Abstract
Development of a safe and effective vaccine for HIV-1 infection is a critical global priority. However, the nature of host-virus interactions that lead to early immunosuppression and CD4 depletion, HIV-1 diversity, and the inability of the immune system to eliminate the latently infected CD4 pool of cells has to date thwarted successful vaccine development. Moreover, both the initial antibody-inducing vaccine (protein envelope gp120) and cell-mediated vaccine (recombinant adenovirus containing HIV-1 genes) strategies have failed in efficacy trials, and the latter cell-mediated vaccine appeared to have caused enhanced HIV-1 acquisition. Thus basic and translational research to understand why current vaccines have failed and elucidation of new mechanisms of virus control at mucosal surfaces is essential for eventual successful development of a preventive HIV-1 vaccine.
Keywords: HIV-1, vaccine, mucosal, gastrointestinal tract, T cells, antibody, innate, adaptive, immunity
Developing a safe and effective preventive HIV-1 vaccine is a critical priority in the overall plan to contain the global AIDS epidemic. However, progress on the development of a vaccine has been slow since the AIDS epidemic was first recognized in 1981. After the HIV vaccine field attempted and failed with most of the strategies that had been used for other successful vaccines, the AIDS vaccine field began to regroup in 2003 with the call for a new global collaboration, the Global HIV Vaccine Enterprise.1 Led by the National Institutes of Health and the Bill and Melinda Gates Foundation, the Enterprise fostered the development of a comprehensive strategic plan for overcoming roadblocks in vaccine development,2 and that plan has recently been updated.3,4 New investments by the National Institute of Allergy and Infectious Diseases and the Gates Foundation have spurred renewed efforts in AIDS vaccine discovery work. However, the recent failure in 2007 of the recombinant adenovirus type 5 (rAd5) HIV-1 vaccine candidate developed by Merck has dealt the vaccine development field another major setback.5 The rAd5 vaccine is comprised of an E1-deleted replication-incompetent rAd5 containing clade B gag, pol, and nef genes that was administered intramuscularly 3 times over the course of the vaccination period. Last October, the HVTN502 phase IIb clinical trial, called the Step Study, was stopped because an interim analysis showed that there was no efficacy of the vaccine and that in trial participants with preexisting immunity to rAd5, there was a strong trend toward enhanced acquisition of HIV-1 infection in those who had received the vaccine but not the placebo.5 Moreover, in addition to preexisting immunity to rAd5, a second predisposing factor for enhanced acquisition was being an uncircumcised man who received the Merck vaccine.6
The rAd5 vaccine was designed to induce CD8+ anti-HIV-1 T-cell responses and to help the host control viral load once infected.7 The cause of the failure of the Merck vaccine to have any effect on viral load in those who received the vaccine and became infected is not known. Nor is it understood why in those with pre-existing immunity to rAd5 and in uncircumcised men the vaccine predisposed to enhanced acquisition of HIV-1. Because virtually all HIV-1 transmission in the HVTN502 trial occurred through mucosal surfaces, understanding why the experimental vaccine failed and why it enhanced infectivity will be best achieved by in-depth study of the biology of the HIV-1 transmission event and by study of the effect of immunogens on immune cells that home to mucosal tissues.
Recently, the Global HIV Vaccine Enterprise updated the 2005 Global HIV Vaccine Enterprise strategic plan, with additions to recommendations to solve the problem of induction of broadly reactive neutralizing antibodies to the HIV-1 envelope3 and new recommendations for critical areas of study of mucosal immunity.4 For the study of mucosal immunity, these recommendations encompassed a broad range of basic and translational studies toward understanding HIV-1–host interactions at mucosal surfaces (Table I). This review will highlight some of these critical areas of research and discuss some of the roadblocks to development of an AIDS vaccine.
Table I.
Priorities for mucosal immunity research for the Global HIV-1 Vaccine Enterprise
| Define the sequence of events required to establish mucosal infection |
| Elucidate acute mucosal events that need to be prevented by HIV vaccines |
| Develop tools for measuring mucosal immune responses: assay development, standardization, and validation |
| Define the role of the common mucosal immune system in protection against HIV transmission |
| Characterize protective mucosal antibody responses |
| Define the role of T-cell responses in protection from HIV transmission |
| Learn how to harness dendritic cells, Toll-like receptors, and non–Toll-like receptors in HIV vaccine development |
| Understand the role of natural antiviral factors and innate immune cells in mediating the interface between innate and adaptive immunity to HIV |
| Understand the role of innate immunity in early HIV infection |
Data from Shattock RJ, Haynes BF, Pulendran B, Flores J, Esparza J, on behalf of a Working Group convened by the Global HIV Vaccine Enterprise. Improving defences at the portal of entry: Mucosal and innate immunity (Summary Report from a Global HIV Vaccine Enterprise Working Group). PLoS Med 2008;5:e81.4
HIV-1 Transmission
HIV-1 is remarkably diverse, with a reverse transcriptase enzyme that has a high error rate such that the average HIV-1 genome differs from its parent by at least 1 mutation and results in HIV-1 consisting of quasispecies or a “swarm” of related viruses.8-10 As a result, HIV-1 has evolved over time into a number of subtypes, or clades, with different clades in different locations worldwide.11 Moreover, HIV-1 can diversify by recombining among virus strains when 2 or more virus strains infect a person.12 Thus clade B predominates in the United States and Europe, clade C in South America and southern Africa, AE recombinants in southeast Asia, and subtypes A, C, and AC recombinants in China.11 The greatest heterogeneity of clades is in central Africa because HIV-1 originated from a simian immunodeficiency virus (SIV) recombinant virus and likely infected human subjects from an infected chimpanzee in central Africa approximately 70 to 80 years ago.13,14
Although mucosal surfaces are challenged with a myriad of HIV-1 quasispecies at each exposure, the infection rate is low and is proportional to the viral load of the donor partner.15,16 Thus the probability of HIV-1 transmission from male subjects to male subjects is 1/10 to 1/600 per coital act, that for male subjects to female subjects is 1/200 to 1/2000 per coital act, and for female subjects to male subjects is 1/200 to 1/10,000 per coital act.16,17 Plasma and semen viral loads are the highest soon after transmission, and for male-to-female transmission, the risk of acute infected males with high viral loads for transmission of HIV-1 to women has been estimated to be 1 transmission event per 53 coital acts.16 Thus the risk of infecting a partner by a donor is high during acute HIV-1 infection in the donor.
Interestingly, even though a donor with chronic HIV-1 might have a myriad of HIV-1 quasispecies in semen or vaginal fluids, in heterosexual HIV-1 transmission, usually only 1 or a few quasispecies of HIV-1 are transmitted.18 Furthermore, although HIV can use one of 2 coreceptors (CCR5 or CXCR4) after CD4 binding for viral entry, mucosal transmission is almost exclusively restricted to viruses that use CCR5.19 The reasons for the transmission bottleneck are not known but might relate to characteristics of the transmitted virus quasispecies that make it particularly fit for transmission across mucosal surfaces. Nonetheless, the heterogeneity of transmitted HIV-1 strains that do traverse the mucosal barrier is quite diverse, and this viral heterogeneity poses considerable problems for HIV-1 vaccine development. Computational biology approaches to HIV-1 diversity appear to be the best current strategies with potential to overcome HIV-1 diversity regarding the design of T-cell immunogens and include vaccine designs of consensus, ancestral, and mosaic HIV-1 genes whose designs aim to induce broad T-cell responses across the spectrum of HIV-1 clades.20-24 However, these experimental vaccine designs do not fully address the considerable problems associated with inducing broadly neutralizing antibodies to HIV-1.25
The Neutralizing Antibody Problem
A number of rare human mAbs have been isolated from HIV-1–infected patients that indeed do broadly neutralize diverse HIV-1 strains, such as mAbs 2F5 and 4E10 against the gp41 membrane proximal region and mAb 1b12 reactive with the gp120 CD4-binding site.26-28 HIV-1 envelope constructs made in the laboratory express the binding sites of these antibodies (ie, they are antigenic), but when these HIV envelopes are injected into animals or human subjects, they do not induce broadly neutralizing antibodies (ie, they are not immunogenic).27,28 The reasons for failure of forms of the HIV-1 envelope to induce broadly neutralizing antibodies with specificities like the rare human mAbs is not fully known, but the causes of poor Env immunogenicity might be multifactorial. The HIV-1 envelope is heavily glycosylated, with up to 40% of envelope mass carbohydrate creating an envelope glycan shield.27 The HIV-1 envelope is quite flexible and confers a considerable energy barrier to B cells that would recognize broadly neutralizing epitopes.27 Some of the vulnerable envelope regions, such as the membrane-proximal region of the gp41 envelope, are involved in the virus-to-cell fusion process, and vulnerable epitopes might only be transiently expressed and not be available to antibody for sufficient periods of time.29 Many of the vulnerable sites on the envelope are covered, either in conformational masking, glycan masking, or, in the case of vulnerable envelope regions near the virus membrane, virion lipids.30-32 Finally, several of the rare broadly neutralizing antibodies have unusual antibody traits, with long hydrophobic CDR3 regions that are reminiscent of autoantibodies. Indeed, 3 of these rare broadly neutralizing mAbs are polyspecific and cross-react with either host lipids or DNA, raising the notion that some of these types of antibodies might not be made because of immunoregulatory tolerance mechanisms.33-35 One broadly neutralizing mAb (mAb 2G12) reacts with a conformational epitope of the HIV-1 envelope carbohydrate.36 Host enzymes catalyze the glycosylation of the HIV envelope, and thus the envelope carbohydrates are host derived and are likely also recognized as self.37 Thus for a variety of reasons, even with highly immunogenic envelope constructs, the human B-cell arm of the immune system prefers to not recognize the vulnerable region epitopes of the HIV-1 envelope but rather prefers to recognize regions of the envelope that induce nonneutralizing antibodies.38,39
A number of other types of anti-HIV-1 antibody responses could potentially help control HIV-1 if they were present at the time of transmission, including antibodies that aggregate virions, thus preventing virion movement across mucosal epithelia40; inhibit transcytosis41; fix complement and lyse virions42; inactivate virus through macrophage Fc-mediated uptake; and mediate antibody-dependent cellular cytotoxicity (ADCC).43 The latter response might be critically important for targeting infected cells because neutralizing antibodies might be inefficient at preventing viral cell-cell transmission.44
An additional problem for induction of an adequate antibody responses to HIV-1 is the propensity of the HIV-1 envelope to induce polyclonal B-cell class switching, resulting in many B cells producing antibody in chronic HIV-1 infection, although with few of the activated B cells making anti-HIV-1 anti-bodies.45,46 Polyclonal B-cell activation in HIV-1 infection can be seen in peripheral blood B cells,45,46 as well as in gut and bone marrow B cells (K. Hwang and B. F. Haynes, unpublished observations). Potential mediators of HIV-1–induced polyclonal B-cell activation include IL-6,47 IL-15,48 B cell–activating factor,49 HIV-1 envelope gp120,49 and gut flora LPS released from HIV-1–induced gut epithelial cell dysfunction.50,51 Thus antigen-specific mucosal IgA and IgG anti-HIV-1 responses that might be able to control HIV-1 infection are inefficient, arrive too late, and are not sufficiently robust to control HIV-1.
HIV-1 and The Latent Pool of CD4+ T Cells
Because many of the strategies used for successful vaccines have now been tried and failed in the quest for a preventive AIDS vaccine, the field has now turned to more basic and translational research areas to understand what is needed to make a vaccine against an integrating lentivirus and, indeed, to determine whether such a vaccine is possible. Because HIV-1 is an integrating retrovirus that forms a latent pool of infected cells that is sheltered from both antiretroviral therapy and from host immune responses, the concept of producing a vaccine that induces sterilizing immunity is daunting. An alternative to a totally preventive AIDS vaccine is a vaccine that assists the immune system to control plasma virus load and virus-infected cell production. Such a vaccine would not prevent infection but rather would prevent or retard disease. Considerable preclinical evidence is available in the SIV model of infection in rhesus monkeys to suggest that this type of vaccine is feasible to develop.52 However, it is this latter kind of vaccine that the Merck rAd5 vaccine was designed to be, yet it failed.5
Johnston and Fauci53 and Wong Justin and Scilicano54 have called attention to the window of opportunity that any potentially successful HIV-1 vaccine has in which to work to extinguish the transmitted virus. This time period is the time of transmission to the establishment of the latent pool of infected CD4+ T cells (Fig 1).53-55 Because the latent pool of CD4+ T cells, once established, is refractory to antiretroviral treatment and anti-HIV-1 immune responses, for a preventive vaccine to be successful, it must work within this time period to extinguish the transmitted virus.
Fig 1.
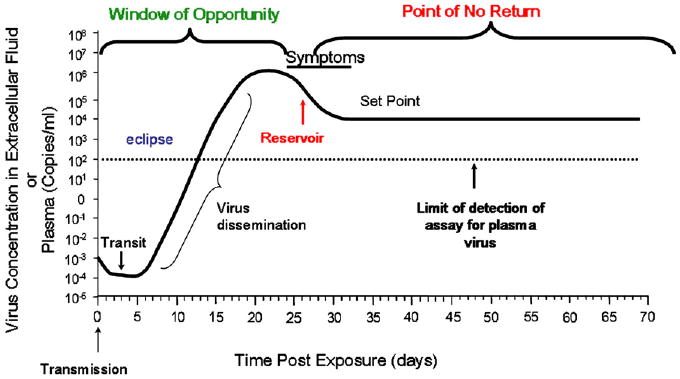
Time course of events after acute HIV-1 infection. Adapted with permission from Wong Justin and Siliciano.54
The Sequence of Transmission Events at The Mucosal Surface
Pope and Haase56 have summarized their work and the work of others in defining what are thought to be the HIV-1 transmission events across mucosal surfaces (Fig 2). HIV-1 crosses the mucosal barrier in 2 to 6 hours and, during the first 3 to 6 days, disseminates locally and reaches draining lymph nodes. This might have been even faster if infected cells within an infectious ejaculate can gain entry through genital ulceration, appearing in draining lymph nodes within 24 hours and distal sites (mesenteric and auxiliary lymph nodes) 2 to 3 days after exposure.57 Systemic dissemination occurs during the period of 6 to 25 days, with the time of first appearance of virus in blood occurring at approximately 10 days (range, 7-21 days).56 Once in the blood, virus replication occurs at a high rate and is proportional to the number of infected CD4+ T cells. The exact time of establishment of the latent pool of CD4+ T cells is not known but is thought to occur early on in infection and has been documented to be established by the time of seroconversion at about 20 to 25 days after transmission. Although the exact location of the first cells infected within mucosal tissue remains controversial, it is generally accepted that these include CD4+ T cells, dendritic cells, and macrophages.58 However, it is the crosstalk between CD4+ T cells and dendritic cells or macrophages59 that appears to drive localized viral replication.56 Furthermore, the uptake and dissemination of virus by dendritic cells to draining lymph nodes might prove an important strategy for avoiding antibody recognition.60,61
Fig 2.
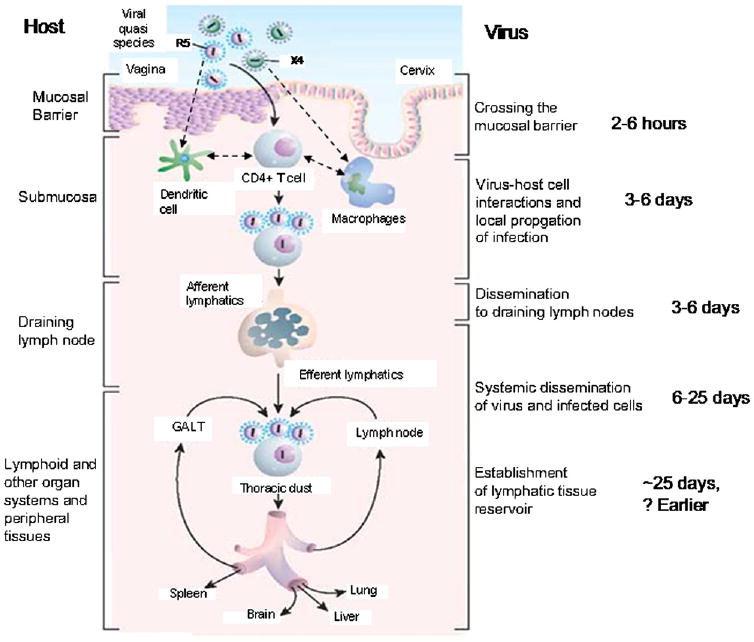
Sequence of local and systemic events that occur after HIV-1 transmission at mucosal surfaces. Adapted with permission from Pope and Haase.56
The Correlates of Immunity to HIV-1 at Mucosal Surfaces
For induction of sterilizing immunity to HIV-1, most agree that a vaccine must induce anti-HIV-1 neutralizing antibody at mucosal surfaces at the time of transmission, induce cytolytic T lymphocytes (CTLs) in mucosal submucosal areas that can rapidly kill virus-infected cells, or a combination of both.62 Unfortunately, in the unvaccinated subject both anti-HIV-1 antibodies and anti-HIV-1 CTLs usually arise too late after transmission to be effective, arising between 20 and 25 days after transmission.63,64 Thus for an AIDS vaccine to be effective, it must induce secondary (memory) CTLs and neutralizing antibody responses to either be present at the time of transmission or to prime for secondary responses that arise within hours to days after transmission. In 2005, the VAXGEN company sponsored a phase III clinical trial of an HIV-1 envelope gp120 vaccine in the United States in which the vaccine was comprised of 2 gp120 clade B envelopes, MN and GNE8.65 Although this antibody-inducing vaccine was immunogenic, the anti-gp120 antibodies induced were not protective, and the vaccine trial failed.66
Live attenuated (nef-deleted) SIV does not consistently protect against disease in rhesus monkeys when challenged with virulent SIV, but live attenuated (nef-deleted) SIV does provide a considerable measure of protection against SIV disease.66 In this setting it has been difficult to determine the basis of the protection, and attempts to define antibody or cellular controls of SIV have been inconclusive.66 However, in a number of settings, depletion of CD8+ T cells has shown that CD8+ T cells can directly contribute to control of SIV-infected cells and control SIV viral load.67 Letvin et al52 have demonstrated that a DNA prime, rAd5 boost containing gag, pol, nef, and env genes do not protect rhesus monkeys against infection with SIVmac251 but does prolong life with SIVand protect against CD4+ central memory cell loss. Thus robust CD8+ memory T-cell induction is likely a component of the correlates of protective immunity for SIV and, by inference, for HIV-1.
Mascola68 and Hessell et al69 have demonstrated that infusion of rhesus monkeys with high levels of human mAbs to neutralizing sites on HIV-1 gp120 can provide sterilizing immunity against mucosal challenge with chimeric simian-human immunodeficiency viruses (ie, SIV viruses with HIV-1 envelopes). Thus if the right kind of antibody and high levels of CD8+ CTLs that recognize diverse HIV-1 isolates could be induced to be present at the time of transmission or induced to arise in a very rapid (hours to days) secondary response after vaccination, it is plausible that a vaccine could prevent establishment of permanent infection with HIV-1. Unfortunately, to date, widely diverse CD8+ CTL cellular responses to HIV-1 have been somewhat difficult to induce,70 and broadly neutralizing antibodies to HIV-1 have been impossible to induce.27,28
The Mucosal Targets and Barriers for HIV-1
The linings of the gastrointestinal and genitourinary tracts are covered in mucus containing both IgA and IgG that forms a natural protective barrier against pathogen invasion, including HIV-1 virions.17,71 Natural mucosal defenses to HIV-1 include the production of molecules including α and β defensins72,73 and secretory leukocyte protease inhibitor.74 Although the earliest events controlling viral transmission across intact mucosal surfaces remain controversial,58 genital infections, mucosal trauma resulting in breaks in the mucosal surface, inflammation, and exposure to high concentrations of virus all predispose to acquisition of HIV-1 infection.16,57 HIV-1 replicates in both resting and activated CD4+ CCR5+ T cells and induces cell death by direct infection and bystander cell killing, resulting in massive CD4+ cell loss.75 At the peak of viremia, more than 80% of CD4+ memory T cells can die, of which only a fraction of cells are infected.76 The time to AIDS has been suggested to be correlated with the level of depletion of the gut CD4+ memory T-cell pool: the more CD4 cells lost in acute infection, the faster the progression to AIDS.77 TNF apoptosis-inducing ligand is an apoptosis-inducing molecule produced by monocytes and CD4+ T cells that is induced early on in HIV-1 infection, binds to uninfected immune cells, and might be responsible for much of the initial bystander killing of immune cells (N. Gasper-Smith and B. F. Haynes, unpublished data).78 Thus, early on in HIV-1 infection, the immune system appears to be trying simultaneously to respond to HIV-1 and, as well, to recover from HIV-1–induced cell death and HIV-1–induced immune suppression. CD4+ CCR5+ T cells are eliminated by multiple mechanisms, including by direct HIV-1 infection, bystander killing of uninfected immune cells, and normal cell death of CD4+ T cells as they become activated to respond to HIV-1 in an accelerated manner by high viral loads.76
What Does a Successful HIV-1 Vaccine Need to do?
A successful, sterilizing, preventive AIDS vaccine must induce protective antibodies that are present at the time of transmission at sufficient concentrations to prevent virion movement from the epithelial surface to dendritic cells and induce anti–HIV-1 CD4+ and CD8+ T cells in the submucosa. Whether the latent pool of CD4+ T cells is established sufficiently late after transmission to allow time for a memory B-cell response to be effective is not known. That postexposure prophylaxis is not fully effective in SIV-challenged monkeys 24 hours after challenge raises the possibility that the latent pool of CD4 cells is established quite early in SIV infection.79 It stands to reason that the presence of CD8+ T cells at the time of transmission would be salutary for immediate elimination of those HIV-1–infected CD4 cells that might evade neutralizing or otherwise infection-inhibitory antibodies and for assistance in control of viral load if sterilizing immunity does not occur.
If a vaccine can act before HIV-1 induction of massive cell death, then it will have a chance to evade the immunosuppressive sequelae of cell death–derived components, such as apoptotic microparticles.80 Otherwise, a successful vaccine will need to overcome the loss of CD4+ T cells brought on by cell death and overcome any immune downregulation by cell death products.
Finally, the innate immune system traditionally responds earliest to infections but lacks immune memory. If possible, a successful vaccine might need to harness innate immunity in some as yet unknown manner to prevent HIV-1 transmission. Components of the innate immune system described with some type of memory include natural killer/T cells,81 T-cell receptor γδ T cells,82 and natural antibody-producing B1 and marginal zone B cells.83 Whether these latter cell types can be recruited by an AIDS vaccine to respond rapidly and effectively to transmitted HIV-1 at mucosal surfaces remains to be determined.
Summary
A number of difficult obstacles continue to stand in the way of making a successful preventive HIV-1 vaccine, including HIV-1 diversity, the early formation of a latently infected CD4+ T-cell pool, the resulting narrow window of time for a vaccine to induce immune responses that might extinguish the transmitted virus, the inability of current envelope proteins to induce broadly neutralizing anti-HIV-1 antibodies, and HIV-1 infection of CD4+ T cells and induction of massive CD4 cell death. The recent failure of the Merck rAd5 vector HIV-1 vaccine trial further complicates this difficult task with the specter of vaccine-mediated enhanced acquisition of HIV-1 infection in those with either preexisting antibody to rAd5 or in those male subjects who were not circumcised. The way forward for successful vaccine development is for the field to perform the basic research to (1) work to understand why the Merck trial failed to protect and why the rAd5 vaccine might result in enhanced HIV-1 acquisition, (2) work to induce high levels of long-lived plasma cells making broadly neutralizing antibodies to HIV-1 at mucosal surfaces, and (3) develop HIV-1 immunogens that overcome the diversity of HIV-1. In addition, it is important to bring the newest and best technology to the problem to provide the field with the best discovery effort available to explore as yet unknown aspects of innate and adaptive immunity. These technologies include genome-wide association study technologies,84 new host and viral DNA and RNA sequencing technology, and genome-wide functional studies of genes involved in HIV-1 replication.85 Finally, it is clear after 25 years of combating HIV-1 that the work to end AIDS will need to be continued by the next generation of clinical and basic investigators, and we must work to ensure that the next generation of investigators are mentored and supported to continue this work until the AIDS epidemic is brought under control.
Acknowledgments
We thank Myron Cohen, Joseph Sodroski, Norman Letvin, Andrew McMichael, Stuart Shapiro, and George Shaw for comments and insightful discussions and Kim R. McClammy for expert secretarial assistance.
Supported by National Institutes of Health grant AI-0678501, the Center For HIV/AIDS Vaccine Immunology (B.H. and R.S.), and Collaboration for AIDS Vaccine Discovery (B.H.) and Grand Challenge (R.S.) grants from the Bill and Melinda Gates Foundation and Europrise (R.S.) network of excellence on vaccines and microbicides funded by the European Commission.
Abbreviations used
- CTL
Cytolytic T lymphocyte
- HVTN
HIV Vaccine Trials Network
- rAd5
Recombinant adenovirus type 5
- SIV
Simian immunodeficiency virus
References
- 1.Klausner RD, Fauci AS, Corey L, Nabel GJ, Gayle H, Berkley S, et al. Medicine. The need for a global HIV vaccine enterprise. Science. 2003;300:2036–9. doi: 10.1126/science.1086916. [DOI] [PubMed] [Google Scholar]
- 2.The Global HIV/AIDS Vaccine Enterprise: scientific strategic plan. Coordinating Committee of the Global HIV/AIDS Vaccine Enterprise.The Global HIV/AIDS Vaccine Enterprise: Scientific Strategic Plan. PLoS Med. 2005;2:e25. doi: 10.1371/journal.pmed.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montefiori D, Sattentau Q, Flores J, Esparza J, Mascola J. Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS Med. 2007;4:e348. doi: 10.1371/journal.pmed.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shattock RJ, Haynes BF, Pulendran B, Flores J, Esparza J on behalf of a Working Group convened by the Global HIV Vaccine Enterprise. Improving defences at the portal of entry: Mucosal and innate immunity (Summary Report from a Global HIV Vaccine Enterprise Working Group) PLoS Med. 2008;5:e81. doi: 10.1371/journal.pmed.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bubnoff A. The 15th annual meeting had its share of sobering news from clinical trials, but researchers also reported many exciting advances. IAVI Rep. 2008;12:1–5. [Google Scholar]
- 7.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 8.Korber BT, Allen EE, Farmer AD, Myers GL. Heterogeneity of HIV-1 and HIV-2. AIDS. 1995;9(suppl A):S5–18. [PubMed] [Google Scholar]
- 9.Peeters M, Toure-Kane C, Nkengasong JN. Genetic diversity of HIV in Africa: impact on diagnosis, treatment, vaccine development and trials. AIDS. 2003;17:2547–60. doi: 10.1097/01.aids.0000096895.73209.89. [DOI] [PubMed] [Google Scholar]
- 10.Keele B, Giorgi E, Salazar-Gonzalez J, Decker J, Pham K, Salazar M, et al. Identification of the transmitted HIV-1 envelope. Proc Natl Acad Sci U S A. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stebbing J, Moyle G. The clades of HIV: their origins and clinical significance. AIDS Rev. 2003;5:205–13. [PubMed] [Google Scholar]
- 12.Robertson DL, Sharp PM, McCutchan FE, Hahn BH. Recombination in HIV-1. Nature. 1995;374:124–6. doi: 10.1038/374124b0. [DOI] [PubMed] [Google Scholar]
- 13.Korber B, Bhattacharya T, Theiler J, Gupta R, Lapedes A, Hahn BH, et al. Search for the origin of HIV and AIDS. Science. 2000;289:1140–1. doi: 10.1126/science.289.5482.1140. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–41. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 16.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–45. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 18.Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 19.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: the “gatekeeper” problem resolved? Nat Rev Microbiol. 2006;4:312–7. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 20.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Korber BT, Weaver E, Liao HX, Hahn BH, Haynes BF. Centralized immunogens as a vaccine strategy to overcome HIV-1 diversity. Expert Rev Vaccines. 2004;3(suppl):S161–8. doi: 10.1586/14760584.3.4.s161. [DOI] [PubMed] [Google Scholar]
- 22.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–6. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 23.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–82. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickle DC, Rolland M, Jensen MA, Pond SL, Deng W, Seligman M, et al. Coping with viral diversity in HIV vaccine design. PLoS Comput Biol. 2007;3:e75. doi: 10.1371/journal.pcbi.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao F, Liao HX, Hahn BH, Letvin NL, Korber BT, Haynes BF. Centralized HIV-1 envelope immunogens and neutralizing antibodies. Curr HIV Res. 2007;5:572–7. doi: 10.2174/157016207782418498. [DOI] [PubMed] [Google Scholar]
- 26.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A. 2005;102:14943–8. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines. 2006;5:579–95. doi: 10.1586/14760584.5.4.579. [DOI] [PubMed] [Google Scholar]
- 29.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–30. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 30.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 31.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 32.Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, et al. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 34.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 35.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–35. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 37.Scanlan CN, Ritchie GE, Baruah K, Crispin M, Harvey DJ, Singer BB, et al. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. J Mol Biol. 2007;372:16–22. doi: 10.1016/j.jmb.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Crooks ET, Moore PL, Franti M, Cayanan CS, Zhu P, Jiang P, et al. A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120. Virology. 2007;366:245–62. doi: 10.1016/j.virol.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–25. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Outlaw MC, Dimmock NJ. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990;71:69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- 41.Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165:5170–6. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 42.Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederost B, et al. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 2006;3:e441. doi: 10.1371/journal.pmed.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinhold KJ. Anti-HIV-1 ADCC: clinical and therapeutic implications. Biotechnol Ther. 1991;2:147–57. [PubMed] [Google Scholar]
- 44.Ganesh L, Leung K, Loré K, Levin R, Panet A, Schwartz O, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–7. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–45. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest. 1992;89:561–6. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 48.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 49.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–41. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 50.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 51.Haynes BF. Gut microbes out of control in HIV infection. Nat Med. 2006;12:1351–2. doi: 10.1038/nm1206-1351. [DOI] [PubMed] [Google Scholar]
- 52.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–3. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356:2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 54.Wong Justin SB, Siliciano RF. Biology of early infection and impact on vaccine design. In: Koff WC, Kahn P, Gust ID, editors. AIDS Vaccine Development Challenges and Opportunities. Chapter 3. Norwich (UK): Horizon Scientific Press; 2007. pp. 17–22. [Google Scholar]
- 55.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–52. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 57.Weiler AM, Li Q, Duan L, Kaizu M, Weisgrau KL, Friedrich TC, et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated SIVmac239. J Virol. 2008;82:4154–8. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 59.Loré K, Smed-Sörensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–33. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen P, Hübner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–95. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Montfort T, Nabatov AA, Geijtenbeek TB, Pollakis G, Paxton WA. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4+ T lymphocytes. J Immunol. 2007;178:3177–85. doi: 10.4049/jimmunol.178.5.3177. [DOI] [PubMed] [Google Scholar]
- 62.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–35. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 65.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok. Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 66.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 68.Mascola JR. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine. 2002;20:1922–5. doi: 10.1016/s0264-410x(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 69.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:29–30. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 70.Weaver EA, Lu Z, Camacho ZT, Moukdar F, Liao HX, Ma BJ, et al. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. J Virol. 2006;80:6745–56. doi: 10.1128/JVI.02484-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macpheron A, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 72.Furci L, Sironi F, Tolazzi M, Vassena L, Lusso P. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood. 2007;109:2928–35. doi: 10.1182/blood-2006-05-024489. [DOI] [PubMed] [Google Scholar]
- 73.Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–29. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–64. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chase A, Zhou Y, Siliciano RF. HIV-1-induced depletion of CD4+ T cells in the gut: mechanism and therapeutic implications. Trends Pharmacol Sci. 2006;27:4–7. doi: 10.1016/j.tips.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1080–1. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 77.Brenchley JM, Know KS, Asher AI, Price DA, Kohli LM, Gostick E, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Nature. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–8. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emau P, Jiang Y, Agy MB, Tian B, Bekele G, Tsai CC. Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention. AIDS Res Ther. 2006;28:29. doi: 10.1186/1742-6405-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huber LC, Jüngel A, Distler JH, Moritz F, Gay RE, Michel BA, et al. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007;12:363–74. doi: 10.1007/s10495-006-0622-7. [DOI] [PubMed] [Google Scholar]
- 81.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 82.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–50. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Telenti A, Goldstein DB. Genomics meets HIV-1. Nat Rev Microbiol. 2006;4:865–73. doi: 10.1038/nrmicro1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–6. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]


