer+/TCRmid iNKT cells (cells that had divided at least once) were assessed by dilution of CFSE staining. Data is representative of 3 experiments. B. Proliferation of CD1d-αGalCer+/TCRmid iNKT cells versus CD1d-αGalCer−/TCRβhigh CD4 SP T cells was assessed (as in A) following stimulation with CD1d-αGalCer. Data is representative of 3 experiments. C. Cultured iNKT cells were stimulated for 20 min with either unloaded or αGalCer loaded CD1d. Data show western blot analysis of PKB and TSC2 and is representative of 2 such experiments. D. CD1d-αGalCer+/TCRmid iNKT cell proliferation was analysed (as in A) +/− the PKB inhibitor, AKT-1/2i (1μM). Data is mean +/− S.E.M of 7 experiments.
Discussion
The present study uses two independent genetic strategies to study the role of PDK1 in the differentiation and maturation of DP thymocytes. One strategy is to delete PDK1 floxed alleles in DPs with cre recombinase. The second strategy is to study the development of DP thymocytes where wild type PDK1 alleles are substituted by a PDK1 allele with a single point mutation that prevents ATP binding to the catalytic site. This latter approach ablates PDK1 kinase activity while retaining any scaffold function of the protein. It thus provides a more precise model for studying the role of PDK1 kinase activity in T cell development. It is also an approach that avoids some of the fundamental caveats of gene deletion models, namely that removal of a protein can alter the balance of multiple signaling pathways. A key finding is that loss of PDK1 catalytic function in DP thymocytes causes a profound defect in intrathymic iNKT cell development but allows the maturation of conventional α/β thymocytes and regulatory T cells in the thymus.
Why would the development of iNKT cells be dependent on PDK1? While initial positive selection of iNKT cell progenitors proceeds normally in the absence of PDK1 it is clear that PDK1 signaling is required for subsequent iNKT cell development involving robust proliferative expansion of stage 1 progenitors. Thus, PDK1-null stage 1 iNKT cells are small and have failed to upregulate the expression of crucial nutrient receptors CD71 and CD98. The present data show that the PDK1 substrate PKB is essential for the proliferative responses of iNKT cells to multiple signals that are crucial iNKT cell development, namely IL15 and the Vα14 TCR. The molecular basis for the failed iNKT cell development caused by loss of PDK1 catalytic activity can thus be attributed to the essential role of the PDK1/PKB signaling pathway for the proliferative burst of iNKT cells that is driven by Vα14 TCR/IL15 signaling and accompanies the positive selection of these cells. The data identify PDK1 as a kinase that integrates cytokine and antigen receptor signaling to support the proliferative expansion of iNKT cells in the thymus. This is reminiscent of a similar PDK1/PKB requirement for the preTCR/Notch induced changes in thymocyte metabolism that support the proliferative expansion that occurs as T cell progenitors transit from DNs to DPs (16, 17, 19-21).
The PDK1 independence of conventional α/β T cells at the DP to SP stage is indicative that there are distinct metabolic requirements for the positive selection of conventional α/β T cells versus iNKT cells, probably reflecting that conventional α/β T cells only proliferate modestly at this stage (49). However, the PDK1 independence of the DP/SP transition of conventional α/β T cells and regulatory T cells was unexpected because this stage of thymus development is controlled by the strength of TCR signaling and triggering of the T cell antigen receptor activates multiple PDK1 controlled kinases. Moreover, PDK1/PKB signaling in peripheral T cells modulates their differentiation: for example strong activation of PKB can impair Th17 and Treg cell differentiation (24, 25). Conversely, premature termination of PKB activity promotes the differentiation of regulatory T cells (23). Nevertheless, PDK1 was dispensable for the production of regulatory and conventional α/β T cells in the thymus. This does not mean that there is no role for PDK1 in SP thymocytes. In this respect, we could clearly demonstrate that constitutive PDK1 signaling programs the biology of SPs. The present data thus show that PDK1 ‘fine tunes’ expression of the coreceptor CD8 in peripheral T cells and controls the expression of adhesion molecules and chemokine receptors including CD44, CD62L and CCR7. PDK1 null SPs thus fail to express CD44 but show increased expression of CD62L and CCR7. The expression of CD62L and CCR7 is controlled by the transcription factor Foxo1 which has high basal activity in quiescent cells. Foxo1 is phosphorylated and inactivated by the PDK1 substrate PKB following T cell activation. The present data show that in naïve T cells, Foxo1 transcriptional activity can be increased by loss of PDK1. This result affords the insight that Foxo activity in SP T cells is dynamic and restrained by constitutive PDK1 signaling pathways.
The importance of PDK1 signaling for conventional T cells was emphasized by the universal deficit of T cell subsets in peripheral lymphoid tissues in both Pdk1fl/fl CD4-cre and Pdk1K111A/fl CD4-cre mice. The inability of PDK1 null T cells to undergo homeostatic proliferation induced proliferation in a lymphopenic environment may explain why the PDK1-null α/β SP thymocytes fail to effectively populate the periphery. Thus, the physiologic lymphopenic environment existing in neonatal mice supports the proliferative expansion of T cells that then populate the peripheral lymphoid tissues (50). In the absence of PDK1, thymic emigrants might not be able to meet the metabolic demands of this proliferative phase. In this respect, it has recently been shown that basal PI3K/PKB/Foxo signaling initiated by the BCR is crucial for peripheral homeostasis of mature B cells (51). While PDK1 signalling is not required for IL7 mediated T cell peripheral survival, weak TCR signalling, TCR “tickling”, is also important for T cell peripheral homeostasis (52). Therefore, basal TCR mediated PDK1/PKB signaling may also have a role in maintaining T cell homeostasis in the periphery.
The present data are consistent with a model that PDK1 is necessary for T cells whenever there is a metabolic demand on the cells imposed by a phase of rapid proliferation. PDK1 catalytic activity is thus essential for all T cells but at precise developmental stages depending on the metabolic demands exerted upon the developing T cell.
Supplementary Material
Acknowledgments
This work was supported by a Welcome Trust Principal Research Fellowship and Program Grant (065975/Z/01/A0).
Abbreviations
- PDK1
Phosphoinositide Dependent Kinase 1
- iNKT cells
invariant Vα14 Natural Killer T cells
- PKB
Protein Kinase B
- PKC
Protein Kinase C
- Treg
regulatory T cell
- DP
Double Positive
- DN
Double Negative
- SP
Single Positive
- RSK
90 kilodalton Ribosomal S6 Kinase
- S6K1
70 kilodalton S6 ribosomal protein Kinase 1
- αGalCer
α-galactocerimide
- TSC2
Tuberous sclerosis protein 2
- PLZF
Promyelocytic leukaemia zinc finger protein
Footnotes
Disclosures
The authors declare no competing financial interests.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in the Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI. Holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found a www.jimmunol.org.
References
- 1.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Current opinion in immunology. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Current opinion in immunology. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nature reviews. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nature reviews. 2009;9:106–115. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Bosselut R. CD4-CD8 lineage differentiation: Thpok-ing into the nucleus. J Immunol. 2009;183:2903–2910. doi: 10.4049/jimmunol.0901041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nature medicine. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. The Journal of experimental medicine. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. The Journal of experimental medicine. 2003;197:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. The Journal of experimental medicine. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 13.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 14.Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, MacDonald HR. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182:4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. The EMBO journal. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nature immunology. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- 18.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 19.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, Horie K, Gaitanaris GA, Fidanza V, Ludwig T, Wiest DL, Gounari F, Tsichlis PN. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178:5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- 21.Fayard E, Gill J, Paolino M, Hynx D, Hollander GA, Hemmings BA. Deletion of PKBalpha/Akt1 Affects Thymic Development. PloS one. 2007;2:e992. doi: 10.1371/journal.pone.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nature immunology. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183:6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- 25.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of experimental medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn CR, Lucocq JM, Gray GA, Jovanovic A, Alessi DR. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. Embo J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman DR, Lee PP, Wilson CB, Held W, MacDonald HR, Radtke F. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nature immunology. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 28.Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. The EMBO journal. 2003;22:4202–4211. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nature immunology. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 30.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, Cantrell DA. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. The Journal of experimental medicine. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science (New York, N.Y. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 34.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. The Journal of experimental medicine. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science (New York, N.Y. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 36.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Y, Du X, Ye J, Han M, Xu T, Zhuang Y, Tao W. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J Immunol. 2009;183:3865–3872. doi: 10.4049/jimmunol.0900678. [DOI] [PubMed] [Google Scholar]
- 38.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–1238. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]
- 39.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature immunology. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nature cell biology. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 41.Lee KY, D’Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science (New York, N.Y. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 42.Kim N, Saudemont A, Webb L, Camps M, Ruckle T, Hirsch E, Turner M, Colucci F. The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood. 2007;110:3202–3208. doi: 10.1182/blood-2007-02-075366. [DOI] [PubMed] [Google Scholar]
- 43.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 44.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science (New York, N.Y. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V alpha 14i NKT cells. Nature immunology. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 46.Minagawa M, Watanabe H, Miyaji C, Tomiyama K, Shimura H, Ito A, Ito M, Domen J, Weissman IL, Kawai K. Enforced expression of Bcl-2 restores the number of NK cells, but does not rescue the impaired development of NKT cells or intraepithelial lymphocytes, in IL-2/IL-15 receptor beta-chain-deficient mice. J Immunol. 2002;169:4153–4160. doi: 10.4049/jimmunol.169.8.4153. [DOI] [PubMed] [Google Scholar]
- 47.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, Malinowski J, McAvoy EM, Nahas DD, Robinson RG, Huber HE. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. The Biochemical journal. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green CJ, Goransson O, Kular GS, Leslie NR, Gray A, Alessi DR, Sakamoto K, Hundal HS. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. The Journal of biological chemistry. 2008;283:27653–27667. doi: 10.1074/jbc.M802623200. [DOI] [PubMed] [Google Scholar]
- 49.Hare KJ, Jenkinson EJ, Anderson G. CD69 expression discriminates MHC-dependent and -independent stages of thymocyte positive selection. J Immunol. 1999;162:3978–3983. [PubMed] [Google Scholar]
- 50.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin B, Becourt C, Bienvenu B, Lucas B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood. 2006;108:270–277. doi: 10.1182/blood-2006-01-0017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.