Abstract
Unsaturated vitamin B12-binding capacity (UBBC) of human serum is not reproducibly measurable because it increases variably in vitro in relation to time, temperature, and, in the case of plasma, anticoagulant present before removal of cells. This variable increase proved to be due to variable release in vitro of transcobalamin III (TC III) from granulocytes. UBBC increase was greatest (up to fourfold normal levels) in the presence of lithium, which is the heparin salt used in many laboratories doing UBBC studies. In vitro increase was least when blood was collected in EDTA at 0°C and immediately centrifuges at 0°C (T0 sample); results equivalent to T0 were obtained at room temperature even after several hours delay when 47 mM fluoride was present; either cold temperature or 47 mM fluoride appeared to prevent TC III release from granulocytes. The measured levels of the three transcobalamins with T0 methods of collection, which presumably reflect most closely the in vivo circulating levels, suggest that TC I and TC III in normal plasms are of the same order of magnitude and together normally comprise less than 10% of the UBBC.
Approximately 90% of the UBBC content of sonicates of peripheral blood granulocytes and of bone marrow aspirates of normal individuals appears to be TC III, with the rest being TC I. Thus, normal myelocytes, like normal granulocytes, appear to contain mainly TC III. No TC II was present in any of the sonicates.
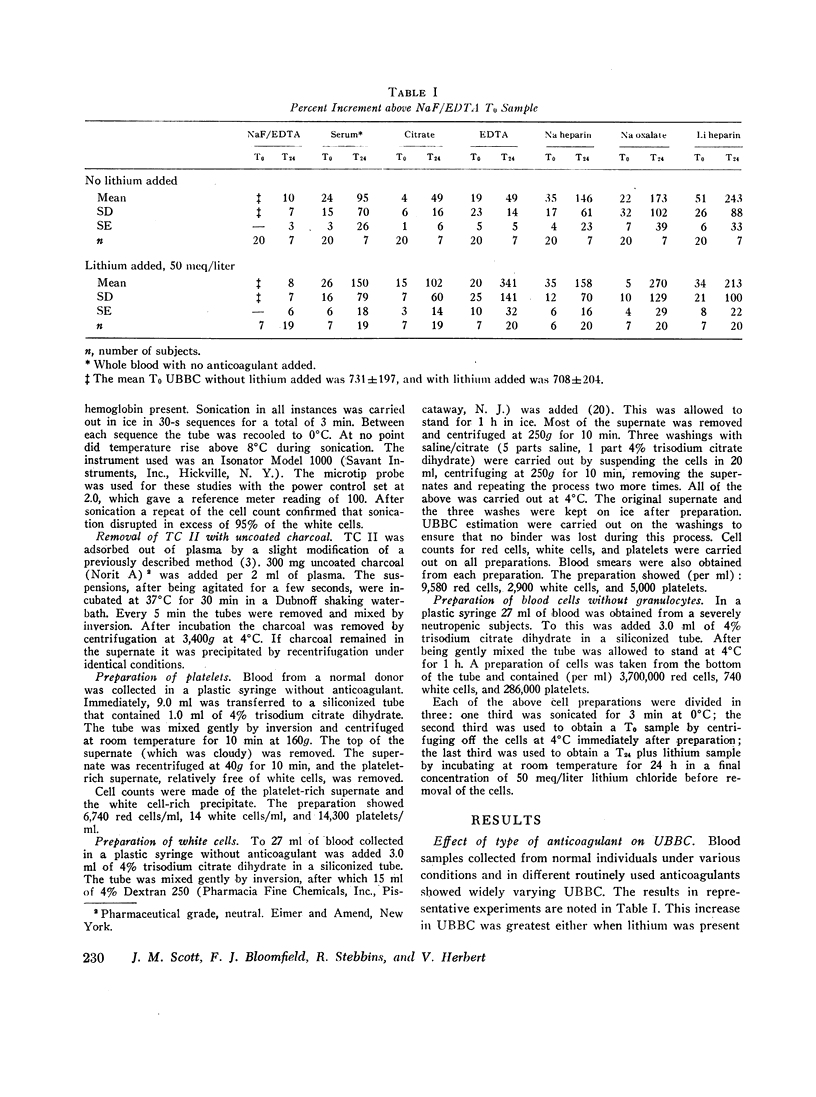
The general practice in most laboratories has been to determine serum UBBC. Because in vitro increments of up to 119% were found to occur in serum, this practice should be replaced by collection using methods that prevent such increments. Blood collected in EDTA-47 mM NaF had a stable, reproducible UBBC with no significant in vitro increment with time.
EDTA-NaF UBBC was 640±168 (range 380-921 pg B12 bound/ml plasma) for 12 normal adult men and 809±232 (range 505-1208) for normal adult women. It presumably approximates circulating UBBC and is substantially below the serum UBBC mean of 935±262 (range 611-1506 for the same 12 men) and 1273±355 (range 811-2306 for the same 10 women).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H., Majerus P. W. Isolation of vitamin B12-binding proteins using affinity chromatography. II. Purification and properties of a human granulocyte vitamine B12-binding protein. J Biol Chem. 1972 Dec 10;247(23):7702–7708. [PubMed] [Google Scholar]
- Bloomfield F. J., Scott J. M. Identification of a new vitamin B 12 binder (transcobalamin 3) in normal human serum. Br J Haematol. 1972 Jan;22(1):33–42. doi: 10.1111/j.1365-2141.1972.tb08784.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield F. J., Scott J. M., Somerville J. J., Weir D. G. Levels in normal, pathological, and foetal sera of the three transcobalamins. Ir J Med Sci. 1973 Mar;142(2):51–57. doi: 10.1007/BF02949990. [DOI] [PubMed] [Google Scholar]
- Bloomfield F. J., Weir D. G., Scott J. M. Some properties of transcobalamin 3 from normal human serum. Br J Haematol. 1972 Sep;23(3):289–295. doi: 10.1111/j.1365-2141.1972.tb08875.x. [DOI] [PubMed] [Google Scholar]
- Carmel R., Herbert V. Deficiency of vitamin B12-binding alpha globulin in two brothers. Blood. 1969 Jan;33(1):1–12. [PubMed] [Google Scholar]
- Carmel R., Herbert V. Vitamin B 12 -binding protein of leukocytes as a possible major source of the third vitamin B 12 -binding protein of serum. Blood. 1972 Oct;40(4):542–549. [PubMed] [Google Scholar]
- Chanarin I., England J. M., Rowe K. L., Stacey J. A. Role of third serum vitamin B 12 binding protein in vitamin B 12 transport. Br Med J. 1972 May 20;2(5811):441–442. doi: 10.1136/bmj.2.5811.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikkappa G., Corcino J., Greenerg M. L., Herert V. Correation between vario blood white cell pools and the serum B12-binding capaities. Blood. 1971 Feb;37(2):142–151. [PubMed] [Google Scholar]
- Cooper B. A. Complexing of transcobalamin 2 and apparent combination with heparin. Blood. 1970 Jun;35(6):829–837. [PubMed] [Google Scholar]
- Corcino J., Krauss S., Waxman S., Herbert V. Release of vitamin B12--binding protein by human leukocytes in vitro. J Clin Invest. 1970 Dec;49(12):2250–2255. doi: 10.1172/JCI106444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Gilbert H. S., Krauss S., Pasternack B., Herbert V., Wasserman L. R. Serum vitamin B12 content and unsaturated vitamin B12-binding capacity in myeloproliferative disease. Value in differential diagnosis and as indicators of disease activity. Ann Intern Med. 1969 Oct;71(4):719–729. doi: 10.7326/0003-4819-71-4-719. [DOI] [PubMed] [Google Scholar]
- Gizis E. J., Dietrich M. F., Ohoi G., Meyer L. M. A 57Co vitamin B12 binder in normal serum eluted by DEAE-cellulose chromatography with 0. 1m sodium phosphate buffer, pH 5.8. J Lab Clin Med. 1970 Apr;75(4):673–678. [PubMed] [Google Scholar]
- Gullberg R. Influence of plasma preparation technique on the amount of recovered B 12 -binding proteins. Clin Chim Acta. 1971 Jun;33(1):173–177. doi: 10.1016/0009-8981(71)90265-8. [DOI] [PubMed] [Google Scholar]
- Gullberg R. Vitamin B 12 -binding proteins in normal human blood plasma and serum. Scand J Haematol. 1972;9(6):639–647. doi: 10.1111/j.1600-0609.1972.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Hall C. A., Finkler A. E. Measurement of the amounts of the individual vitamin B12 binding proteins in plasma. 1. Studies of normal plasma. Blood. 1966 May;27(5):611–617. [PubMed] [Google Scholar]
- Hall C. A. Transport of vitamin B 12 in man. Br J Haematol. 1969 May;16(5):429–433. doi: 10.1111/j.1365-2141.1969.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Herbert V. Diagnostic and prognostic values of measurement of serum vitamin B12-binding proteins. Blood. 1968 Aug;32(2):305–312. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kennedy E. H., Adams J. F. The effect of anticoagulants on the in vitro binding capacity of serum and plasma for cobalamins. Scand J Haematol. 1967 Dec;4(6):489–492. doi: 10.1111/j.1600-0609.1967.tb01652.x. [DOI] [PubMed] [Google Scholar]
- LAU K. S., GOTTLIEB C., WASSERMAN L. R., HERBERT V. MEASUREMENT OF SERUM VITAMIN B12 LEVEL USING RADIOISOTOPE DILUTION AND COATED CHARCOAL. Blood. 1965 Aug;26:202–214. [PubMed] [Google Scholar]
- Lawrence C. The heterogeneity of the high molecular weight B12 binder in serum. Blood. 1969 Jun;33(6):899–908. [PubMed] [Google Scholar]
- Rachmilewitz B., Rachmilewitz M., Moshkowitz B., Gross J. Serum transcobalamin in myeloid leukemia. J Lab Clin Med. 1971 Aug;78(2):275–288. [PubMed] [Google Scholar]
- Retief F. P., Gottlieb C. W., Kochwa S., Pratt P. W., Herbert V. Separation of vitamin B 12-binding proteins of serum, gastric juice and saliva by rapid DEAE cellulose chromatography. Blood. 1967 Apr;29(4):501–516. [PubMed] [Google Scholar]
- Retief F. P., Gottlieb C. W., Kochwa S., Pratt P. W., Herbert V. Separation of vitamin B 12-binding proteins of serum, gastric juice and saliva by rapid DEAE cellulose chromatography. Blood. 1967 Apr;29(4):501–516. [PubMed] [Google Scholar]
- Rosner F., Schreiber Z. A. Serum vitamin B 12 and vitamin B 12 binding capacity in chronic myelogenous leukemia and other disorders. Am J Med Sci. 1972 Jun;263(6):473–480. doi: 10.1097/00000441-197206000-00008. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Tisman G., Herbert V., Rosenblatt S. Evidence that lithium induces human granulocyte proliferation: elevated serum vitamin B 12 binding capacity in vivo and granulocyte colony proliferation in vitro. Br J Haematol. 1973 Jun;24(6):767–771. doi: 10.1111/j.1365-2141.1973.tb01704.x. [DOI] [PubMed] [Google Scholar]



