Abstract
Background
Although total white blood cell (WBC) count has been associated with hypertension, the association between specific WBC types and blood pressure (BP) levels has not been studied.
Methods
In a cohort of 5,746 middle-aged African-American and white adults free of clinical cardiovascular disease and cancer and not taking hypertension or anti-inflammatory medications, BP was measured at baseline and 3, 6, and 9 years later. Levels of circulating neutrophils, lymphocytes, and monocytes were measured at baseline.
Results
In African-Americans, but much less so in whites, increased neutrophil levels and decreased lymphocyte levels were significantly associated with elevation of BP but did not influence the rate of change of BP over time. The mean BP difference between the highest and lowest quartiles of neutrophils was approximately 8 mmHg for systolic BP (SBP), 4 mmHg for mean arterial pressure (MAP), and 5 mmHg for pulse pressure (PP). The mean BP difference between the lowest and highest quartiles of lymphocytes was approximately 6 mmHg for SBP, 2 mmHg for diastolic BP (DBP), 3 mmHg for MAP, and 4 mmHg for PP.
Conclusions
Increased neutrophils and decreased lymphocytes are significantly correlated with the regulation of BP and the development of hypertension, especially in African-Americans.
Keywords: Inflammation, Hypertension, Neutrophil, Lymphocyte
INTRODUCTION
Hypertension is a well-known risk factor for cardiovascular disease 1,2 and cardiovascular -related death 3. It was recently reported that 29% of adults ≥20 years age in the United States had hypertension and an additional 37% had pre-hypertension 4. Therefore, hypertension continues to be an important public health problem. For the past 200 years, a variety of etiologies for essential hypertension has been proposed 5. However, the mechanisms underlying the pathogenesis of this disease remain unclear. In recent years, inflammation has emerged as a potential mechanism of hypertension and a prospective therapeutic target 6,7. A number of epidemiological studies have shown that markers of systemic low-grade inflammation are increased in hypertensive patients, and their levels predict the onset of hypertension 8. Circulating leukocytes are the most stable, well-standardized, readily available and inexpensive measure of systemic inflammation. The predictive role of circulating total white blood cell (WBC) level in the incidence or prevalence of hypertension has been well documented 9,10,11. Nevertheless, little information is available on the independent contribution of specific WBC types.
Blood pressure (BP) differences between African-Americans and whites have been noted for a long time 12. A recent study found that 35% of African-Americans have hypertension, which accounts for 20% of African-American deaths in the United States - twice the percentage in whites. Compared to whites, hypertension develops earlier in life and average BPs are much higher in African-Americans 13. The reason for these differences is still poorly understood. We hypothesized that circulating levels of specific leukocytes contribute to the development of hypertension, especially in African-Americans. The objectives of this study were to determine: 1) whether there is an independent association between the levels of circulating specific leukocytes (neutrophils, lymphocytes, and monocytes) and BP parameters [systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP) and pulse pressure (PP)]; 2) whether the levels of circulating specific leukocytes are associated with the rate of change of BP with age.
METHODS
Study Population
The ARIC study is a prospective investigation of atherosclerosis and clinical atherosclerotic diseases in four US communities. A population sample totaling 15,792 persons aged 45–64 was selected from Forsyth County, NC, Jackson, Miss., the northwest suburbs of Minneapolis, MN, and Washington County, MD, using a published study design, sampling strategy, and examination techniques 14. Each community's cohort was a probability sample, except that only African-Americans were sampled in Jackson. A baseline examination (visit 1) was conducted from 1986 to 1989, with response rates of 46% in Jackson and 65–67% in the other three communities. Participants were re-examined every three years (visit 2, 1990-92; visit 3, 1993-95; visit 4, 1996-98). At each clinic visit, sociodemographic characteristics, medical history, medication use, diet, and physical activity were assessed, and a variety of biochemical, physiologic, and anthropomorphic measures were obtained.
For the cross-sectional analysis component of this study we analyzed data from visit 1. We excluded 48 participants who were neither white nor African-American and 55 African-Americans in the Minneapolis, MN and Washington County, MD field centers (due to their small numbers); 874 with a history of cancer; 766 with prevalent coronary heart disease CHD; 284 with prior stroke; 401 who had had heart/arterial surgery; 261 who had had balloon angioplasty; 635 with a history of peripheral artery disease; 16 persons missing BP at visit 1; and 4,846 persons on hypertension medications or whose medication status was unknown. In addition, 173 persons on steroids, 2,842 on Ibuprofen or other non-steroidal anti-inflammatory drugs, and 4,251 on aspirin/salicylates were also excluded because of the anti-inflammatory actions of these drugs. Finally, we excluded persons with cell counts in the top 1% of the leukocyte distributions [total WBC >12,000 (n=155), neutrophils >78% (n=114), lymphocytes >63% (n=62), monocytes >14% (n=102)] because these extreme levels could represent occult disease. This left 5,746 participants available for study. For the longitudinal analysis, the BP readings at visits 2, 3, or 4 were marked as missing if the participant reported use of hypertension medications in the 2 weeks prior to that visit. All participants with at least 1 BP reading (at visit1) were used in the longitudinal analysis. Thirteen percent had only the visit 1 BP reading, 16% had 2 BP readings, 18% had 3 BP readings, and 53% had all 4 BP readings (mean 3.1 readings per person).
Clinical measurements and definitions
All clinical measures were assessed at baseline using standardized instruments and a strict protocol. SBP and DBP were taken in the sitting position by trained technicians using a random-zero sphygmomanometer after a 5-minute rest, and the average of the last two values was computed. Hypertension was defined based on Joint National Committee VII guidelines as SBP ≥140 mmHg or DBP ≥90 mmHg, or self-reported use of anti-hypertensive medication within the 2 weeks before the exam, or a history of physician diagnosis. Pre-hypertension was defined as SBP ≥120 mmHg but <140 mmHg or DBP ≥80 mmHg but < 90 mmHg. Diabetes was defined based on the American Diabetes Association guidelines as fasting serum glucose of ≥126 mg/dL (7 mmol/L) or non-fasting glucose of ≥200 mg/dL (11.1 mmol/L), or self-reported use of diabetic medications within two weeks of the clinic visit, or a history of physician-diagnosed diabetes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Self-reported smoking and drinking status were categorized into 3 levels (current, former, never). Fasting serum total lipoprotein cholesterol concentration was assessed with Roche enzymatic methods using a Cobras centrifuge analyzer (Hoffman-La Roche), with the laboratory certified by the CDC-NHLBI Lipid Standardization Program. Physical activity was assessed on a scale 0-5 using the sport score from the modified Baecke instrument used in the ARIC Study 15. Usual dietary sodium intake was collected at baseline using a semi-quantitative, interviewer-administered food frequency questionnaire (FFQ). The ARIC FFQ contains 66-items and was based on the original Willett 61-item FFQ 16.
Statistical Analysis
Because we were interested in examining differences between race groups, and because in almost all cases the interaction term for race*quartile of leukocyte level was statistically significant, results are presented separately for African-Americans and whites. Descriptive data are presented by race in Table 1 as mean ± standard error (SE) for continuous variables and percentages for categorical variables. In the cross-sectional analysis, BP was regressed on leukocyte level (as a continuous variable) in a linear regression model, with adjustment for age, sex, center, diabetes, smoking (current, former, never), drinking (current, former, never), BMI, total cholesterol, physical activity, and usual dietary sodium intake. Also, differences in mean BP by quartile of leukocyte level (tertile of monocyte level because of the more restricted range of values) were tested in an ANOVA model, with adjustment for the same covariates.
Table 1.
Clinical characteristics of study population by race.
| Characteristic | African- Americans (n=1,605) |
Whites (n=4,141) |
P for difference |
|---|---|---|---|
| Systolic BP, mmHg | 126.1 (0.51) | 116.0 (0.25) | <.0001 |
| Diastolic BP, mmHg | 78.9 (0.30) | 70.8 (0.15) | <.0001 |
| MAP, mmHg | 94.6 (0.34) | 85.8 (0.17) | <.0001 |
| PP, mmHg | 47.2 (0.36) | 45.2 (0.18) | <.0001 |
| Hypertension, % | 26.6 | 8.5 | <.0001 |
| Age, yrs [min, max] | 52.4 (0.14) | 53.7 (0.09) | <.0001 |
| Women, % | 54.6 | 48.0 | <.0001 |
| Diabetes, % | 12.2 | 6.1 | <.0001 |
| Current cigarette smoking, % |
31.6 | 24.8 | <.0001 |
| Current drinking, % | 37.2 | 64.6 | <.0001 |
| BMI, kg/m2 | 28.4 (0.14) | 26.4 (0.07) | <.0001 |
| Total serum cholesterol, mmol/L |
5.5 (0.03) | 5.5 (0.02) | 0.91 |
| Sport index | 2.2 (0.02) | 2.6 (0.01) | <.0001 |
| Usual dietary sodium intake, mg |
1,384.6 (14.3) | 1,525.7 (9.75) | <.0001 |
| Neutrophils, % | 48.1 | 60.2 | <.0001 |
| Lymphocytes, % | 38.9 | 31.4 | <.0001 |
| Monocytes¶ | 5.64 (1.01) | 5.47 (1.01) | 0.01 |
Values are means (standard error) or percentages.
Geometric mean (because of the highly skewed distribution).
Abbreviations: BP, blood pressure; MAP, mean arterial pressure; PP, pulse pressure; BMI, body mass index.
Analysis of longitudinal change in BP by quartile of leukocyte level (tertile of monocyte level) was done using linear mixed models (SAS PROC MIXED) with a random intercept and slope and an unstructured correlation matrix 17. Age was used as the metameter for time and was centered at age 55, the approximate median age of the study population. When age was decomposed into baseline age and time since baseline, both terms were found to be statistically significant (indicating both cross-sectional and longitudinal associations between age and BP) and were retained in all models 18. The equation of the basic fitted model is: E(Y) = β1 + β2(baseline age) + β3(time) + β4(Q1) + β5(Q2) + β6(Q3) + β7(time*Q1) + β8(time*Q2) + β9(time*Q3), where E(Y) is mean BP and β1 represents the intercept, which is the mean BP of the study population with all covariates set to zero (for baseline age, this is equivalent in this study to baseline age = 55 years). Dummy variables were used to code quartiles, where Q1 is the highest quartile and Q4, the lowest, is the reference quartile. The coefficient for each quartile term represents the additive effect of that quartile, relative to the reference quartile, on the intercept of the BP trajectory over time. The coefficient of each time*quartile interaction term represents the additive effect of that quartile, relative to the reference quartile, on the slope of the overall average trajectory of the BP line, that is, on the average annual rate of change in BP over time. (“Quartile” is replaced by “tertile” in the model formulation for monocytes.) Adjustment was made for the same covariates as in the cross-sectional analysis. All data were analyzed using SAS v. 9.1 (SAS Institute, Cary, NC).
RESULTS
The study population comprised 49.8% women and 27.9% African-Americans, with a mean age of 53.3 years (range 44 - 66 years, SD 5.6 years); 7.8% had diabetes, 26.7% were current smokers, 57% were current drinkers, and the mean values for BMI, total cholesterol, sport index, and dietary sodium intake were 27.0 kg/m2 (SD 4.9), 5.5 mmol/L (SD 1.0), 2.5 (SD 0.8), and 1,486.8 mg (SD 608.5) respectively. The mean SBP, DBP, MAP, and PP were 118.8 mmHg (SD 17.8), 73.0 mmHg (SD 11.0), 88.3 mmHg (SD 12.3), and 45.8 mmHg (SD 12.4), respectively. The clinical characteristics of white and African-Americans participants are compared in Table 1. The group of African-Americans had a higher % of women, persons with hypertension, persons with diabetes, and smokers, and a higher mean BMI. The group of whites had a higher % of drinkers and higher mean values for age, sport index, and usual dietary sodium intake. The absolute levels of all BP measures were always higher in African-Americans than in whites. In addition, relative neutrophil levels were higher in whites than in African-Americans, whereas relative lymphocyte and monocyte levels were higher in African-Americans than in whites.
Cross-sectional analysis
In all BP groups neutrophil levels were higher in whites than in African-Americans, whereas lymphocyte and monocyte levels were higher in African-Americans than in whites (Table 2). In African-Americans there was a statistically significant trend of increasing mean neutrophil levels and decreasing mean lymphocyte levels going from normal to hypertension groups. In whites, no statistically significant trends in neutrophil or lymphocyte level by BP group were seen. No association was seen between monocyte levels and BP group in either race.
Table 2.
Multivariable-adjusted* mean leukocyte levels by BP group, by race.
| HT group |
P value for trend |
|||
|---|---|---|---|---|
| Normal | Pre-HT | HT | ||
| Whites | ||||
| Neutrophils | 60.2 (0.24) | 60.6 (0.31) | 60.5 (0.52) | 0.30 |
| Lymphocytes | 32.1 (0.22) | 31.9 (0.28) | 31.2 (0.48) | 0.09 |
| Monocytes¶ | 5.55 (1.01) | 5.33 (1.02) | 5.48 (1.03) | 0.16 |
| African-Americans | ||||
| Neutrophils | 49.7 (0.67) | 50.4 (0.67) | 51.4 (0.79) | 0.04 |
| Lymphocytes | 38.7 (0.60) | 37.9 (0.59) | 36.6 (0.70) | 0.005 |
| Monocytes¶ | 5.59 (1.03) | 5.66 (1.03) | 5.87 (1.03) | 0.15 |
Values are means (standard error).
Geometric mean (because of the highly skewed distribution).
in a general linear model, adjusted for age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake.
Abbreviations: HT, hypertension; Pre-HT, pre-hypertension; BMI, body mass index.
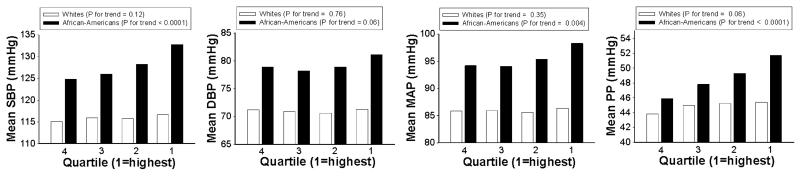
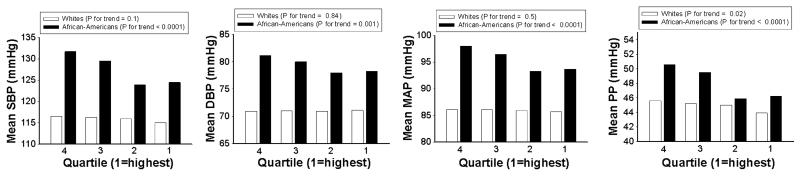
In African-Americans there was a statistically significant positive association between neutrophil level and 3 of the 4 BP measures (SBP, MAP, and PP) (Figure 1). These associations remained after adjustment for age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake (Table 3). The adjusted mean difference between the highest and lowest quartiles of neutrophils was approximately 8 mmHg for SBP, 4 mmHg for MAP, and 5 mmHg for PP. An absolute increase of 10% in the neutrophil level was associated with an increase of about 1.7 mmHg in mean SBP, 0.8 mmHg in mean MAP, and 1.3 mmHg in mean PP. An inverse association was seen between lymphocytes and all 4 BP measures (Figure 2), which again remained after multivariable adjustment (Table 3). The adjusted mean difference between the highest and lowest quartiles of lymphocytes was approximately 6 mmHg for SBP, 2 mmHg for DBP, 3.4 mmHg for MAP, and 4 mmHg for PP. An absolute increase of 10% in the lymphocyte level was associated with a decrease of about 2.3 mmHg in mean SBP, 0.7 mmHg in mean DBP, 1.2 mmHg in mean MAP, and 1.6 mmHg in mean PP. There was no clear pattern of association of any BP measure with monocyte tertiles, and in most cases the differences were not statistically significant (Table 3).
Figure 1. Unadjusted mean BP measures by quartile of neutrophil level, by race.
Q1=highest quartile, Q4=lowest; Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
Table 3.
Multivariable-adjusted* mean BP levels by quartile/tertile and by increment of leukocyte level, by race.
| SBP |
DBP |
MAP |
PP |
|||||
|---|---|---|---|---|---|---|---|---|
| African- Americans |
Whites | African- Americans |
Whites | African- Americans |
Whites | African- Americans |
Whites | |
| Neutrophils | ||||||||
| Q1 | 129.8 | 116.9 | 77.7 | 70.9 | 95.0 | 86.2 | 52.1 | 46.0 |
| Q2 | 124.8 | 115.7 | 75.4 | 70.2 | 91.9 | 85.4 | 49.4 | 45.4 |
| Q3 | 123.6 | 115.8 | 74.9 | 70.6 | 91.1 | 85.7 | 48.7 | 45.2 |
| Q4 | 121.9 | 115.1 | 75.1 | 70.8 | 90.7 | 85.6 | 46.8 | 44.3 |
| P for trend | <.0001 | 0.06 | 0.06 | 0.86 | 0.002 | 0.31 | <.0001 | 0.02 |
| BP change (SE) per | 1.65 | 0.54 | 0.40 | −0.02 | 0.82 | 0.17 | 1.25 | 0.55 |
| 10% increment | (0.44) | (0.36) | (0.26) | (0.22) | (0.29) | (0.24) | (0.31) | (0.26) |
| P value | 0.0002 | 0.13 | 0.12 | 0.95 | 0.006 | 0.49 | <.0001 | 0.03 |
| Lymphocytes | ||||||||
| Q1 | 122.0 | 115.2 | 74.9 | 70.4 | 90.6 | 85.3 | 47.2 | 44.8 |
| Q2 | 121.4 | 115.7 | 74.7 | 70.5 | 90.3 | 85.6 | 46.8 | 45.2 |
| Q3 | 126.8 | 116.1 | 76.3 | 70.6 | 93.1 | 85.8 | 50.5 | 45.6 |
| Q4 | 128.2 | 116.9 | 76.9 | 70.7 | 94.0 | 86.1 | 51.3 | 46.2 |
| P for trend | <.0001 | 0.03 | 0.01 | 0.58 | 0.0002 | 0.17 | <.0001 | 0.01 |
| BP change (SE) per | −2.26 | −0.82 | −0.66 | −0.14 | −1.19 | −0.36 | −1.60 | −0.68 |
| 10% increment | (0.49) | (0.38) | (0.29) | (0.24) | (0.33) | (0.26) | (0.34) | (0.28) |
| P value | <.0001 | 0.03 | 0.02 | 0.57 | 0.0003 | 0.17 | <.0001 | 0.01 |
| Monocytes | ||||||||
| T1 | 123.8 | 115.3 | 76.2 | 70.1 | 92.1 | 85.1 | 47.6 | 45.2 |
| T2 | 123.4 | 116.8 | 74.7 | 70.9 | 90.9 | 86.2 | 48.7 | 46.0 |
| T3 | 123.8 | 115.6 | 75.0 | 70.4 | 91.3 | 85.5 | 48.8 | 45.2 |
| P for trend | 0.99 | 0.49 | 0.11 | 0.35 | 0.35 | 0.37 | 0.19 | 0.88 |
| BP change (SE) per | 2.56 | −0.77 | 2.63 | −0.53 | 2.60 | −0.61 | −0.06 | −0.24 |
| 10% increment | (1.97) | (1.25) | (1.16) | (0.77) | (1.33) | (0.86) | (1.39) | (0.91) |
| P value | 0.19 | 0.54 | 0.02 | 0.49 | 0.05 | 0.48 | 0.96 | 0.79 |
Values are means (standard error).
in a linear mixed model, adjusted for age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake.
Q1=highest quartile, Q4=lowest; T1=highest tertile, T3=lowest. The reference category was Q4 (lowest quartile) or T3 (lowest tertile).
Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SE, standard error.
Figure 2. Unadjusted mean BP measures by quartile of lymphocyte level, by race.
Q1=highest quartile, Q4=lowest; Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
In whites, differences in the BP measures between quartiles of neutrophils and of lymphocytes were, in most cases, much smaller than those seen in African-Americans (Figures 1 and 2), and after multivariable adjustment, statistically significant associations were seen only between quartiles of neutrophils and PP and between quartiles of lymphocytes and SBP and PP (Table 3).
Longitudinal analysis
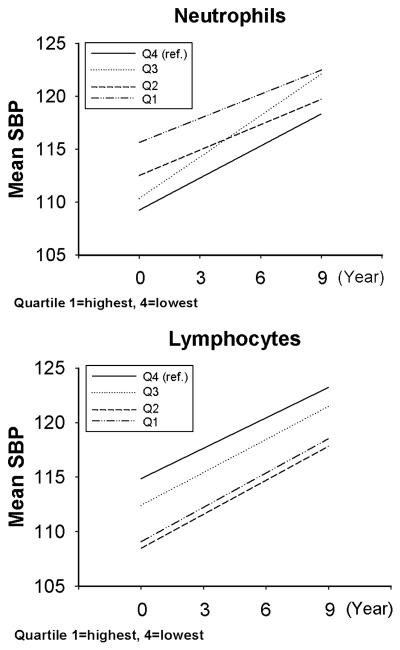
The crude (unadjusted) average intercept and slope for each BP trajectory over time is shown, by race, in Table 4. The additive effect of leukocyte quartile (using the lowest quartile (Q4) as the reference group) on these average intercepts and slopes, after adjustment for baseline age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake, is shown in Table 5. In African-Americans the trajectories of all 4 BP measures showed a trend of increasing average intercept with increasing quartile of neutrophils, consistent with the cross-sectional findings. Persons in the highest quartile of neutrophils had an increase in the average intercept of 6.4 mmHg for SBP, 1.6 mmHg for DBP, 3.2 mmHg for MAP, and 4.7 mmHg for PP. Persons in the second highest quartile of neutrophils had an increase in the average intercept of 3.3 mmHg for SBP, 1.3 mmHg for DBP, 1.9 mmHg for MAP, and 2.1 mmHg for PP. All quartiles of lymphocytes were associated with the opposite effect on the average intercept, again consistent with the cross-sectional findings, although there was no clear trend by increasing quartile. Persons in the highest quartile of lymphocytes had a decrease in the average intercept of 5.8 mmHg for SBP, 2.2 mmHg for DBP, 3.4 mmHg for MAP, and 3.7 mmHg for PP. Persons in the second highest quartile of lymphocytes had a decrease in the average intercept of 6.4 mmHg for SBP, 2.4 mmHg for DBP, 3.8 mmHg for MAP, and 4 mmHg for PP. The effect of neutrophil and lymphocyte quartiles on the average slope of the trajectories was statistically significant only for the highest 2 quartiles of neutrophils which were associated with a decrease of about 0.3 - 0.4 in the slope of the PP trajectory. To better illustrate the results of the regression models, the predicted average intercept and slope are shown for 2 situations, SBP by quartile of neutrophils and SBP by quartile of lymphocytes, in African-Americans with the baseline age set at 55 years (Figure 3).
Table 4.
Unadjusted average intercept and slope coefficient for each BP trajectory over time, by race.
| African-Americans |
Whites |
|||
|---|---|---|---|---|
| Intercept (SE) |
Slope coefficient (SE) |
Intercept (SE) |
Slope coefficient (SE) |
|
| SBP | 126.73 (0.52) | 1.01 (0.6) | 115.93 (0.24) | 1.13 (0.03) |
| DBP | 77.8 (0.31) | −0.28 (0.04) | 70.7 (0.15) | −0.01 (0.02) |
| MAP | 94.1 (0.35) | 0.15 (0.04) | 85.8 (0.17) | 0.37 (0.02) |
| PP | 48.8 (0.36) | 1.2 (0.05) | 45.2 (0.16) | 1.1 (0.02) |
Values are means (standard error).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SE, standard error.
Table 5.
Multivariable-adjusted* estimates of the additive effect of quartile/tertile of leukocytes on mean intercept and slope of BP trajectories over time, by race. Values are means (standard error).
| African-Americans |
Whites |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile/Tertile (1=highest) |
Intercept (SE) |
P for trend |
Slope coefficient (SE) |
P for trend |
Intercept (SE) |
P for trend |
Slope coefficient (SE) |
P for trend |
|
| SBP | |||||||||
| Neutrophils | Q1 | 6.39 (1.71) | −0.25 (0.22) | 1.30 (1.01) | 0.001 (0.12) | ||||
| Q2 | 3.27 (1.63) | 0.0001 | −0.21 (0.22) | 0.31 | 0.56 (1.02) | 0.91 | 0.01 (0.13) | 0.04 | |
| Q3 | 1.09 (1.26) | 0.30 (0.16) | −0.16 (1.03) | −0.01 (0.13) | |||||
| Lymphocytes | Q1 | −5.78 (1.50) | 0.12 (0.20) | −1.68 (0.89) | 0.08 (0.11) | ||||
| Q2 | −6.39 (1.69) | <.0001 | 0.11 (0.23) | 0.58 | −1.72 (0.74) | 0.02 | 0.002 (0.09) | 0.61 | |
| Q3 | −2.46 (1.80) | 0.08 (0.25) | −1.16 (0.67) | 0.03 (0.08) | |||||
| Monocytes | T1 | 0.47 (1.29) | 0.66 | −0.04 (0.17) | 0.69 | −0.57 (0.69) | 0.31 | −0.06 (0.09) | 0.53 |
| T2 | −0.54 (1.32) | 0.15 (0.17) | 0.91 (0.68) | −0.12 (0.08) | |||||
| DBP | |||||||||
| Neutrophils | Q1 | 1.61 (0.99) | 0.12 (0.14) | 0.14 (0.62) | −0.002 (0.07) | ||||
| Q2 | 1.28 (0.94) | 0.08 | 0.09 (0.14) | 0.19 | 0.06 (0.62) | 0.51 | −0.02 (0.08) | 0.93 | |
| Q3 | −0.87 (0.73) | 0.28 (0.10) | −0.21 (0.63) | −0.01 (0.08) | |||||
| Lymphocytes | Q1 | −2.16 (0.87) | −0.12 (0.12) | −0.06 (0.55) | 0.05 (0.06) | ||||
| Q2 | −2.43 (0.98) | 0.01 | −0.11 (0.14) | 0.30 | −0.51 (0.45) | 0.51 | −0.05 (0.05) | 0.93 | |
| Q3 | −0.85 (1.04) | −0.03 (0.15) | −0.04 (0.41) | 0.02 (0.05) | |||||
| Monocytes | T1 | 1.15 (0.75) | 0.08 | −0.08 (0.10) | 0.33 | −0.22 (0.42) | 0.51 | −0.05 (0.05) | 0.51 |
| T2 | −0.52 (0.76) | 0.11 (0.25) | 0.36 (0.41) | −0.12 (0.05) | |||||
| MAP | |||||||||
| Neutrophils | Q1 | 3.22 (1.14) | 0.01 (0.15) | 0.53 (0.69) | −0.00 (0.08) | ||||
| Q2 | 1.94 (1.09) | 0.003 | −0.01 (0.15) | 0.73 | 0.22 (0.70) | 0.15 | −0.01 (0.08) | 0.90 | |
| Q3 | −0.21 (0.84) | 0.28 (0.11) | −0.20 (0.71) | −0.01 (0.08) | |||||
| Lymphocytes | Q1 | −3.37 (1.01) | −0.05 (0.14) | −0.61 (0.61) | 0.06 (0.07) | ||||
| Q2 | −3.75 (1.13) | 0.22 | −0.05 (0.15) | 0.68 | −0.93 (0.50) | 0.11 | −0.03 (0.06) | 0.80 | |
| Q3 | −1.38 (1.21) | −0.02 (0.17) | −0.42 (0.46) | 0.03 (0.05) | |||||
| Monocytes | T1 | 0.92 (0.87) | 0.22 | −0.07 (0.11) | 0.41 | −0.34 (0.47) | 0.38 | −0.05 (0.06) | 0.47 |
| T2 | −0.52 (0.88) | 0.12 (0.12) | 0.55 (0.46) | −0.12 (0.05) | |||||
| PP | |||||||||
| Neutrophils | Q1 | 4.72 (1.19) | −0.40 (0.17) | 1.17 (0.73) | −0.002 (0.10) | ||||
| Q2 | 2.05 (1.13) | <.0001 | −0.34 (0.17) | 0.01 | 0.55 (0.74) | 0.02 | 0.03 (0.10) | 0.94 | |
| Q3 | 1.90 (0.87) | 0.02 (0.13) | 0.07 (0.75) | 0.01 (0.11) | |||||
| Lymphocytes | Q1 | −3.66 (1.05) | 0.31 (0.16) | −1.65 (0.64) | 0.05 (0.09) | ||||
| Q2 | −4.01 (1.18) | 0.0003 | 0.30 (0.18) | 0.06 | −1.19 (0.53) | 0.01 | 0.06 (0.08) | 0.43 | |
| Q3 | −1.66 (1.25) | 0.19 (0.19) | −1.11 (0.48) | 0.02 (0.07) | |||||
| Monocytes | T1 | −0.65 (0.90) | 0.45 | 0.05 (0.13) | 0.74 | −0.32 (0.50) | 0.43 | −0.01 (0.07) | 0.88 |
| T2 | −0.06 (0.92) | 0.07 (0.14) | 0.53 (0.49) | −0.01 (0.07) | |||||
in a linear mixed model, adjusted for age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake.
Q1=highest quartile, Q4=lowest; T1=highest tertile, T3=lowest. The reference category was Q4 (lowest quartile) or T3 (lowest tertile).
Abbreviations: BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SE, standard error.
Figure 3.
Multivariable-adjusted* predicted average intercept and slope of SBP trajectory, by quartile of neutrophils and by quartile of lymphocytes (African-Americans only, baseline age set at 55 years). Q1=highest quartile, Q4=lowest; Abbreviations: SBP, systolic blood pressure; * in a linear mixed model, adjusted for age, sex, center, diabetes, BMI, total cholesterol, smoking, drinking, physical activity, and dietary sodium intake.
In whites, the quartiles of neutrophils and lymphocytes were associated with much smaller effects on the intercept and slope of all 4 BP trajectories, which in most cases were not statistically significant (Table 5). The only statistically significant effects were seen with neutrophils and SBP (a very small change in the slope coefficient), neutrophils and PP, lymphocytes and SBP, and lymphocytes and PP.
In both African-Americans and whites there was no consistent effect of monocyte tertile on either intercept or slope of the trajectory of any BP measure.
DISCUSSION
Our study has shown that in African-Americans, but much less so in whites, increased neutrophil levels and decreased lymphocyte levels are associated with elevated BP but do not influence the rate of change of BP over time. The most striking finding in the current study was the inverse association between the BP parameters and lymphocyte levels, especially in African-Americans. To our knowledge, this is the first report suggesting that there is a negative relationship between lymphocytes and BP.
Previous epidemiologic studies have suggested that clinical hypertension and systemic inflammation are associated 8. Even though the relationship between circulating total WBC and hypertension has been well documented 9,10,11, the association of different specific circulating leukocytes with BP and their roles in the regulation of BP and development of hypertension remain unknown. Our cross-sectional data showed that some of the BP parameters in the white group and nearly all the BP parameters in the African-Americans group were positively and significantly associated with increased quartiles of neutrophils even after adjustment for the main cardiovascular risk factors, indicating that neutrophils are significantly correlated with the regulation of BP and the development of hypertension. The results of our longitudinal analysis are consistent with a report that neutrophil count is a predictor of hypertension. 19. However, the question remains: what is the role of neutrophils in the pathogenesis of hypertension?
Since Guyton et al 20 established that the kidney has an overriding influence in arterial pressure regulation and hypertension, support for a key role for the kidney in the pathogenesis of hypertension has come from transplant studies in experimental models of hypertension 21,22,23 and in humans 24. Using animal models, we and others have suggested that increased renal oxidative stress 25,26,27, renal inflammation 28,29, and renal local angiotensin II (Ang II) activity 30,31,32 are all important in the pathogenesis of hypertension 33. The combination and interaction of these detrimental renal factors, especially through the inactivation of NO bioavailability 34,35 by oxidative stress, favors sodium retention and the development and maintenance of hypertension.
Evidence has shown that increased renal oxidative stress precedes the occurrence of hypertension in genetic hypertensive animals 36,37,38. The source of oxidative stress could be renal in origin. However, it is possible that primary systemic oxidative stress caused by elevated and activated circulating leukocytes, capable of releasing reactive oxygen species (ROS), can be directly delivered into kidney by these mobile blood-borne cells. In animal studies, Shen et al. 39 have found a highly significant elevation in the total leukocyte, neutrophil and monocyte counts, as well as in the level of activated neutrophils and monocytes in Dahl salt-sensitive rat, a salt-sensitive hypertensive model, but not in Dahl salt-resistance rats. Besides, in both spontaneously hypertensive rats 40 and Sabra rats 41, another model of salt-sensitive hypertension, elevation and activation of leukocytes and increased superoxide (O2−.) release from polymorphonuclear leukocytes (PMNLs) precede the occurrence of experimental hypertension 41. In human studies, elevated leukocyte count and enhanced PMNL activation are also correlated with the development and progress of hypertension and cardiovascular disease 42. Oxidative stress in hypertensive patients' neutrophils is evidenced by an increased NADPH oxidase production and lipid peroxidation and decreased cytosolic and mitochondrial superoxide dismutase concentration 42,43,44,45. Recently, Ramasamy et al.46 evaluated the effect of neutrophil oxidative burst activity in essential hypertensive patients. They found that freshly isolated neutrophils from uncontrolled human subjects diagnosed with essential hypertension acquire the ability of producing a higher amount of ROS in response to stimuli indicating that oxidative bust activity is impaired.
Previous studies have shown that African-Americans have a lower mean neutrophil count 47, 48 but the reason is unknown. Our results confirmed that neutrophil levels in general and at all BP levels (normal, pre-hypertension and hypertension) in the African-American group were lower than in the white group. The reason, we believe, is due to the PMNLs oxidative burst and consequent apoptosis 49 and self-necrosis 50. Study on PMNLs in essential hypertensive patients has shown that survival of PMNLs in vitro decreases linearly with the increased rate of superoxide (O2−.) release 50. Therefore, there could be more neutrophil death and more ROS release from neutrophils in African-Americans than in the whites and this may partly explain why the BP in African-Americans is worse than in whites in all BP levels. In addition, our study found that neutrophils were positively and significantly associated with increases in BP in the African-American group (but much less so in the whites). This latter finding seemingly conflicts with the former deduction. However, it has been well established that PMNL apoptosis and necrosis further adds to the inflammation and promotes chemotaxis and PMNL recruitment: for example, PMNL counts in essential hypertensive patients were significantly higher than the normal control indicating necrosis and recruitment 50. Therefore, we believe that with the progression of necrosis, neutrophils will be continuously recruited, and this causes the positive association between BP and neutrophils, which is stronger in African-Americans than in whites (Table 2).
The innate immune system mainly includes granulocytes and macrophages as well as Toll-like receptors (TLRs) 6. Once the innate immune system has been activated and renal damage initiated, it is possible that low-grade inflammation in tubulointerstitial areas of the kidney could be maintained by autoimmune reactivity 51. It has been found that following oxidative stress-induced renal vasoconstriction and kidney damage, oxidatively-modified proteins can serve as autoantigens leading to an auto-inflammatory response 6. Tubulointerstitial infiltration of lymphocytes and macrophages appears to be universally present in experimental models of salt-sensitive hypertension 52. However, the connection between this renal infiltration of lymphocytes, the major component of the autoimmune system, and circulating lymphocytes has not been explained. Recently, the pivotal role of the T-cells in hypertension was demonstrated by Guzik et al 53 in mice lacking B- and T-cells. These genetically altered mice do not develop hypertension or vascular damage. When T-cells are transferred to these mice, hypertension returns. This evidence supports an important role of T-lymphocytes in the pathogenesis of hypertension, and it seems that circulating lymphocytes should be positively associated with an increase in BP. In our study, however, circulating lymphocytes actually had an obvious inverse relationship with BP, especially in African-Americans. The importance of this finding and the apparent inconsistency between our study and previous animal studies could be explained as follows.
We propose that the presence of normally functioning lymphocytes is a prerequisite for the genesis of hypertension. In the pathological condition, once the autoimmune system is activated, lymphocytes may be attacked by autoantibodies54. Lymphocyte destruction may release ROS and Ang II through NADPH oxidase and AT1 receptors 53. Concurrently, lymphocytes infiltrate into adventitia and adventitial fat and produce TNFα, IFNγ and tissue-homing receptors which stimulate vascular O2−. release. At the same time, vascular endothelial cells produce more intercellular adhesion molecule-1 and RANTES 53 which could attract more lymphocytes into tissue including the kidneys. Therefore, as circulating lymphocytes decrease due to autoantibody-mediated destruction and infiltration into tissue including the renal tubulointerstitial area 52, BP increases and/or hypertension occurs. Hence, our finding that circulating lymphocytes have an inverse relationship with BP does not decrease the importance of lymphocytes in the pathogenesis of hypertension. Instead, this finding further implies that lymphocytes contribute to the development of human hypertension. It is of note that in contrast to neutrophils, the relative lymphocyte counts in general and in all BP groups (normal, pre-hypertension and hypertension) are higher in African-Americans than in the whites. This may partly explain why the BP in African-Americans is higher than in whites in all quartiles of lymphocytes. Higher absolute levels of both lymphocytes and dysfunctional lymphocytes are associated with higher BP in African-Americans.
To further discuss the detail role of circulating lymphocytes in the pathogenesis of hypertension, the subsets of lymphocyte should be considered. Even though the information of lymphocyte subsets were not available in the ARIC datasets, studies have shown that T lymphocyte CD4+ (T helper cell) and CD 8+ (Cytotoxic T cell) are involved in the Ang II-induced hypertensive cardiac hypertrophy and fibrosis and adoptive immunosupressive CD4+, CD 25+ regulatory T cell transfer resulted in a marked reduction of these cells' infiltration and the improvement of cardiac damage 55. Moreover, in the aorta of Dahl salt-sensitive hypertensive rats, CD4+ mRNA was increased compared with the Brown Norway normotensive rat 56. Most interestingly, recent studies on pulmonary arterial hypertension suggested that alteration in circulating T cell subsets, particularly CD8+ T lymphocytes may contribute to disease pathogenesis. In these studies, significantly decreased CD8+ T cells were found in the peripheral blood of patients compared to control 57 together with a preponderance of CD3+ and CD8+ T cells infiltration in the patients' lung 58. This offers strong evidence to support our above speculation for the mechanism of lymphocytes in the tissue damage-related pathogenesis of hypertension.
In our longitudinal analysis, levels of specific leukocytes types did not influence the rate of change of BP with age. In other words, once the innate and autoimmune systems were activated, the increase in arterial pressure with age did not depend on the level of leukocytes at baseline. Instead, it may depend on the inflammation-related consequences such as tissue or organ damage or other factors. We believe that renal damage is initiated by activation of the innate immune system and exacerbated by activation of autoimmune system. Once inflammation-associated renal damage has been initiated, however, inflammation may no longer be a dominant factor in the further development of hypertension.
Limitations of the above study include its cross-sectional component, which limits our ability to infer a causal relationship between increased neutrophils and decreased lymphocytes and elevation of BP. Therefore, the data do not prove that the increased neutrophils or decreased lymphocytes cause the increase in BP. Besides, due to the limitation of ARIC data, we were not able to evaluate sodium excretion. This may limit our ability to interpret BP changes. In addition, although we controlled for the major cardiac risk factors, the existence of unrecognized confounding is always possible.
Our findings may have important clinical implications. Our data show that neutrophils and lymphocytes have an important association with BP, especially SBP in African-Americans. The mean difference in SBP among between the highest and lowest quartiles of neutrophils was approximately 8 mmHg, and the mean difference in SBP between the highest and lowest quartiles of lymphocytes was approximately 6 mmHg. A meta-analysis of over 1 million patients calculated that even a small 2 mmHg decrease in SBP could lower stroke and coronary and other vascular related mortality by 10% and 7%, respectively,3 indicating that neutrophils and lymphocytes could be important biomarkers not only for predicting higher BP but also for identifying persons at greater risk of hypertension-related cardiovascular events, who might benefit from earlier intervention. Interestingly, the T-cell-modulating agent mycophenolate mofetil has been shown to lower BP in experimentally-induced 59,60 and genetic hypertensive models 29,61 and in humans with psoriasis and rheumatoid arthritis (which are T-cell-dependant autoimmune disorder) 62. Acute or short-term treatment by immune-modulation in the initial stage of hypertension may be worth exploring further.
Acknowledgement
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: the authors have no conflicts of interest to declare
REFERENCES
- 1.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Palmer A, Bulpitt C, Beevers G, Coles E, Fletcher A, Ledingham J, Petrie J, Webster J, Dollery C. Risk factors for ischaemic heart disease and stroke mortality in young and old hypertensive patients. J Hum.Hypertens. 1995;9:695–697. [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control--continued disparities in adults: United States, 2005-2006. NCHS.Data Brief. 2008:1–8. [PubMed] [Google Scholar]
- 5.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26:381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr.Cardiol.Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B, Johnson RJ. Role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial.Transplant. 2006;21:260–263. doi: 10.1093/ndt/gfi319. [DOI] [PubMed] [Google Scholar]
- 8.Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial.Transplant. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- 9.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 10.Kim DJ, Noh JH, Lee BW, Choi YH, Chung JH, Min YK, Lee MS, Lee MK, Kim KW. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J Korean Med.Sci. 2008;23:193–198. doi: 10.3346/jkms.2008.23.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar A, Klein BE, Klein R. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2004;17:233–239. doi: 10.1016/j.amjhyper.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Gillum RF. Pathophysiology of hypertension in blacks and whites. A review of the basis of racial blood pressure differences. Hypertension. 1979;1:468–475. doi: 10.1161/01.hyp.1.5.468. [DOI] [PubMed] [Google Scholar]
- 13.Cooper RS, Rotimi CN, Ward R. The puzzle of hypertension in African-Americans. Sci.Am. 1999;280:56–63. doi: 10.1038/scientificamerican0299-56. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC investigators The Atherosclerosis Risk in Communities(ARIC) Study:design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int.J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Fitzmaurice GM, laird NM, Ware JH. Applied longitu-dinal analysis. John Wiley; Hoboken, NewJersey: 2004. [Google Scholar]
- 18.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol.A Biol.Sci.Med.Sci. 2009;64:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatsukawa Y, Hsu WL, Yamada M, Cologne JB, Suzuki G, Yamamoto H, Yamane K, Akahoshi M, Fujiwara S, Kohno N. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens.Res. 2008;31:1391–1397. doi: 10.1291/hypres.31.1391. [DOI] [PubMed] [Google Scholar]
- 20.Guyton AC, Coleman TG, Cowley AV, Jr., Scheel KW, Manning RD, Jr., Norman RA., Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am.J.Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin.Sci.Mol.Med. 1974;47:435–448. doi: 10.1042/cs0470435. [DOI] [PubMed] [Google Scholar]
- 22.Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ.Res. 1975;36:692–696. doi: 10.1161/01.res.36.6.692. [DOI] [PubMed] [Google Scholar]
- 23.Rettig R, Folberth C, Kopf D, Stauss H, Unger T. Role of the kidney in the pathogenesis of primary hypertension. Clin.Exp.Hypertens.A. 1990;12:957–1002. doi: 10.3109/10641969009073513. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N.Engl.J Med. 1983;309:1009–1015. doi: 10.1056/NEJM198310273091702. [DOI] [PubMed] [Google Scholar]
- 25.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 26.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD., Jr. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J.Hypertens. 2006;24:2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 27.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul.Integr.Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am.J.Physiol Heart Circ.Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 29.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ.Physiol. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem.Biophys.Res.Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L, Phillips WE, Manning RD. Intrarenal Angiotensin ii is associated with inflammation, renal damage and dysfunction in dahl salt-sensitive hypertension. J Am Soc Hypertens. 2009;3:306–314. doi: 10.1016/j.jash.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat.Clin.Pract.Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am.J Physiol Regul.Integr.Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 35.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 36.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 37.Meng S, Roberts LJ, Cason GW, Curry TS, Manning RD., Jr. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am.J.Physiol Regul.Integr.Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 38.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 39.Shen K, DeLano FA, Zweifach BW, Schmid-Schonbein GW. Circulating leukocyte counts, activation, and degranulation in Dahl hypertensive rats. Circ.Res. 1995;76:276–283. doi: 10.1161/01.res.76.2.276. [DOI] [PubMed] [Google Scholar]
- 40.Schmid-Schonbein GW, Seiffge D, DeLano FA, Shen K, Zweifach BW. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 41.Sela S, Mazor R, Amsalam M, Yagil C, Yagil Y, Kristal B. Primed polymorphonuclear leukocytes, oxidative stress, and inflammation antecede hypertension in the Sabra rat. Hypertension. 2004;44:764–769. doi: 10.1161/01.HYP.0000144480.10207.34. [DOI] [PubMed] [Google Scholar]
- 42.Hopps E, Lo PR, Caimi G. Pathophysiology of polymorphonuclear leukocyte in arterial hypertension. Clin.Hemorheol.Microcirc. 2009;41:209–218. doi: 10.3233/CH-2009-1173. [DOI] [PubMed] [Google Scholar]
- 43.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc.Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 44.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 45.Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr.Opin.Nephrol Hypertens. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 46.Ramasamy R, Maqbool M, Mohamed AL, Noah RM. Elevated neutrophil respiratory burst activity in essential hypertensive patients. Cell Immunol. 2010;263:230–234. doi: 10.1016/j.cellimm.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Freedman DS, Gates L, Flanders WD, Van Assendelft OW, Barboriak JJ, Joesoef MR, Byers T. Black/white differences in leukocyte subpopulations in men. Int.J Epidemiol. 1997;26:757–764. doi: 10.1093/ije/26.4.757. [DOI] [PubMed] [Google Scholar]
- 48.Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, Mullikin J, Hsueh WC, Cheng CY, Coresh J, Boerwinkle E, Li M, Waliszewska A, Neubauer J, Li R, Leak TS, Ekunwe L, Files JC, Hardy CL, Zmuda JM, Taylor HA, Ziv E, Harris TB, Wilson JG. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS.Genet. 2009;5:e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elbim C, Lizard G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry A. 2009;75:475–481. doi: 10.1002/cyto.a.20726. [DOI] [PubMed] [Google Scholar]
- 50.Kristal B, Shurtz-Swirski R, Chezar J, Manaster J, Levy R, Shapiro G, Weissman I, Shasha SM, Sela S. Participation of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation in patients with essential hypertension. Am J Hypertens. 1998;11:921–928. doi: 10.1016/s0895-7061(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 51.Caetano EP, Zatz R, Praxedes JN. The clinical diagnosis of hypertensive nephrosclerosis--how reliable is it? Nephrol Dial.Transplant. 1999;14:288–290. doi: 10.1093/ndt/14.2.288. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Iturbe B, Quiroz Y, Herrera-Acosta J, Johnson RJ, Pons HA. The role of immune cells infiltrating the kidney in the pathogenesis of salt-sensitive hypertension. J Hypertens.Suppl. 2002;20:S9–14. [PubMed] [Google Scholar]
- 53.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp.Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeichi N, Ba D, Kobayashi H. Natural cytotoxic autoantibody against thymocytes in spontaneously hypertensive rats. Cell Immunol. 1981;60:181–190. doi: 10.1016/0008-8749(81)90258-6. [DOI] [PubMed] [Google Scholar]
- 55.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 56.Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ.Physiol. 2010;298:H938–H944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 57.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration. 2008;75:272–280. doi: 10.1159/000111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin ED, Rock MT, Mosse CA, Vnencak-Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir.Med. 2010;104:454–462. doi: 10.1016/j.rmed.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293:F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 60.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul.Integr.Comp Physiol. 2008;294:R1234–R1239. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 61.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 62.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]