Abstract
We describe an alkylative dearomatization/acid-mediated adamantane annulation sequence which allows facile access to type A polyprenylated acylphloroglucinol (PPAP) natural products including plukenetione A. Introduction of the 2-methyl-1-propenyl moiety was achieved via stereodivergent SN2 and SN1 cyclizations of allylic alcohol substrates.
Introduction
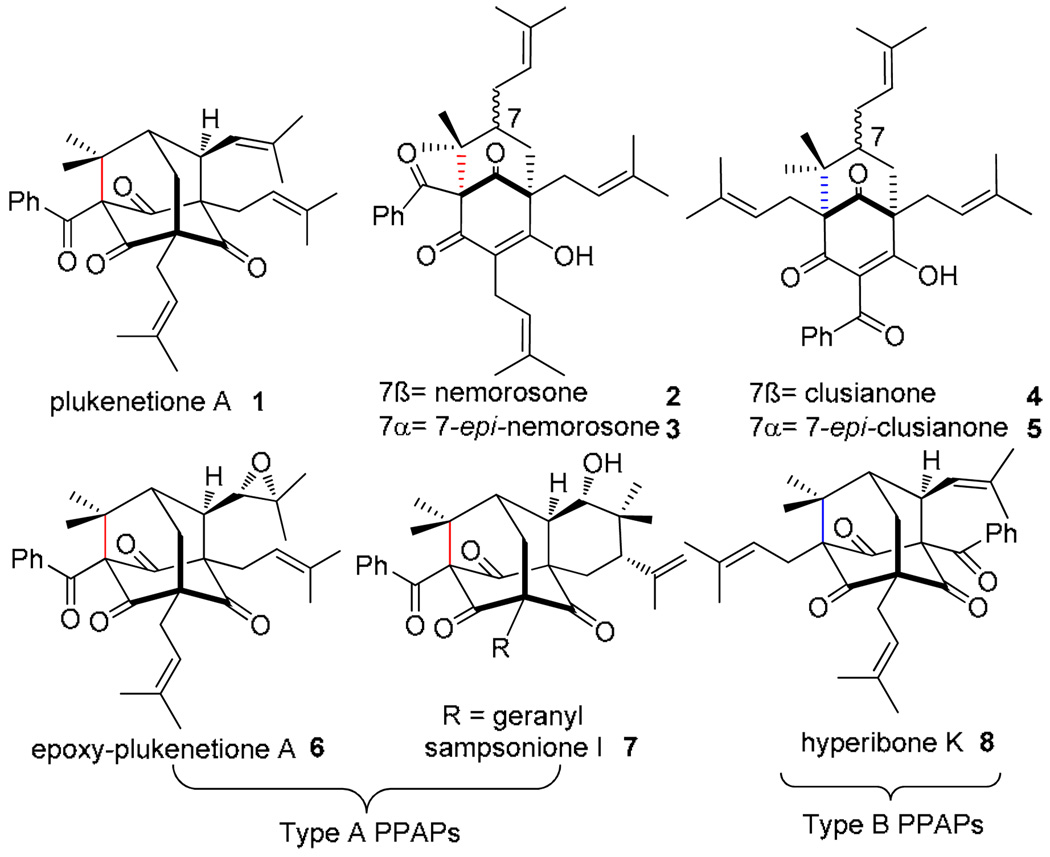
The polycyclic polyprenylated acylphloroglucinol (PPAP) plukenetione A 1 1 (Figure 1) was isolated by Jacobs and coworkers in 19962 from Clusia plukenetii (Guttiferae) and is the first natural product bearing an adamantane framework isolated from plant sources. In addition to its unique structure, 1 has been found to inhibit the enzymatic activities of both topoisomerase I and DNA polymerase.3 Plukenetione A may be biogenetically derived from oxidation of 7-epi-nemorosone 3. 4 , 5 Further oxidation of 1 may also lead to the densely functionalized derivatives 66 and 7.5 Compounds 1– 3 and related derivatives7 are described as type A PPAPs.8 Clusianone 4, its C7 epimer 5, 9 and the adamantane hyperibone K 8 10 are categorized as isomeric type B PPAPs. In light of their challenging structures and promising biological activities, the synthetic chemistry community has shown significant attention to the PPAP family with a number of chemical synthesis efforts reported to date,11 including a recent synthesis of the plukenetione core.11i
Figure 1.
Polycyclic polyprenylated acylphloroglucinol natural products
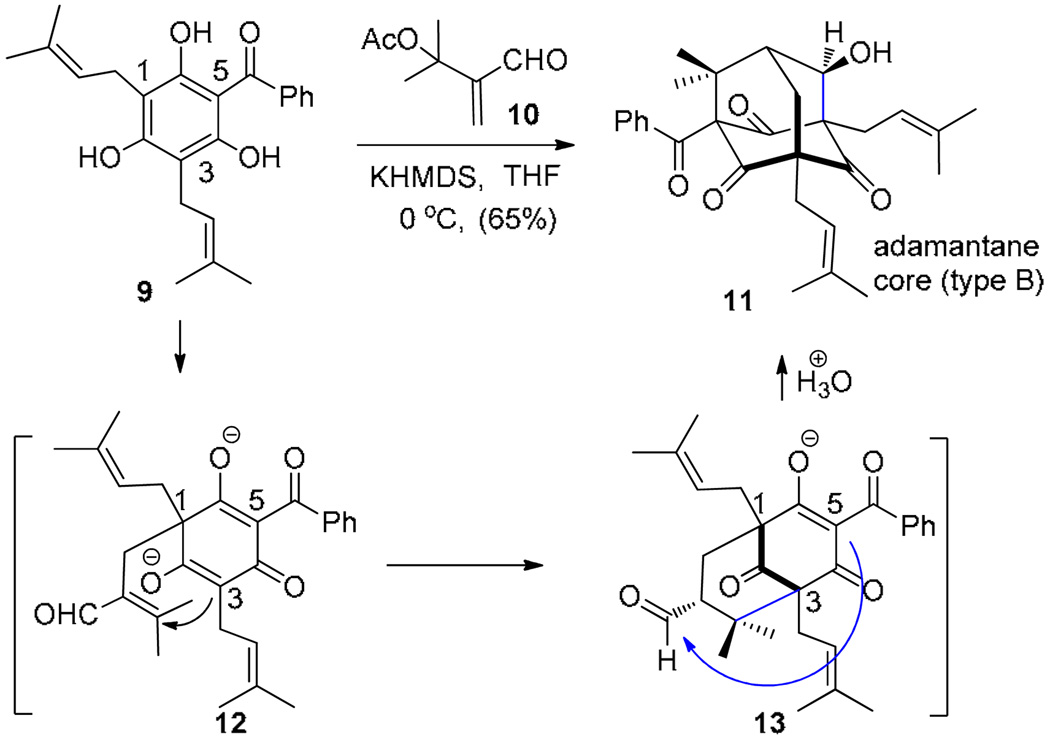
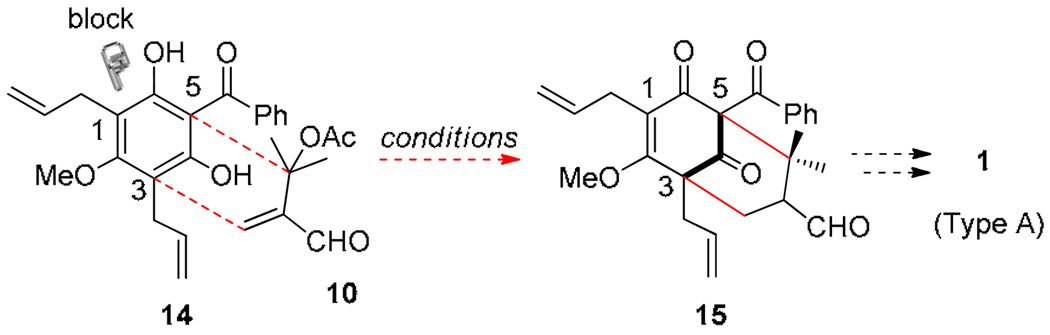
We have previously reported the synthesis of the type B PPAP clusianone 4 employing a tandem alkylative dearomatization-annulation process (Scheme 1). 12 During these studies, we found that treatment of the acylphloroglucinol clusiaphenone B 9 with α-acetoxy enal 10 under basic conditions led to the production of adamantane 11 via a tandem Michael addition–elimination-Michael (MEM)-aldol sequence proceeding through anionic intermediates 12 and 13. In subsequent work, we have developed an enantioselective variant of this transformation employing chiral phase transfer catalysis and have applied the methodology to the enantioselective synthesis of hyperibone K 8. 13 For acylphloroglucinol substrate 9, alkylative dearomatization/annulation occurred chemoselectively at carbons 1 and 3. In the present work, we considered whether a protected acylphloroglucinol such as clusiaphenone methyl ether 14 may block initial alkylative dearomatization at C1 and redirect annulation to C3 and C5 (Figure 2). Successful achievement of this approach would afford the type A PPAP framework 15 which may be further elaborated to 1 and related natural products. In this article, we report development of methodology along these lines to access the isomeric type A PPAPs leading to the total synthesis of the complex adamantane plukenetione A 1.
Scheme 1.
Synthesis of the type B adamantane core
Figure 2.
Alkylative Dearomatization Approach to type A PPAPs
Results and Discussion
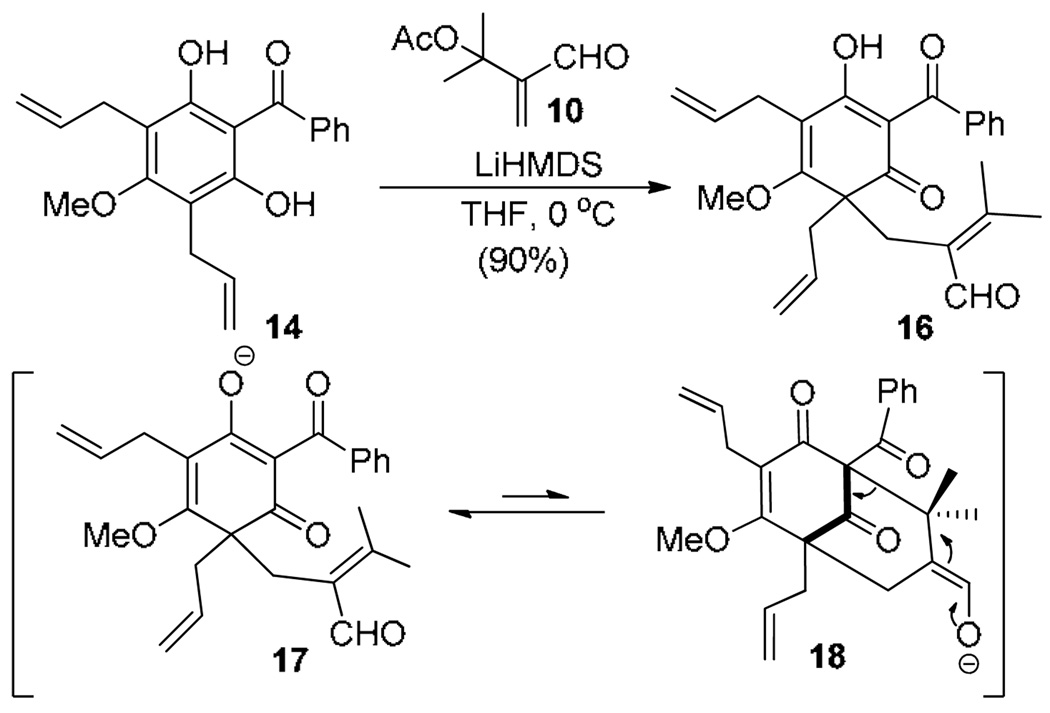
We initiated our investigation by examining alkylative dearomatization of 14 (prepared in two steps from 5-methoxyresorcinol)14 with α-acetoxy enal 1012 (Scheme 2) under basic conditions.15 In initial studies, we found that the desired annulation product 15 was not observed and only mono-dearomatized adduct 16 was obtained. The inability of enolate intermediate 17 to further cyclize to a bicyclo [3.3.1] ring system under basic conditions as previously observed in the type B series (cf. Scheme 1) is likely related to facile and reversible retro-Michael addition of 18 due to high thermodynamic stability of enolate 17.
Scheme 2.
Attempted alkylative dearomatization/annulation
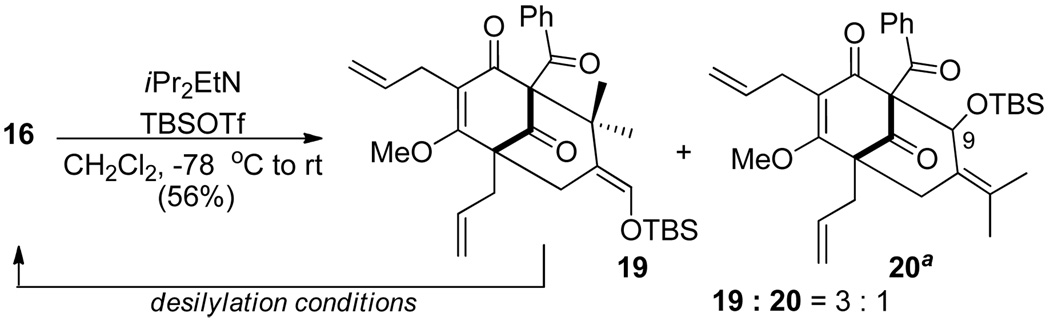
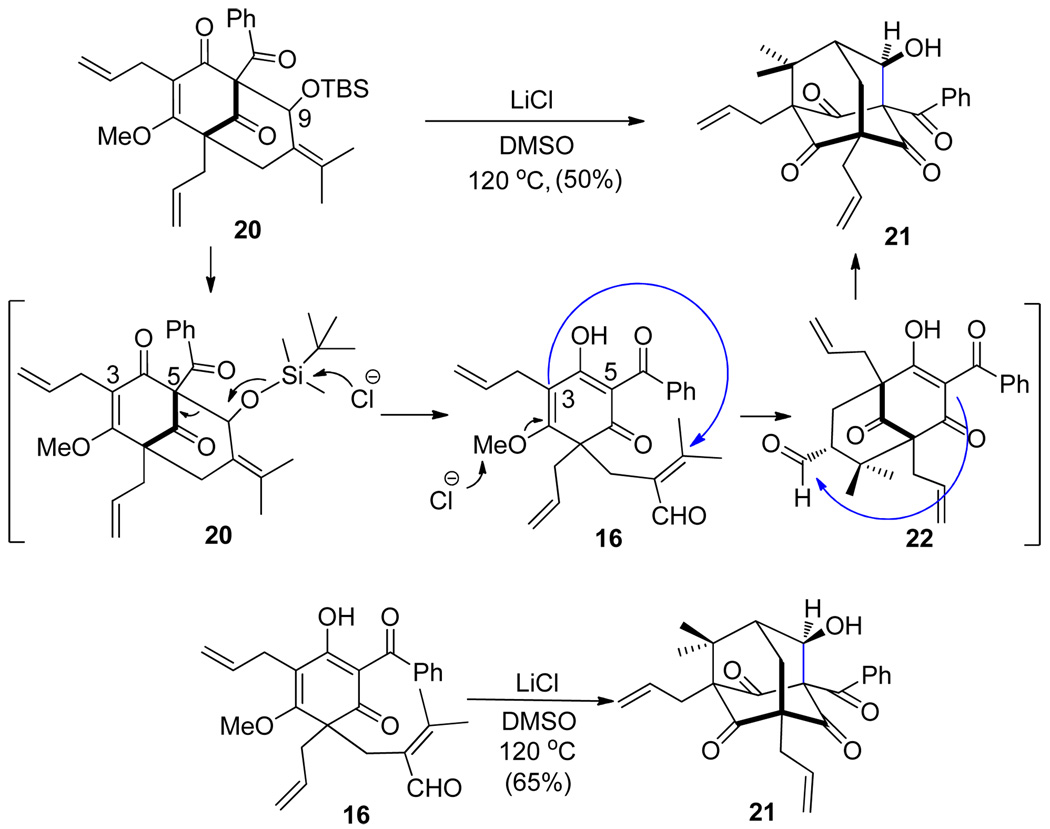
In an effort to identify irreversible cyclization conditions, silylative cyclization of 16 with TBSOTf and N,N-diisopropylethylamine (DIEA)16 yielded the cyclized products 19 and 20 as a 3 : 1 mixture of structural isomers (Scheme 3). Based on this result, we proceeded to evaluate cyclizations of 19 and 20 under demethylation conditions to access an adamantane structure (Scheme 4). Interestingly, treatment of silyl ether 20 using conditions reported by Krapcho and coworkers 17 unexpectedly led to the formation of the type B adamantane 21. In this case, the chloride ion may remove the silyl protecting group triggering retro-aldol fragmentation and formation of ring-opened aldehyde 16. Further nucleophilic demethylation, followed by intramolecular Michael addition, provides aldehyde 22 which participates in intramolecular aldol cyclization to 21. In order to further probe the proposed mechanism, treatment of dearomatized substrate 16 under identical reaction conditions led to clean formation of adamantane alcohol 21 as a single product.
Scheme 3.
Silylative cyclization to the bicyclo [3.3.1] ring system
a C9 stereochemistry unassigned. See Supporting Information
Scheme 4.
Unexpected production of the type B adamantine core
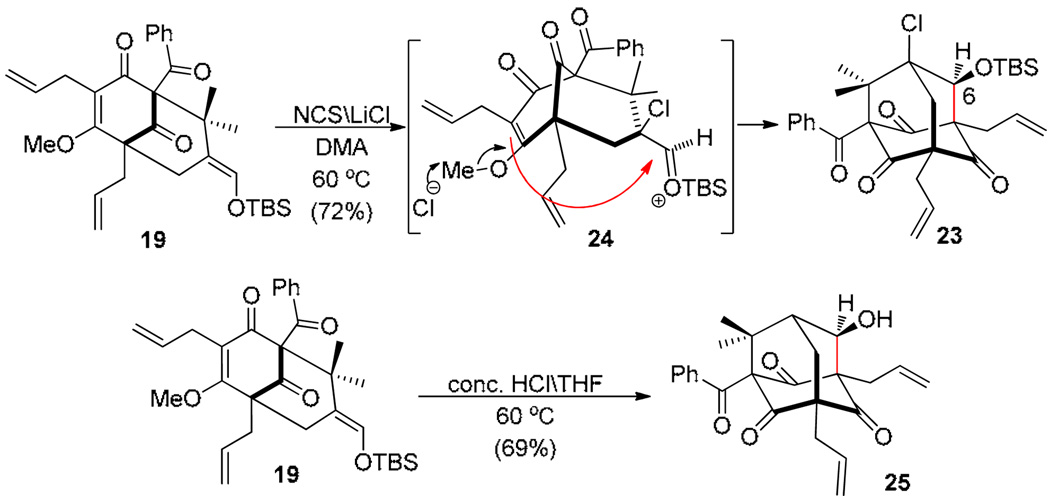
We also evaluated chlorinative cyclization18 of silyl enol ether 19 with the expectation that this reaction mode would allow us to directly access an adamantane framework (Scheme 5). To our delight, treatment of 19 with N-chlorosuccinimide (NCS) 18 in the presence of LiCl to promote demethylation17 (DMA, 60 °C) led to the formation of chloroadamantane 23 as a single diastereomer, likely thru cyclization of silyloxonium (siloxycarbinyl cation)19 24. Unfortunately, chloroadamantane derivative 23 was found to be resilient to desilylation and led to either no reaction or formation of byproducts under either acidic or basic fluoride mediated reaction conditions.
Scheme 5.
Synthesis of the type A adamantane framework
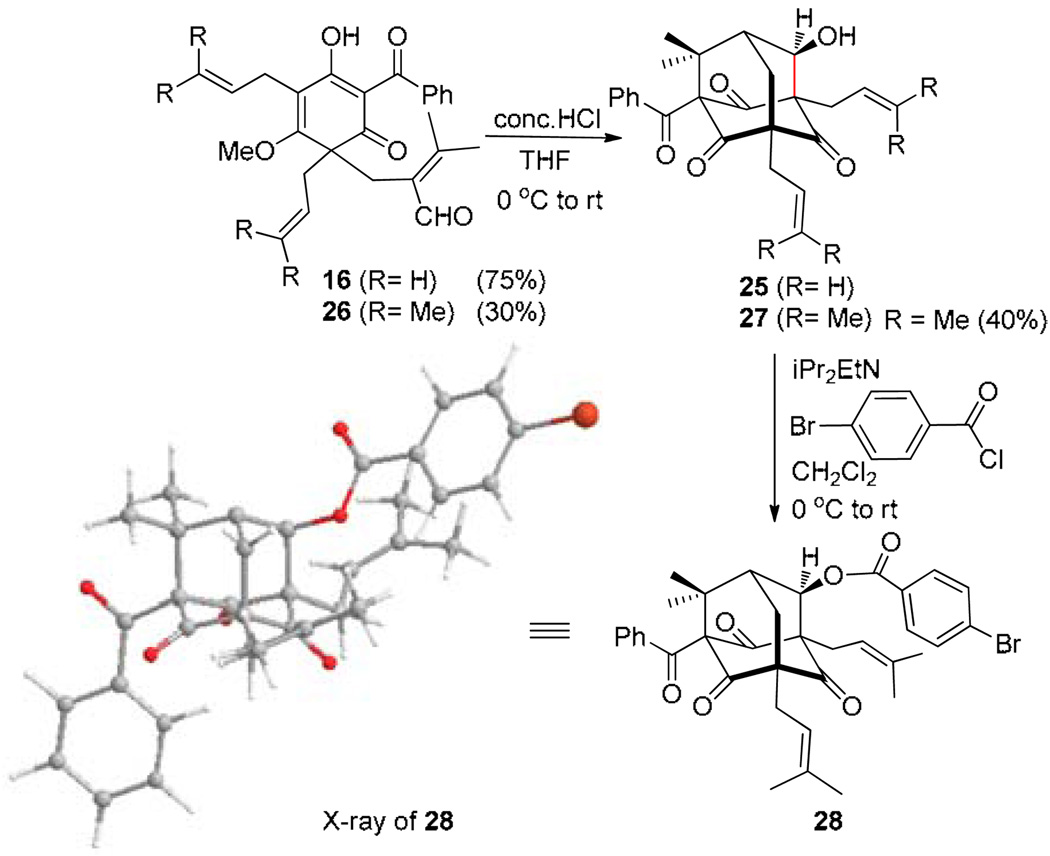
The latter observation led us to reevaluate the possibility for protonation/cyclization of 19 to directly access the adamantane core of plukenetione A. Subjection of silyl enol ether 19 to conventional desilylation conditions, Lewis, and Bronsted acids (e.g. TFA and AcOH) generally led to formation of ring-opened product 16 or rearomatized acylphloroglucinol 14 (Scheme 3).20 After a survey of conditions, we found that exposure of 19 to conc. HCl in THF (4 equiv) led to the production of adamantane alcohol 25 in 69% yield as a single diastereomer (cf. Scheme 1). As we suspected hydrolysis of 19 to enal 16 under the reaction conditions, we found that treatment of both 16 or its the bis-prenylated variant 26 with conc. HCl in THF led directly to adamantanes 25 and 27 (Scheme 6)21 enabling rapid construction of the plukenetione A core in two steps from acylphloroglucinol 14. The structure of adamantane 27 was confirmed by acylation to p-bromobenzoate 28 and X-ray crystal structure analysis.15
Scheme 6.
Direct access to the adamantane core of 1.
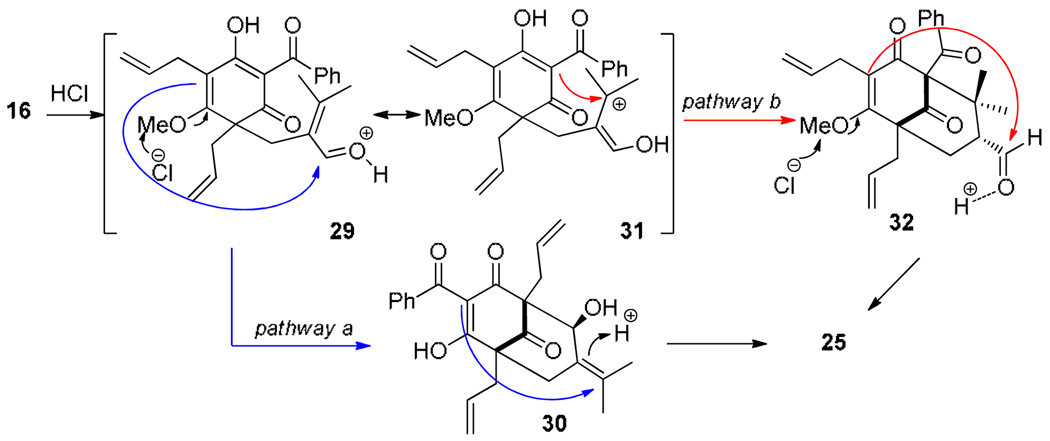
Two possible pathways for the acid-mediated adamantane cyclization are shown in Figure 3. In pathway a, the protonated aldehyde 29 may undergo demethylative aldol cyclization22 to 30 which may be followed by protonation of the tetrasubstituted alkene and cationic cyclization11i to afford 25. In pathway b, the allylic cation resonance structure 31 derived from 29 may participate in cationic cyclization11i leading to the production of bicyclo [3.3.1] derivative 32 which may undergo final demethylative aldol cyclization22 to afford adamantane 25. 23
Figure 3.
Possible reaction mechanisms for adamantane formation
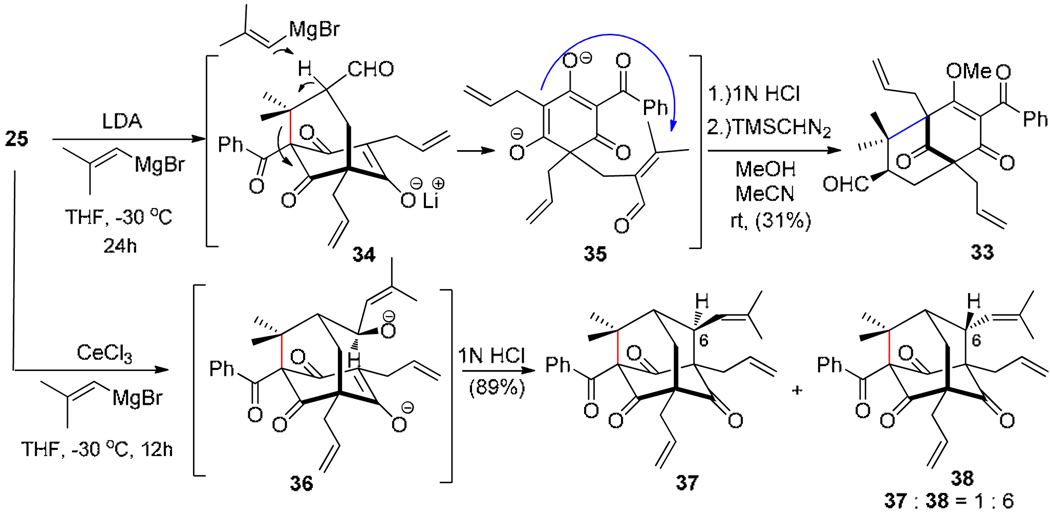
We next investigated whether adamantane alcohol 25 could be converted to plukenetione A using the retro-aldol/alkenyl metal addition protocol employed in our hyperibone K synthesis.13 Unexpectedly, exposure of 25 to LDA followed by addition of 2-methyl-1-propenyl magnesium bromide led to fragmentation to provide the clusianone (Type B PPAP) aldehyde 33 after enol methylation of the crude product (Scheme 7). This cascade process likely occurs by deprotonation of derived aldehyde 34 followed by retro-Michael reaction to intermediate 35 which may undergo subsequent intramolecular Michael addition. The structure of 33 was further confirmed by treatment with Sc(OTf)3 in CH3NO2 to afford the type B PPAP adamantane derivative 21.13,15 After surveying a number of base/nucleophile combinations, the fragmentation process was circumvented by cerium(III) chloride-promoted reaction of the alkenyl Grignard reagent24 which cleanly afforded the desired adduct 36 without noticeable side reactions. Interestingly, quenching of the organocerium reaction with 1N HCl led to the production of adamantanes 37 and 38 as a 1 to 6 mixture of isomers favoring the undesired stereoisomer 38.
Scheme 7.
Retro-aldol/organocerium addition
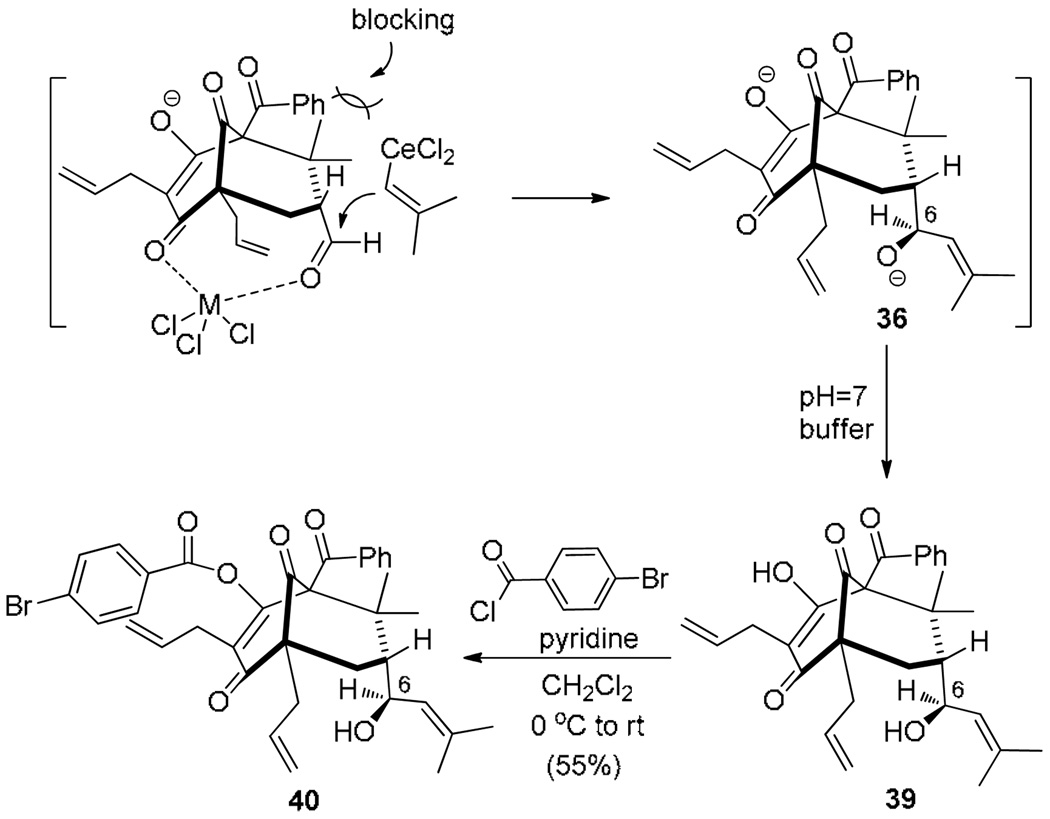
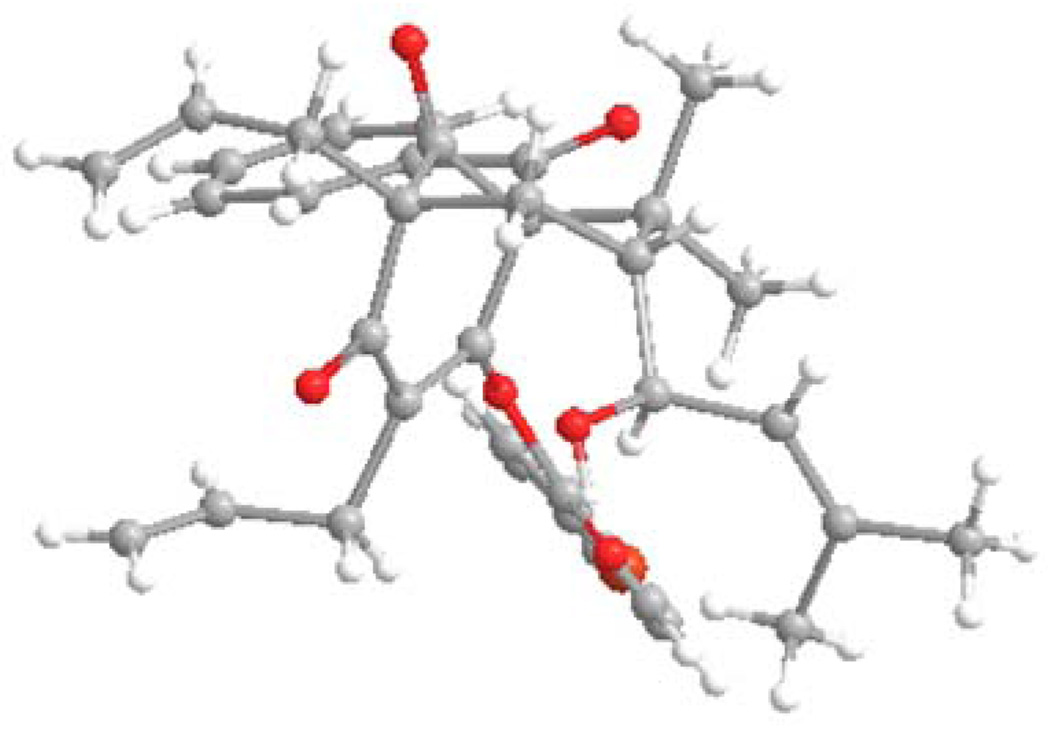
Based on these results, we continued to probe the reaction mechanism leading to adamantanes 37 and 38 and the possibility to reverse the selectivity. We found that quenching of organocerium addition with pH 7 buffer led to formation of an enol intermediate 39 which was acylated directly with p-bromobenzoyl chloride to provide enol ester 40 (Scheme 8) along with its enol ester isomer. The structure of 40 was confirmed by single X-ray crystal structure analysis.15 Inspection of this structure indicates that the configuration at C6 of the allylic alcohol of 36 may be derived from long range chelation between Ce(III) (or Mg (II)) of the enol/ketone25 moiety and the aldehyde followed by facially selective addition of the alkenyl metal reagent to the face away from the gem-dimethyl moiety.
Scheme 8.
Proposal for diastereoselectivity in the Grignard addition
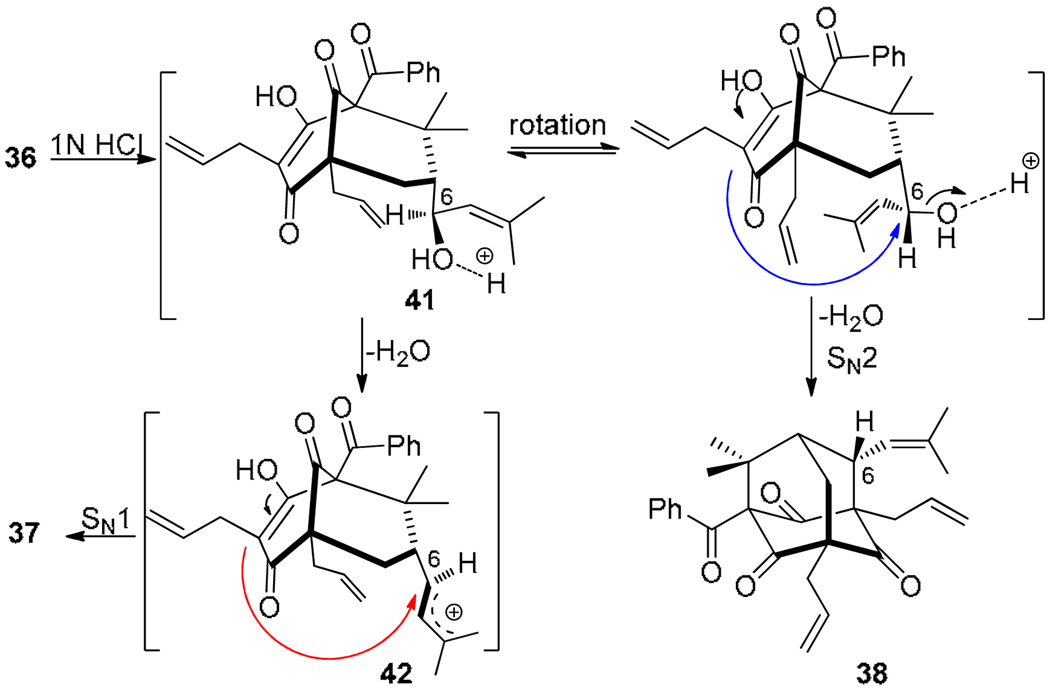
Based on the cyclization results and the observed stereochemistry at C6, we propose a rationale for stereo-selectivity of the adamantane cyclization as shown in Scheme 9. Quenching of intermediate 36 with 1N HCl should lead to protonated enol 41 which is properly situated for SN2 cyclization26 leading to formation of the C6-epi derivative 38. Alternatively, ionization of allylic alcohol 41 should afford allylic cation 42 which may undergo cationic (SN1) cyclization leading to 37.13
Scheme 9.
Rationale for stereoselectivity
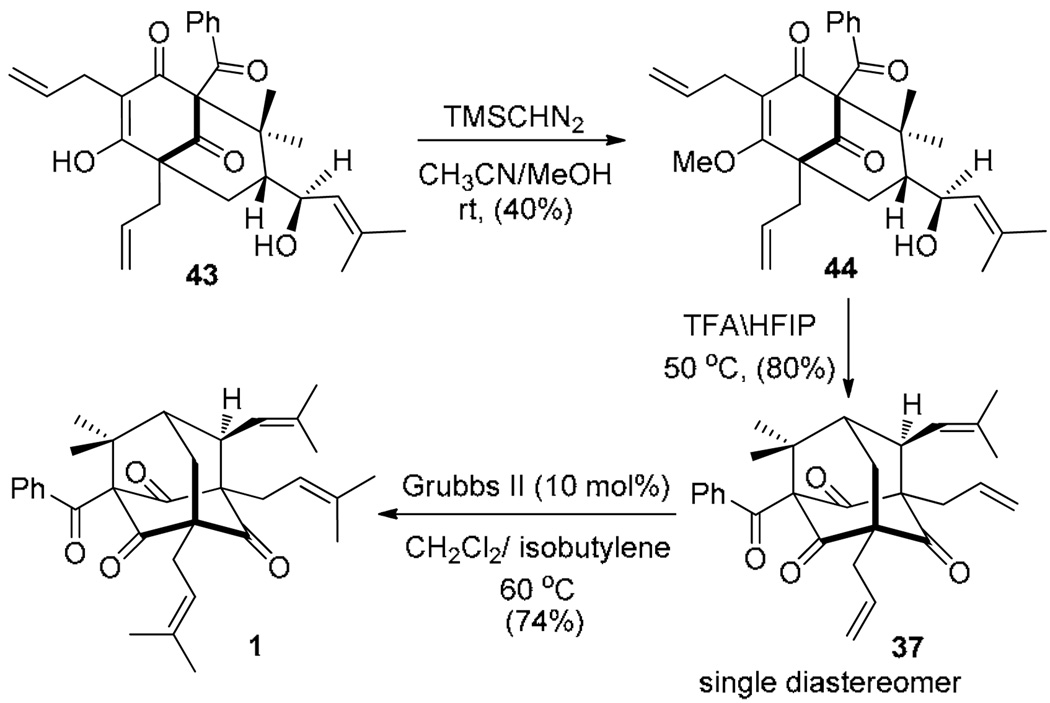
Given the high reactivity of the free enol/allylic alcohol 39/43, we proceeded to methylate the crude enol 39/43 in an effort to lower its nucleophilicity in the form of vinylogous ester 44 and access an SN1 cyclization mode (Scheme 10). Gratifyingly, treatment of 44 with TFA (10 equiv) in hexafluoroisopropanol (HFIP) 27 as solvent led to selective formation of adamantane derivative 37. Other cyclization conditions were also attempted on substrate 44 including use of Sc(OTf)3 and the cation-stabilizing solvent nitromethane (MeNO2) which afforded 37 and 38 in a 1: 3 ratio. Olefin cross-metathesis of 37 using isobutylene in the presence of the Grubbs II28 metathesis catalyst afforded plukenetione A 1 in 74% yield. Spectral data for 1 (1H NMR, 13C NMR, mass spectrum) were in full agreement with data reported for the natural product.2, 15
Scheme 10.
Completion of the synthesis of plukenetione Aa
a For 44, one enol ether isomer shown for clarity.
Conclusion
In summary, we have developed an expedient approach to the type A PPAP natural products including plukenetione A 1. The strategy relies on introduction of an ether blocking group on an acylphloroglucinol substrate to direct the regiochemistry for alkylative dearomatization/annulation and acid-mediated cyclization to construct the adamantane core. Introduction of the 2-methyl-1-propenyl moiety of 1 was achieved via stereodivergent SN2 and SN1 cyclizations of allylic alcohol substrates. Studies to further probe the mechanism of the acid-mediated adamantane cyclization as well as the synthesis and biological evaluation of other type A PPAP natural product targets are currently underway and will be reported in due course.
Supplementary Material
Figure 4.
X-ray structure of enol ester 40
Acknowledgment
We thank the National Institutes of Health (GM-073855) and the National Science Foundation (0848082) for research support and Profs. Gary Molander (University of Pennsylvania) and Aaron Beeler (Boston University) for helpful discussions. We also thank Prof. Helen Jacobs (University of West Indies, Kingston, Jamaica) for providing NMR spectra of natural plukenetione A.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all new compounds. X-ray crystal structure coordinates and files in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Presented in part at the 238rd American Chemical Society National meeting; August 18–23, 2009; Washington DC. ORGN abstract 595. [Google Scholar]
- 2.Henry GE, Jacobs H, Sean Carrington CM, McLean S, Reynolds WF. Tetrahedron Lett. 1996;37:8663. [Google Scholar]
- 3.Diaz-Carballo D, Malak S, Bardenheuer W, Freistuehler M, Peter R. Bioorg. Med. Chem. 2008;16:9635. doi: 10.1016/j.bmc.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira CMA, Porto ALM, Bittrich V, Marsaioli AJ. Phytochemistry. 1999;50:1073. [Google Scholar]
- 5.Hu L, Sim K. Tetrahedron. 2000;56:1379. [Google Scholar]
- 6.Christian OE, Henry GE, Jacobs H, McLean S, Reynolds WF. J. Nat. Prod. 2001;64:23. doi: 10.1021/np000321o. [DOI] [PubMed] [Google Scholar]
- 7.Cuesta-Rubio O, Velez-Castro H, Frontana-Uribe B, Cardenas J. Phytochemistry. 2001;57:279. doi: 10.1016/s0031-9422(00)00510-0. [DOI] [PubMed] [Google Scholar]
- 8.Ciochina R, Grossman R. Chem. Rev. 2006;106:3963. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 9.Piccinelli A, Cuesta-Rubio O, Chica M, Mahmood N, Pagano B, Pavone M, Barone V, Rastrelli L. Tetrahedron. 2005;61:8206. [Google Scholar]
- 10.Tanaka N, Takaishi Y, Shikishima Y, Nakanishi Y, Bastow K, Lee KH, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O. J. Nat. Prod. 2004;67:1870. doi: 10.1021/np040024+. [DOI] [PubMed] [Google Scholar]
- 11.For select syntheses of type A PPAPs, see: Spessard SJ, Stoltz BM. Org. Lett. 2002;4:1943. doi: 10.1021/ol025968+. Ciochina R, Grossman RB. Org. Lett. 2003;5:4619. doi: 10.1021/ol0357907. Kraus GA, Nguyen TH, Jeon I.Tetrahedron Lett 20034465916951711 Kuramochi A, Usuda H, Yamatsugu K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2005;127:14200. doi: 10.1021/ja055301t. Nicolaou KC, Carenzi GEA, Jeso V. Angew. Chem. Int. Ed. 2005;44:3895. doi: 10.1002/anie.200500776. Siegel DR, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:1048. doi: 10.1021/ja057418n. Tsukano C, Siegel DR, Danishefsky SJ. Angew. Chem. Int. Ed. 2007;46:8840. doi: 10.1002/anie.200703886. Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Blake AJ. J. Org. Chem. 2007;72:4803. doi: 10.1021/jo070388h. Takagi R, Inoue Y, Ohkata K. J. Org. Chem. 2008;73:9320. doi: 10.1021/jo801595y. Couladouros EA, Dakanali M, Demadis KD, Vidali VP. Org. Lett. 2009;11:4430. doi: 10.1021/ol901781n. Shimizu Y, Shi S, Usuda H, Kanai M, Shibasaki M. Angew. Chem. Int. Ed. 2010;49:1103. doi: 10.1002/anie.200906678. Simpkins N, Taylor J, Weller M, Hayes C. Synlett. 2010;4:639. Abe M, Saito A, Nakada M. Tetrahedron Lett. 2010;51:1298..
- 12.Qi J, Porco JA., Jr. J. Am. Chem. Soc. 2007;129:12682. doi: 10.1021/ja0762339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi J, Beeler AB, Zhang Q, Porco JA., Jr J. Am. Chem. Soc. 2010;132 doi: 10.1021/ja1057828. xxxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitasev B, Porco JA., Jr. Org Lett. 2009;11:2285. doi: 10.1021/ol900590t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.See Supporting Information for complete experimental details.
- 16.Kozikowski AP, Jung S. J. Org. Chem. 1986;51:3400. [Google Scholar]
- 17.Krapcho A, Weimaster J, Eldridge J, Jahngen E, Lovey A, Stephens W. J. Org. Chem. 1978;43:138. [Google Scholar]
- 18.For a review of NCS-mediated reactions, see: Golebiewski WM, Gucma M. Synthesis. 2007;23:3599..
- 19.a) Hambly GF, Chan TH. Tetrahedron Lett. 1986;27:2563. [Google Scholar]; b) Ohkata K, Mase M, Akiba K. J. Chem. Soc., Chem. Commun. 1987;22:1727. [Google Scholar]
- 20.For rearomatization of dearomatized acylphloroglucinols, see: Raikar S, Nuhant P, Delpech B, Marazano C. Eur. J. Org. Chem. 2008;8:1358. doi: 10.1021/jo9019545..
- 21.Anhydrous HCl was also examined with substrates 16 and 19 at room temperature leading to recovery of starting materials after 2 days.
- 22.For demethylative aldol reactions, see: Murakami M, Minamikawa H, Mukaiyama T. Chem. Lett. 1987:1051..
- 23.According to the insightful recommendation of a reviewer, demethylation of intermediates 29 and 32 may also occur by hydrolysis of oxonium intermediates produced after cyclization.
- 24.Imamoto T, Takiyama N, Nakamura K, Hatajima T, Kamiya Y. J. Am. Chem. Soc. 1989;111:4392. [Google Scholar]
- 25.For 8-membered ring chelation using Sm(III) see: Molander G, McKie J. J. Am. Chem. Soc. 1993;115:5821.. (b) Mg(II): Chung K, Chu C, Chang M. Bull. Korean Chem. Soc. 2004;25:417..
- 26.For SN1 v.s. SN2 reactivity in metal-triflate catalyzed alkylations, see: Noji M, Konno Y, Ishii Y. J. Org. Chem. 2007;72:5161. doi: 10.1021/jo0705216..
- 27.For solvolytic reactions in fluorinated alcohols, see: Bentley TW, Llewellyn G, Ryu Z. J. Org. Chem. 1998;63:4654. For a review on fluorinated solvents, see: Shuklov IA, Dubrovina NV, Börner A. Synthesis. 2007:2925..
- 28.Chatterjee AK, Sanders DP, Grubbs RH. Org. Lett. 2002;4:1939. doi: 10.1021/ol0259793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.