Abstract
Background & Aims
Aldehydes that are produced following the breakdown of ethanol (acetaldehyde) and lipid peroxidation of membranes (malondialdehyde) have been shown to bind (adduct) proteins. Additionally, these two aldehydes can combine (MAA) on non-syngeneic and syngeneic proteins to initiate numerous immune responses to the unmodified part of the protein in the absence of an adjuvant. Therefore, these studies provide a potential mechanism for the development of antigen-specific immune responses resulting in liver damage should syngeneic liver proteins be adducted with MAA.
Methods
This study sought to test whether MAA modified syngeneic liver cytosolic proteins administered daily in the absence of adjuvant into C57BL/6 mice abrogates tolerance to initiate a MAA induced autoimmune-like hepatitis (MIAH).
Results
In mice immunized with MAA modified cytosols there was an increase in liver damage as assessed by AST/ALT levels that correlated with liver pathology scores and the presence of the pro-fibrotic factors; smooth muscle actin (SMA), TGF-β, and collagen. IgG antibodies and T-cell proliferative responses specific for cytosolic proteins were also detected. Pro-inflammatory cytokines were produced in the livers of animals exposed to MAA-modified cytosols. Finally, transfer of immunized T-cells to naïve animals caused biochemical and histological evidence of liver damage.
Conclusions
These data demonstrate that a disease with an autoimmune-like pathophysiology can be generated in this animal model using soluble MAA modified syngeneic liver cytosols as the immunogen. These studies provide insight into potential mechanism(s) that the metabolites of alcohol may play in contributing to the onset of an autoimmune-like disease in ALD patients.
Introduction
A number of different studies suggest that the onset of alcoholic liver disease (ALD) is initiated in part by immune mechanisms. The detection of circulating antibodies and lymphocytes with specificity to hepatic antigens in patients with ALD strongly supports this hypothesis (Cook, 1998; Duryee et al., 2004b; Laskin et al., 1990; Paronetto, 1993). However, the mechanism(s) by which proteins from the liver break immunologic tolerance and induce these autoimmune responses have not been identified. Studies have shown that aldehyde modified proteins are present in the tissue of humans and animals consuming ethanol. Also, the modification of proteins with aldehydes makes them antigenic (Israel et al., 1986; Lin et al., 1990; Niemela et al., 1991; Terabayashi and Kolber, 1990).
Reports from our laboratories have demonstrated the development of antibodies and T cell responses to exogenous proteins modified with the combination of metabolically-derived aldehydes (MAlondialdehyde and Acetaldehyde) or MAA (Tuma et al., 1996; Willis et al., 2003; Xu et al., 1997) in the absence of any adjuvant (Thiele et al., 1998), making the immunogenicity of those biotransformed proteins relevant. Circulating antibodies to the MAA adduct have been detected in the serum of both humans and rats chronically consuming alcohol (Rolla et al., 2000; Xu et al., 1998). In humans, anti-MAA antibodies have correlated both with the presence and severity of ALD. Additionally, alcohol fed rats generated antibodies that responded to unmodified liver self-proteins, suggesting that MAA adducts induce an anti-self immune response (Xu et al., 1998).
A number of animal models of autoimmune hepatitis have been developed in order to study the underlying mechanisms of disease initiation (Lohse et al., 1990; Peters, 2002; Tiegs, 1997). However, most rely on the use of both strong adjuvants and high doses of antigen to initiate autoimmunity (Howell and Yoder, 1994; Kohda et al., 1990; Tiegs, 1997). For example, chemical modifications of self proteins have been shown to generate an autoimmune humoral response to the modified and carrier protein when administered in adjuvants (Abraham et al., 1997; Abraham et al., 1995; Thiele et al., 1998). Additionally, lipid peroxidation products given with adjuvants have been shown to break tolerance to syngeneic proteins and generate T cell reponses (Wallberg et al., 2007; Wuttge et al., 1999).
There is a wide spectrum of alcohol-related pathology in humans and animals including; hepatitis, steatosis, non-specific steatohepatitis, apoptosis, and centrilobular, periportal and pericellular fibrosis (French and Tsukamoto, 1989; Lieber and DeCarli, 1982; Song et al., 2002). However, no one animal model has totally mimicked the classical pattern of alcoholic hepatitis observed in humans. Importantly, these animal models have been invaluable in evaluating the individual (LPS, fatty liver, oxidative stress, etc.) potential pathogenic mechanisms that potentially contribute to the development and/or progression of ALD. Therefore, this study elucidates the contribution of aldehdye-modified proteins in initiating immune responses that may play a role in this process. In these studies, mice were immunized in the absence or adjuvants with MAA-modified syngeneic proteins. A daily course of immunizations was initiated in order to mimic the exposure that an alcoholic would encounter over time to these aldehyde-modified proteins. Importantly, these studies were developed in order to determine whether liver self-proteins modified with aldehydes can break tolerance in the absence of adjuvant and provide a model to study the immune responses involved in ALD.
Materials and Methods
Animals
Six week old female C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) and maintained on a Purina chow diet. All animals were allowed free access to their food and/or water up to 1 hour prior to sacrifice. All procedures were approved by the Animal Subcommittee of the Omaha VA Medical Center, and are in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Preparation of Malondialdehyde-Acetaldehyde Modified S100 (S100-MAA)
Syngeneic C57BL/6 mice were sacrificed daily, livers removed, and a S100 cytosolic fraction prepared according to the method of Lohse et al (Lohse et al., 1990). These materials were prepared daily in order to retain the antigenicity of the liver specific proteins (LSPs) as previously described (Lohse et al., 1998). Protein concentration of the S100 fraction was determined using a BCA Protein Assay kit from Pierce (Rockford, IL). Daily modifications of the S100 fraction with MAA was performed by incubating 4mM malondialdehyde and 1mM acetaldehyde in 0.1 M phosphate buffer (PB) with 2 milligrams of S100 at 37°C overnight (Tuma et al., 1996). These concentrations of aldehydes, while not physiological, were chosen to modify the protein with MAA in the shortest possible time to maintain protein immunogenicity (Lohse et al., 1998). Importantly, these concentrations of AA and MDA resulted in the modification of syngeneic liver cytosols to the same level as using physiological concentrations for a longer period of time. Therefore, the adducted proteins should be biologically relevant. In order to guarantee modification of cytosolic proteins with MAA had occurred, immuno-blot analysis used, and a polyclonal rabbit anti-MAA antibody as the probe (Xu et al., 1997). Also, fluorescence for the dihydropyridine structure (2:1 MAA) was detected using a spectrophotometer at 398 nm (excitation) and 460 nm (emission) and used in these studies. All samples were prepared under endotoxin-free conditions and were determined to be endotoxin-free using the Limulus Amebocyte Lysate assay from BioWhittaker (Walkersville, MD).
MAA Induced Autoimmune Hepatitis (MIAH)
Induction of MAA induced autoimmune hepatitis (MIAH) was performed by daily intraperitoneal immunizations of 0.1M phosphate buffer (PB), 100 μg of S100 or 100 μg S100 modified with MAA (S100-MAA) for 3 weeks. Intraperitoneal injection was chosen as drainage should occur through the peritoneal lymph nodes and most likely mimic the mechanism of removal of liver syngeneic proteins following tissue destruction in ALD. The liver, spleen, and serum were removed following the last immunization.
Histology
Livers extracted from PB, S100 or S100-MAA immunized mice were placed in 10% formalin PBS and embedded in paraffin. Sections were stained with hematoxylin/eosin and blindly scored by a pathologist. Chronic hepatitis was graded using the Ludwig and Batts scale from 0-4 (Batts and Ludwig, 1995).
AST/ALT Levels
Serum from animals immunized with PB, S100, or S100-MAA were collected by retro-orbital bleeding just prior to immunization and at the time of sacrifice. Animals receiving PB immunizations were used as controls to determine hemolysis in the serum, and these values were subtracted from the test values. Alanine Aminotransferase (ALT) and aspartate aminotransferase (AST) were measured for liver injury using colorimetric assay kits from Bioquant (San Diego, CA).
Serum Antibody Levels to S100
A direct ELISA was used to determine the circulating antibody titers to the S100 cytosolic fraction. Briefly, S100 or S100-MAA were coated onto (Immulon IV, Dynatech, Chantilly, VA) ELISA plates at 2 μg/well in the presence of bicarbonate buffer, pH 9.6 and incubated overnight at 37°C. Plates were washed, blocked using 2% Casein for 30 minutes, and serum incubated at different dilutions on the plate for 1 hour at room temperature. The plates were then washed and horse-radish peroxidase (HRP) labeled rabbit anti-mouse IgG (H&L) antibody added (Sigma Chemical, Co, St. Louis, MO), incubated for 30 minutes, and developed using TMB substrate. Absorbance was detected after stopping the reaction using 2N H2SO4 and reading at 450 nm using an MRX II Microplate Reader (Dynatech, Chantilly, VA). Data were analyzed using Revelations Software from Dynatech, and expressed in pg/ml using mouse IgG as a standard curve. Serum from PB immunized mice was used as controls and values subtracted from the test samples. In order begin accessing the specific proteins involved in the antibody response, S100 or S100-MAA were subjected to Western Blot technique. Briefly, S100 or S100-MAA were resolved under reducing conditions by SDS-PAGE on 10% gels and blotted onto Immun-Blot™ PVDF membrane (Bio-Rad, Hercules, CA). PVDF membranes were blocked in milk and then incubated with serum from mice immunized with S100-MAA. An alkaline phosphatase goat anti-mouse IgG (H&L) secondary antibody (Jackson Immuno Research Laboratories, Inc., West Grove, PA) was incubated and detected using BCIP/NBT substrate (Sigma Chemical Co., St. Louis, MO).
Proliferative responses to S100
To determine proliferative responses of T-cells from PB, S100 or S100-MAA immunized mice, antigens (S100 or S100 MAA) were added to 96-well flat bottom plates at concentrations ranging from 3.125 to 100 μg/ml. Spleen cells from mice in each of the groups were added at a concentration of (1 × 105 cells/well) and incubated for 48 hours at 37°C in 5% CO2, pulsed with 1.0 μCi/well of [3H] thymidine (GE Healthcare, Piscataway, NJ) for 16 hours, and harvested on a 96-well harvester (Tomtec, Orange, CO). Filter paper containing the incorporated thymidine was placed in scintillation fluid and counted on a 1450 Microbeta Scintillation Counter (Perkin Elmer Life Sciences, Waltham, MA). Whole spleen cell preparations were utilized as both T cells and a source of antigen presenting cells (Dendritic cells and macrophages) are present. T cell proliferations were evaluated by subtracting background levels (BKG) from naïve mice from PB and test samples. Data was expressed as stimulation index (SI) using the following formula:
Passive Transfer of T-cells
In order to determine if cells of the immune system are involved in the detrimental effects of S100-MAA immunization, spleenocytes were isolated from PB, S100, and S100-MAA immunized animals. Briefly, spleens were removed, mechanically disrupted, single cell suspensions prepared, adjusted to 5 × 106 cells/ml and stimulated for 48 hours with concanavalin A (Con A) to stimulate T cell proliferation. These activated cells were washed 3 times with PBS to remove Con A, and transferred into naïve animals via i.p. injection weekly for three weeks. Damage was assessed by serum AST levels and pathology scores.
Liver Cytokine Levels
Livers from PB, S100 or S100-MAA immunized mice were removed and homogenized as outlined in previous studies (Fairweather et al., 2003; Njoku et al., 2005). Homogenates were tested for cytokine levels using the following ELISA kits: IL-1β, TNF-α, and IFN-γ from BD Biosciences (San Diego, CA). Secretion levels are expressed in pg/ml extrapolated from a standard curve supplied by the manufacturer and subtracted from PB animal cytokine levels. Absorbance was detected at 450 nm using an MRX II Microplate Reader (Dynatech, Chantilly, VA) and data analyzed using Revelations Software from Dynatech.
Smooth Muscle Actin and TGF-β Staining
Paraffin embedded sections were cut and subjected to immunohistochemical staining for the increased presence of smooth muscle actin (SMA) and TGF-β. Briefly, following hydration of the samples, antigen retrieval was performed using epitope retrieval buffer from Bethyl as per the protocol (Bethyl Laboratories, Inc, Montgomery, TX). Sections were then incubated with a monoclonal anti-actin, smooth muscle antibody (Sigma, St. Louis, MO), blocked in peroxide, and incubated with an HRP goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). DAB substrate was used as the detection reagent and sections counterstained with hematoxylin.
For the TGF-β samples, antigen retrieval was not performed as it was not necessary for this antigen. Sections were incubated with a rabbit anti-mouse TGF-β antibody (Santa Cruz Biotech, Santa Cruz, CA), blocked in peroxide, and incubated with an HRP goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). DAB substrate was used as the detection reagent and sections counterstained with hematoxylin. Slides were analyzed using a Nikon Eclipse 80i at 200X power and Nis-Elements 3.0 software (Nikon, Melville, NY).
Sirius Red Staining
Paraffin embedded liver tissue were cut and subjected to Sirius Red/fast green staining. Briefly, slides were incubated in 0.1% fast green for 10 minutes and washed in acidified water. Slides were then placed in pico-sirius red for 1 hour, followed by washing in acidified water, rinsed in water, and dehydrated with ethanol. Slides were analyzed using a Nikon Eclipse 80i at 200X power and Nis-Elements 3.0 software (Nikon, Melville, NY).
Results
In order to determine if MAA modified liver cytosol would cause significant liver damage livers from mice immunized with PB, S100 or S100-MAA were removed, placed in formalin, sectioned, and stained with H & E. Slides were scored using the Ludwig and Batts scale of liver damage as described in Materials and Methods. Figure 1 (panel C) (a representation of 5 other slides) demonstrates infiltration and liver damage compared to the S100 (panel B) or PB animals (panel A). These sections were scored in a blinded fashion by a pathologist and determined to have a 2 fold increase in the pathology score (P = 0.032) in the S100-MAA immunized animals over the S100 controls (panel D).
Figure 1.
H & E stains and pathology scores of livers from MIAH mice. C57/Bl6 mice were injected with PB, 100μg S100 or 100μg S100-MAA as described in Materials and Methods. Livers were removed, fixed, sectioned, and stained with H & E. Panel (A) PB injected mouse liver, (B) S100 injected mouse liver, (C) S100-MAA injected mouse liver, (D) pathology scores. H & E sections are representative of 5 slides from separate mouse livers in each group (Magnification 200X). Slides were scored by a blinded pathologist using the Ludwig and Batts scale of liver damage. Data are expressed as the mean ± S.E.M. of five separate animals. Significantly different from S100 immunized control animals *P ≤ 0.04.
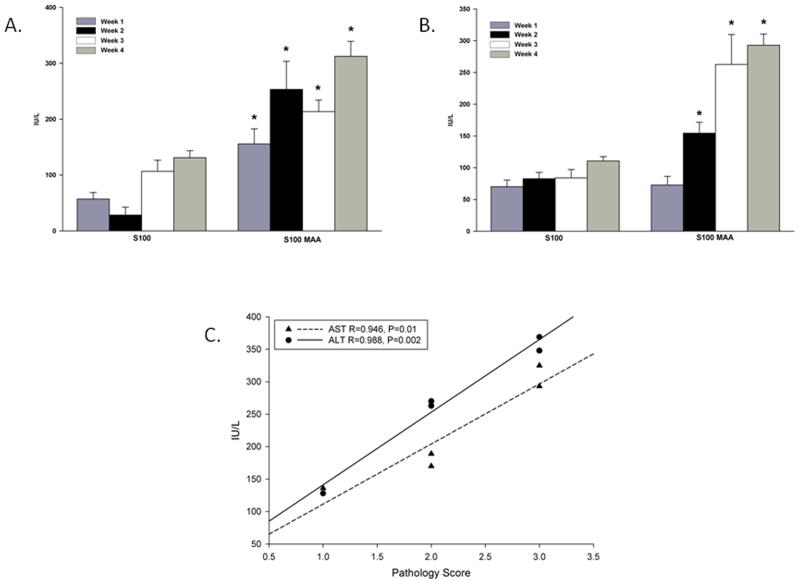
To further assess liver damage in this model, serum was collected from mice immunized with S100 or S100-MAA and tested for AST/ALT liver enzymes. As shown in Figure 2A ALT levels were increased beginning at week 1 and through week 4 in the S100-MAA immunized mice as compared to S100 immunized mice. In contrast AST levels (Figure 2B) did not begin to increase in the serum of S100-MAA immunized mice until week 2, but they continued their increase until the end of the study. PB mice had serum ALT and AST levels < 50 IU/L throughout the study. At week 4, the enzyme levels were correlated to the pathology score and a correlation of 0.946 for AST and 0.988 for ALT was observed indicating that as the liver enzymes increased so did the severity of the pathology score (Figure 2C).
Figure 2.
AST/ALT levels in the serum from immunized mice. C57/Bl6 mice were immunized with PB, 100μg S100 or 100μg S100-MAA as described in the Materials and Methods. Serum was tested weekly for the presence of AST (Panel (A) and Panel (B)) ALT enzyme levels as an indication of liver damage. Background levels of hemolysis have been subtracted out using serum from PB injected mice. Data are expressed as the mean ± S.E.M. of five separate animals. Significantly different from S100 immunized animals, *P ≤ 0.01 for AST and * P ≤ 0.006 for ALT. (Panel C) Correlation of AST/ALT levels to the pathology score. Data generated from pathology scores and AST/ALT enzymes were compared using Pearson Product Moment Correlation test. Correlation Coefficient and P values are reported as; AST (R=0.946, P=0.01), ALT (R=0.988, P=0.002).
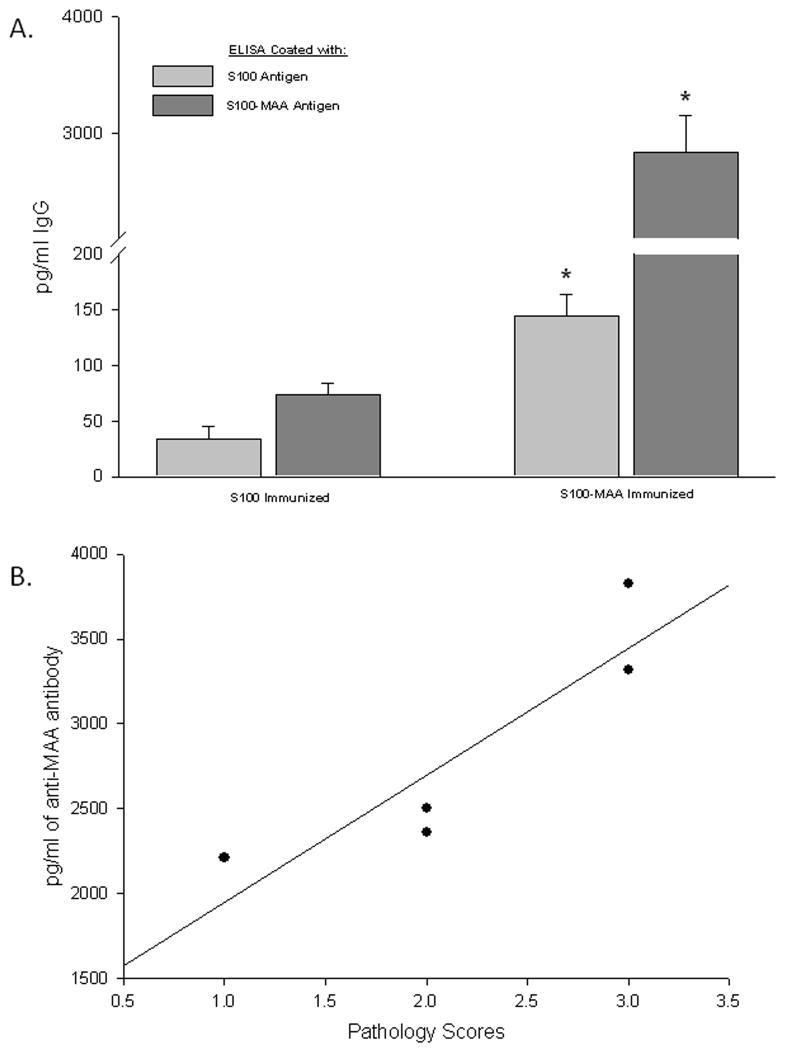
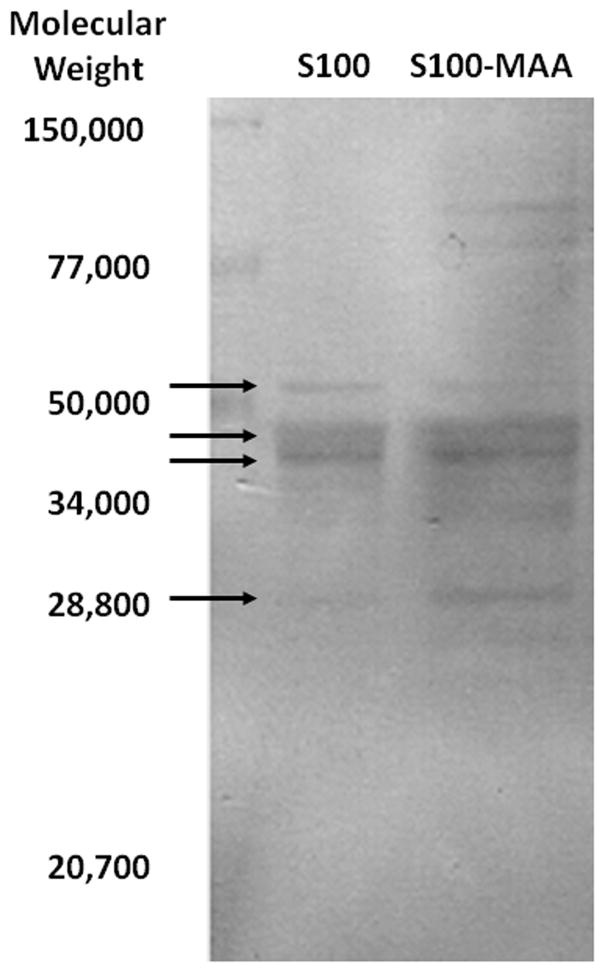
In some studies, organ specific autoimmune disease can be assessed by evaluating the levels of IgG antibodies directed against liver self protein antigens. Examination of IgG specific antibodies to S100 was done using serum from S100 or S100-MAA injected animals. As shown in Figure 3 (panel A) serum antibodies to S100 were increased 5 fold (P < 0.001) in the S100-MAA animals over control S100 animals. When these same sera were screened using S100-MAA as the antigen, a dramatic increase in antibody concentration was observed, indicating antibodies to the S100, MAA and S100-MAA epitopes were initiated. These antibody concentrations were then compared to the pathology scores and found to correlate (0.898, P=0.04) with the severity of the liver disease (Figure 3, panel B). To begin examining the specific proteins to which these antibodies may bind serum from S100-MAA immunized mice were evaluated using immunoblot procedures. Figure 4 shows serum antibodies from S100-MAA mice reacting with bands between 60 and 28 kilo daltons. Serum from PB and S100 immunized mice demonstrated no reactivity (data not shown).
Figure 3.
Serum antibody concentrations to S100 following immunization with MAA haptenated S100 (S100-MAA). C57/Bl6 mice were immunized with PB, 100μg S100 or 100μg S100-MAA as described in the Materials and Methods. (Panel A) Serum was screened against the antigens S100 or S100-MAA followed by subtraction of the values from the serum of PB injected mice. Data are expressed as the mean ± S.E.M. of five separate animals. Significantly different from S100 immunized animals, *P <0.001. (Panel B) Correlation of Anti-MAA Antibodies to Pathology scores. Data generated from antibody titers and pathology scores were compared using Pearson Product Moment Correlation test. Correlation Coefficient and P values are the following: (R=0.898, P=0.04).
Figure 4.
Western blot analysis indicating potential self proteins to antibodies from MIAH mice. Serum collected from C57/Bl6 mice immunized with 100μg of S100-MAA was incubated with S100 or S100-MAA antigens. Representative of five separate animals indicating bands between 60 and 28 kd. PB and S100 immunized mice showed no reactivity and thus are not depicted.
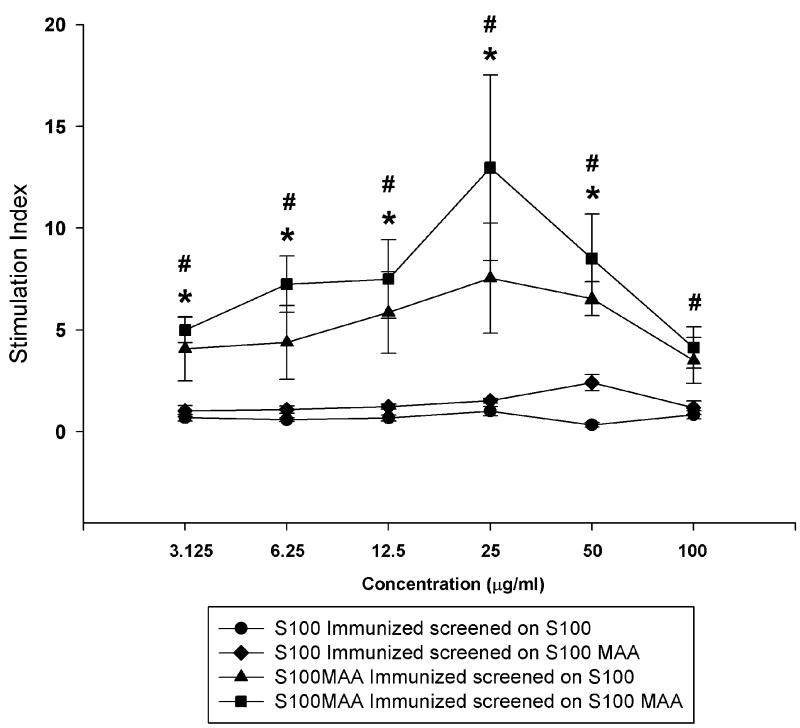
In an effort to begin assessing whether cells of the immune system were involved in these responses, spleen cells from PB, S100 or S100-MAA immunized animals were incubated with S100 or S100-MAA antigens as described in the Materials and Methods. Proliferation assays (Figure 5) showed that cells from the S100 immunized mice showed little or no response as expressed by stimulation index (SI) to S100 or S100-MAA throughout the range of antigens used. In contrast, cells from S100-MAA immunized mice showed SI’s that were significantly elevated throughout the concentrations used compared to SI’s from S100 immunized animals whether the antigen was S100 or S100-MAA. However, it was apparent that the response to S100-MAA was higher at all concentrations of antigens used. Spleen cells from PB mice incubated with S100 or S100-MAA showed no stimulation in these studies. These data suggest that the cellular response is enhanced as either; S100-MAA is processed and presented more efficiently, or there is an additional response to MAA or S100-MAA combination.
Figure 5.
T-cell proliferation in MIAH mice. Spleens from C57/Bl6 mice immunized with PB, 100μg S100 or 100μg S100-MAA as described in the Materials and Methods were collected and incubated with S100 or S100 MAA to determine T-cell proliferation. Data are expressed as the mean ± S.E.M. of the stimulation index (SI) of five separate animals compared to normal animal T-cell proliferation as indicated in the Materials and Methods section. Significantly different from S100 immunized animals, *P ≤ 0.01.
To determine if immune cells from immunized mice would elicit a response in vivo, passive transfer experiments were performed. As shown in Table 1, spleen cells transferred from S100-MAA immunized animals into naïve animals demonstrated over a 2 fold increase in ALT levels compared to naïve animals immunized with S100 or normal liver T-cells. Pathology scores were also increased in the S100-MAA immunized animals. These data strongly suggest that immune cells were involved in the response to self-liver proteins to initiate this autoimmune hepatitis model.
Table 1. Passive Transfer of T Cells From MAA-Induced Autoimmune-Like Hepatitis Mice.
C57/Bl6 mice were injected with PB, 100μg S100 or 100μg S100-MAA. Spleens were extracted and spleenocytes activated for 48 hours with Con A. Activated spleen cells were then injected into naïve mice weekly for 3 weeks. AST and pathology scores were collected as described in Materials and Methods.
| Lymphocytes injected into naïve mice from mice immunized with the following antigens | Alanine aminotransferase levels | Pathology score |
|---|---|---|
| No immunization | 40 ± 5 | 0 |
| S100 control | 42 ± 7 | 0 |
| S100-MAA | 122 ± 34 | 1.5 ± 0.5 |
Data are expressed as the mean ± S.E.M. of five separate animals. Significantly different from S100 immunized or unimmunized animals,
P ≤ 0.05.
Typically, increased cytokine production has been associated with alcohol induced liver diseases (Tiegs, 1997; Tilg and Diehl, 2000). To determine the role of cytokines in this study whole liver homogenates were isolated from PB, S100 or S100-MAA immunized mice and assayed for the presence of common inflammatory cytokines IL-1β, TNF-α, and IFN-γ. Data obtained from the control unimmunized mouse livers was subtracted from S100 or S100-MAA and expressed in pg/ml. As shown in Figure 6, IL-1β was increased from 137.3 pg/ml to 343.56 pg/ml (P = 0.042) in the S100-MAA immunized mice compared to S100 immunized mice. Also, TNF-α was increased from 150.51 pg/ml to 388.21 pg/ml (P = 0.023) over the S100 controls. IFN-γ was increased from 2370.06 pg/ml to 4830.95 pg/ml (P = 0.024) over the S100 controls.
Figure 6.
Release of inflammatory cytokines in liver homogenates from MIAH mice. Liver homogenates from C57/Bl6 mice immunized with PB, 100μg S100 or 100μg S100-MAA as described in the Materials and Methods were collected and analyzed for cytokine release. Serum levels from PB immunized mice were subtracted from the values for S100 or S100 MAA injected mice. Cytokines evaluated were IL-1β, TNF-α, and IFN-γ. Data are expressed as the mean ± S.E.M. of five separate animals. Significantly different from S100 immunized animals, *P ≤ 0.001.
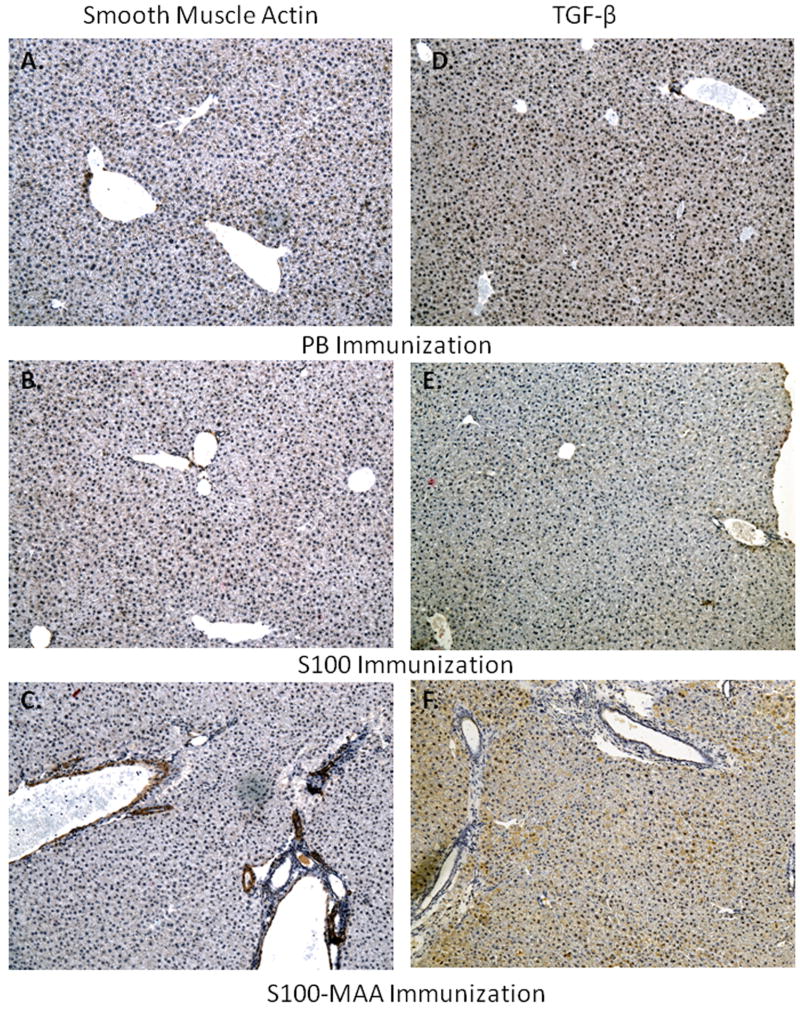
To further examine damage to the liver, paraffin sections were stained for smooth muscle actin (SMA). Figure 7 shows a strong indication of SMA in the S100-MAA (panel C) liver sections from immunized animals. Evidence of this staining is not present in the PB (panel A) or control S100 (panel B) liver sections. There appears to be localization around the portal veins directly behind the endothelial lining, indicative of deposition in these sites following activation of hepatic stellate cells. The secretion of the cytokine TGF-β is known to start the fibrotic process in liver disease or injury. Paraffin embedded liver sections from mice immunized with S100 or S100-MAA was stained for the presence of TGF-β using immunohistochemical techniques. As shown in Figure 7 (panel F), there is an increase in the staining as compared to the PB (panel D) or control S100 immunized (panel E) mouse liver sections. Similar to that observed with SMA, this staining is strongest around the portal veins and areas of damage, and then diffuses out in to the liver parenchyma.
Figure 7.
Smooth muscle actin (SMA) or TGF-β staining of livers from MIAH mice. C57/Bl6 mice were injected with PB, 100μg S100 or 100μg S100-MAA. Liver tissue was sectioned and stained for the presence of SMA or TGF-β and evaluated using a 200x power objective. (Panel A and D) normal mouse liver tissue. (Panel B and E) S100 immunized mice. (Panel C and F) S100-MAA immunized mice. Data is a representative of five separate animals.
Liver damage was also assessed by staining for collagen accumulation using Sirius red. As shown in Figure 8, little to no collagen is present in the PB or S100 immunized mouse livers following Sirius red staining. However, S100-MAA immunized mouse livers have an abundant amount of collagen deposition between the portal tracts.
Figure 8.
Sirius Red staining of livers from MIAH mice. C57/Bl6 mice were injected with PB, 100μg S100 or 100μg S100-MAA. Liver tissue was sectioned and stained for the presence collagen using Sirius red and fast green and evaluated using a 200x objective. Data is a representative of four separate animals.
Discussion
The immune system has been suggested to play a role in the onset/progression of alcoholic liver disease (ALD). However, the mechanism(s) as to how these immune responses are initiated and their relationship to the development of autoimmune liver disease are still under investigation. Studies reported in this manuscript demonstrate that aldehyde modified proteins (alcohol-derived) can abrogate immunologic tolerance, to induce an autoimmune-like liver disease that may be involved in the development and/or progression of ALD.
Previous studies have demonstrated that soluble exogenous proteins modified with reactive aldehydes induce antibody and T cell responses (Abraham et al., 1995; Thiele et al., 1998; Wuttge et al., 1999). The metabolic break down products of ethanol, (AA) (Lin et al., 1990; Ma et al., 2005; Wehr et al., 1993) and lipid peroxidation (MDA) (Chedid et al., 1994; Kurien and Scofield, 2008; Leitinger, 2008; Stewart et al., 2004; Wallberg et al., 2007) have been shown to modify (adduct) proteins and initiate immune responses. We have reported that these reactive aldehydes are capable of combining in a synergistic manner (MAA adduct) to modify proteins and result in the immune system responding as if they were foreign (Rolla et al., 2000; Thiele et al., 1998). Also, the combination of low concentrations of AA and MDA on a non-self protein(s) to form MAA-protein adducts have proven to increase; 1) Cytokine secretion, 2) Cell damage, 3) Fibronectin secretion, and 4) Antibody and T-cell responses (Duryee et al., 2004a; Thiele et al., 2005; Thiele et al., 1998; Willis et al., 2004; Willis et al., 2003). Importantly, the immune responses to MAA-modified proteins have been demonstrated to occur without the use of adjuvants.
Studies reported in this manuscript sought to determine whether, immunization of mice with MAA-modified self-proteins from syngeneic liver in the absence of adjuvant induces an autoimmune-like hepatitis. To accomplish these studies mice were injected with low doses of syngeneic cytosolic fractions of a liver homogenate (S100) modified in vitro with MAA (S100-MAA). In order to assess the level of liver damage caused by immune responses, a pathologist scored the changes in the liver of mice from each group. For example, liver histology and AST/ALT enzyme levels were evaluated and found to be significantly increased in the S100-MAA immunized animals when compared to controls, indicating that tissue damage had been initiated. The fact that the pathology scores significantly correlated with elevated hepatocellular enzyme release, strongly supports the development of pathological changes.
In order to determine whether the immune system was involved in these changes, the presence of circulating antibodies specific for self-proteins was evaluated (Cook, 1998; Czaja, 2008; Duryee et al., 2004b; Laskin et al., 1990; Thiele et al., 2004). Mice immunized with S100-MAA had significantly increased antibody concentrations to unmodified cytosolic liver proteins. It is important to note that these antibodies were generated in the absence of adjuvant utilizing only 100 μg of MAA modified cytosol, as compared to other protocols using 2.5 mg of cytosol in Freunds complete adjuvant. Additionally, these antibodies to cytosolic proteins were found to correlate with the liver pathology scores indicating that antibodies may be involved in the disease process. In previous studies, a dose response was used to determine how much S100-MAA should be administered and for how long. The use of 100 μg gave a reproducible response within a 3-4 week period. It is thought this level was justified as others have shown in models using cytosols that lose their immunogenicity in as little as 24-48 hours (Lohse et al., 1998). In order to modify the cytosol with MAA, it was necessary to incubate with MDA and AA for 16 hours. More than likely this decreases the antigenicity of the cytosol which results in the need for higher levels of protein. Also, the use of 2 mM MDA and 1 mM AA is warranted in order to modify the cytosol with MAA in the shortest amount of time as possible to maintain its antigenicity. The cytosol will modify with lower concentrations of MDA and AA to the same level as when 2 mM MDA and 1mM AA are used, but it takes 2-3 days for this too occur, which would not allow for the retention of the cytosol antigenicity.
It is possible that cytosols are not the best materials for inducing these responses. This is, membrane fractions or other soluble proteins may be better targets. However, this study was designed to determine whether an autoimmune response could be generated, and most of the traditional models of autoimmune hepatitis uses cytosols as the antigen source (Lohse et al., 1990). Future experiments will focus on isolated macromolecules that have been shown to be MAA-modified in vivo, and may result in the use of lower concentrations of these modified proteins.
For example the use of serum from S100-MAA immunized mice in Western Blots using cytosolic proteins showed reactivity to a number of proteins between 60 and 28 kd. Preliminary protein sequencing data identified the heat shock protein binding immunophilin, heat shock protein precursor, vascular endothelial growth factor C precursor, cytochrome P450 3A25, and TGF-Beta receptor type II precursor as candidate self-proteins involved in this response (data not shown). Reports show that heat shock proteins can be modified with 4-hydroxynonenal (4-HNE) or methylglyoxal, and are related to disease states in the retina (Chen et al., 2009), ALD (Carbone et al., 2005), and liver endothelial cells in diabetes (Schalkwijk et al., 2006). Heat shock proteins have also been shown to be involved in the immune response by increasing the release of cytokines (Basu et al., 2000) and activation of dendritic cells (Lumeng and Lin, 1991; Nicholls et al., 1992). Modification of these proteins could explain how MAA-modified proteins are; internalized by dendritic cells, processed and presented, and eventually induce antibody and T-cell responses. Therefore, multiple self-antigens have been identified through this study that could be potential targets for the immune system and will be the focus of future studies.
Typically, liver damage initiated by autoimmune mechanism(s) has been characterized by an infiltration of immune cells into an area by an increase in cytokine secretion by Kupffer, endothelial, and stellate cells (Cook, 1998; Thiele et al., 2004). Mice injected with S100-MAA showed increases in an in vitro cellular proliferation assay when incubated with S100 alone. Involvement of reactive immune cells provides a possible mechanism by which S100 self antigens are processed and presented to stimulate T helper cell responses to aid in the production of B cell differentiation and antibody production. Passive transfer of primed immune cells from S100-MAA immunized animals caused an increase in ALT levels and pathology scores indicating these are partly responsible for the development of this liver damage. Further studies are under investigation to evaluate the role of CD4+ (Th1, Th2, Treg, Th17) and CD8+ T cells in this response. Preliminary dat suggest a strong role for Th17 and Tregs in response to MAA modified proteins. Also, that dendritic cells play a predominate role in the generation of these cells (data not shown).
Release of cytokines in the liver is an indication of a local hepatic immune responses (Cook, 1998; Thiele et al., 2004). Liver homogenates from S100 MAA immunized mice demonstrated an increase in IL-1β, TNF-α, and IFN-γ as compared to homogenates from S100 injected mice, all of which are pro-inflammatory cytokines responsible for the recruitment of immune cells (CD4+, CD8+, macrophages). These cytokines have been shown to be involved in the immunological responses following treatment of MAA-modified proteins (Duryee et al., 2004a; Willis et al., 2004) and are directly related to what has been observed in the progression of ALD.
As mentioned above an increase in the pathology score was observed in the livers of S100-MAA injected mice compared to the unimmunized or S100 injected mice. Lymphocyte clusters around the portal veins with some plasma cells were reported that formed around the portal veins. These clusters may be explained by the ability of sinusoidal liver endothelial cells (SECs), kupffer cells and/or stellate cells to bind MAA modified proteins, up-regulate their adhesion molecules, and increase their secretion of cytokines following incubation with MAA modified proteins (Sacanella and Estruch, 2003; Willis et al., 2003). It is reasonable to hypothesize that MAA modified self-liver proteins are taken up by antigen presenting cells through scavenger mechanisms, traffic to the lymphatic system, initiate antibody and T-cell responses, return and bind to cells of the liver and damage the tissue around the portal veins. Additionally, SECs can release fibronectin EIIIA in response to MAA-modified proteins (Thiele et al., 2005). This release may initiate the fibrogenic process by stellate cells which would release smooth muscle actin (SMA) resulting in an increase in the wound healing type of processes. Livers from S100-MAA immunized mice showed increased levels of SMA and collagen by immunohistochemistry strongly supporting this scenario was initiated in these livers. Also of interest is the positive staining for TGF-β in the S100-MAA immunized animal livers. Increases in TGF-β can activate stellate cells to release collagen which could exacerbate the wound healing responses in the liver and lead to fibrosis and/or cirrhosis (Tsukamoto et al., 1990). Thus, these data further support the concept that S100-MAA injection results in the deposition of collagen between the portal tracts and may play a role in cirrhosis.
While the underlying mechanism(s) of alcoholic liver disease is not clear, these studies provide a potential model to evaluate how the metabolites of ethanol and lipid peroxidation modify self-proteins to induce hepatitis. While the damage observed in these animals was significant, they did not become morbid nor was any mortality seen following these immunizations. This would suggest that additional factors are involved in end stage liver failure. Therefore, more work is needed to identify cofactors such as lipopolysacharide (LPS), genetic polymorphisms, or other toxins which result in ALD in humans. This preclinical model offers the ability to; identify and quantify specific other cofactors, identify and isolate specific self protein epitopes, and define specific immune pathways utilizing the alcohol induced autoimmune hepatitis model.
Acknowledgments
Supported by: National Institutes of Health Grants R01 AA10435, R37 AA07818, and R21 AA15505-01A2. Also supported by the Department of Veterans Affairs National Merit Review Program and the Department of Internal Medicine at the UNMC.
Abbreviations used in this paper
- MIAH
MAA Induced Autoimmune Hepatitis
- MDA
Malondialdehyde
- AA
Acetaldehyde
- ALD
Alcoholic Liver Disease
- SMA
Smooth muscle actin
- S100
cytosol
- MAA
Malondialdehyde-acetaldehyde adduct
Footnotes
All work was performed in the Experimental Immunology Laboratory at the Omaha Veterans Administration Medical Center, Research Services 151, 4101 Woolworth Avenue, Omaha, NE 68105.
Conflict of Interests/disclosures: There are no conflicts of interest to disclose for any of the authors in this manuscript.
References
- Abraham R, Choudhury A, Basu SK, Bal V, Rath S. Disruption of T cell tolerance by directing a self antigen to macrophage-specific scavenger receptors. J Immunol. 1997;158:4029–4035. [PubMed] [Google Scholar]
- Abraham R, Singh N, Mukhopadhyay A, Basu SK, Bal V, Rath S. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J Immunol. 1995;154:1–8. [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- Chedid A, Chadalawada KR, Morgan TR, Moritz TE, Mendenhall CL, Hammond JB, Emblad PW, Cifuentes DC, Kwak JW, Gilman-Sachs A, et al. Phospholipid antibodies in alcoholic liver disease. Hepatology. 1994;20:1465–1471. doi: 10.1002/hep.1840200614. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Chen Y, Sternberg P, Cai J. Phosphatidylinositol 3 kinase pathway and 4-hydroxy-2-nonenal-induced oxidative injury in the RPE. Invest Ophthalmol Vis Sci. 2009;50:936–942. doi: 10.1167/iovs.08-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2008;24:298–305. doi: 10.1097/MOG.0b013e3282f57268. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004a;28:1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, Thiele GM. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Biosci. 2004b;9:3145–3155. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- French SW, Tsukamoto H. Animal models for alcoholic liver disease. Hepatology. 1989;10:898–899. doi: 10.1002/hep.1840100529. [DOI] [PubMed] [Google Scholar]
- Howell CD, Yoder TD. Murine experimental autoimmune hepatitis: nonspecific inflammation due to adjuvant oil. Clin Immunol Immunopathol. 1994;72:76–82. doi: 10.1006/clin.1994.1109. [DOI] [PubMed] [Google Scholar]
- Israel Y, Hurwitz E, Niemela O, Arnon R. Monoclonal and polyclonal antibodies against acetaldehyde-containing epitopes in acetaldehyde-protein adducts. Proc Natl Acad Sci U S A. 1986;83:7923–7927. doi: 10.1073/pnas.83.20.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda H, Sekiya C, Kanai M, Yoshida Y, Uede T, Kikuchi K, Namiki M. Flow cytometric and functional analysis of mononuclear cells infiltrating the liver in experimental autoimmune hepatitis. Clin Exp Immunol. 1990;82:473–478. doi: 10.1111/j.1365-2249.1990.tb05474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin CA, Vidins E, Blendis LM, Soloninka CA. Autoantibodies in alcoholic liver disease. Am J Med. 1990;89:129–133. doi: 10.1016/0002-9343(90)90288-o. [DOI] [PubMed] [Google Scholar]
- Leitinger N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: evidence from animal models and in humans. Subcell Biochem. 2008;49:325–350. doi: 10.1007/978-1-4020-8830-8_12. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lin RC, Lumeng L, Shahidi S, Kelly T, Pound DC. Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol Clin Exp Res. 1990;14:438–443. doi: 10.1111/j.1530-0277.1990.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Lohse AW, Dienes HP, Meyer zum Buschenfelde KH. Suppression of murine experimental autoimmune hepatitis by T-cell vaccination or immunosuppression. Hepatology. 1998;27:1536–1543. doi: 10.1002/hep.510270611. [DOI] [PubMed] [Google Scholar]
- Lohse AW, Manns M, Dienes HP, Meyer zum Buschenfelde KH, Cohen IR. Experimental autoimmune hepatitis: disease induction, time course and T-cell reactivity. Hepatology. 1990;11:24–30. doi: 10.1002/hep.1840110106. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Lin RC. Formation of a 37 kilodalton liver protein-acetaldehyde adduct in vivo and in liver cell culture during chronic alcohol exposure. Ann N Y Acad Sci. 1991;625:793–801. doi: 10.1111/j.1749-6632.1991.tb33921.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Meregalli M, Hodges S, Davies N, Bogdanos DP, Fargion S, Fiorelli G, Vergani D. Alcohol dehydrogenase: an autoantibody target in patients with alcoholic liver disease. Int J Immunopathol Pharmacol. 2005;18:173–182. doi: 10.1177/039463200501800118. [DOI] [PubMed] [Google Scholar]
- Nicholls R, de Jersey J, Worrall S, Wilce P. Modification of proteins and other biological molecules by acetaldehyde: adduct structure and functional significance. Int J Biochem. 1992;24:1899–1906. doi: 10.1016/0020-711x(92)90285-9. [DOI] [PubMed] [Google Scholar]
- Niemela O, Juvonen T, Parkkila S. Immunohistochemical demonstration of acetaldehyde-modified epitopes in human liver after alcohol consumption. J Clin Invest. 1991;87:1367–1374. doi: 10.1172/JCI115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoku DB, Talor MV, Fairweather D, Frisancho-Kiss S, Odumade OA, Rose NR. A novel model of drug hapten-induced hepatitis with increased mast cells in the BALB/c mouse. Exp Mol Pathol. 2005;78:87–100. doi: 10.1016/j.yexmp.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Paronetto F. Immunologic reactions in alcoholic liver disease. Semin Liver Dis. 1993;13:183–195. doi: 10.1055/s-2007-1007348. [DOI] [PubMed] [Google Scholar]
- Peters MG. Animal models of autoimmune liver disease. Immunol Cell Biol. 2002;80:113–116. doi: 10.1046/j.0818-9641.2001.01059.x. [DOI] [PubMed] [Google Scholar]
- Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Arico S, Sartori M, Bellomo G, Klassen LW, Thiele GM, Tuma DJ, Albano E. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31:878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- Sacanella E, Estruch R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict Biol. 2003;8:371–378. doi: 10.1080/13556210310001656376. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006;580:1565–1570. doi: 10.1016/j.febslet.2006.01.086. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Stewart SF, Vidali M, Day CP, Albano E, Jones DE. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39:197–203. doi: 10.1002/hep.20021. [DOI] [PubMed] [Google Scholar]
- Terabayashi H, Kolber MA. The generation of cytotoxic T lymphocytes against acetaldehyde-modified syngeneic cells. Alcohol Clin Exp Res. 1990;14:893–899. doi: 10.1111/j.1530-0277.1990.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Duryee MJ, Freeman TL, Sorrell MF, Willis MS, Tuma DJ, Klassen LW. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593–1600. doi: 10.1016/j.bcp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22:1731–1739. [PubMed] [Google Scholar]
- Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastroenterol Belg. 1997;60:176–179. [PubMed] [Google Scholar]
- Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Gaal K, French SW. Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: status report. Hepatology. 1990;12:599–608. doi: 10.1002/hep.1840120325. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- Wallberg M, Bergquist J, Achour A, Breij E, Harris RA. Malondialdehyde modification of myelin oligodendrocyte glycoprotein leads to increased immunogenicity and encephalitogenicity. Eur J Immunol. 2007;37:1986–1995. doi: 10.1002/eji.200636912. [DOI] [PubMed] [Google Scholar]
- Wehr H, Rodo M, Lieber CS, Baraona E. Acetaldehyde adducts and autoantibodies against VLDL and LDL in alcoholics. J Lipid Res. 1993;34:1237–1244. [PubMed] [Google Scholar]
- Willis MS, Klassen LW, Carlson DL, Brouse CF, Thiele GM. Malondialdehyde-acetaldehyde haptenated protein binds macrophage scavenger receptor(s) and induces lysosomal damage. Int Immunopharmacol. 2004;4:885–899. doi: 10.1016/j.intimp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Willis MS, Thiele GM, Tuma DJ, Klassen LW. T cell proliferative responses to malondialdehyde-acetaldehyde haptenated protein are scavenger receptor mediated. Int Immunopharmacol. 2003;3:1381–1399. doi: 10.1016/S1567-5769(03)00136-X. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self proteins. Immunology. 1999;98:273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology. 1998;115:686–692. doi: 10.1016/s0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]
- Xu D, Thiele GM, Kearley ML, Haugen MD, Klassen LW, Sorrell MF, Tuma DJ. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem Res Toxicol. 1997;10:978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]