Abstract
Iron deficiency anemia (IDA) is a frequent disorder. Also, it may be a sign of underlying serious diseases. Iron deficiency points to an occult or frank bleeding lesion when occurred in men or postmenopausal women. In this study, we aimed to evaluate the diagnostic yield of endoscopy in patients with IDA and to define predictive factors of gastrointestinal (GI) lesions causing IDA. Ninety-one patients (77 women, 14 men; mean age: 43 years) who were decided to have esophago-duodenoscopy and/or colonoscopy for iron deficiency anemia were interviewed and responded to a questionnaire that included clinical and biochemical variables. The endoscopic findings were recorded as GI lesions causing IDA or not causing IDA. Endoscopy revealed a source of IDA in 18.6 % of cases. The risk factors for finding GI lesions causing IDA were as follows: male gender (p= 0.004), advanced age (> 50 years) (p= 0.010), weight loss (over 20% of total body weight lost in last 6 month) (p= 0.020), chronic diarrhea (p= 0.006), change of bowel habits (p= 0.043), epigastric tenderness (p= 0.037), raised carcinoembryonic antigen (CEA) level (normal range: 0-7 ng/mL) (p= 0.039), < 10 gr/dl hemoglobin (Hb) level (p=0.054). None of these risk factors had been present in 21 (23%) women younger than 51 years. In this group, no patient had any GI lesion likely to cause IDA (negative predictive value= 100%). In multivariate analysis, advanced age (p=0.017), male gender (p< 0.01) and weight lost (p=0.012) found that associated with GI lesions in all patients. It may be an appropriate clinical approach to consider these risk factors when deciding for gastrointestinal endoscopic evaluation in iron deficiency anemia.
Keywords: Iron deficiency anemia, gastrointestinal lesions, predictive risk factors, endoscopic investigation.
Introduction
Iron deficiency anemia (IDA) remains the most common cause of anemia and affects about 5-12% of non-pregnant women and 1-5% of men have IDA 1-2. It is a result of blood loss from the gastrointestinal tract or the uterus and is a requiring further investigation due to sign of serious underlying disease. While menstrual blood loss is the commonest cause of IDA in pre-menopausal women, blood loss from the gastrointestinal (GI) tract is the commonest cause in adult men and post-menopausal women 3-6.
Laboratory tests used to make the diagnosis have not changed in many decades, their interpretation has, and this is possibly due to the availability of extensive testing in key populations. A loss of 10 ml of blood per day is usually required for a positive based fecal occult blood test (FOBT), although FOBT positivity is highly dependent on the locus of the bleeding source. Bleeding lesions in the GI tract are identified in about 50% of patients with IDA 7-8. Laboratory findings in IDA include elevated total iron-binding capacity (TIBC), low transferrin saturation, and low serum iron level 9. Those with a mixed diagnosis (an addition vitamin B12, folic acid deficiency or chronic disease anemia), the use of transferrin saturation in the diagnosis of IDA have been discouraged 9. When the diagnosis remains ambiguous after laboratory results are analyzed, a bone marrow biopsy should be considered in order to make a definitive diagnosis. The absence of stainable iron is the “gold standard”, for diagnosis of IDA. Marrow examination shows, in addition to the absence of hemosiderin iron, a decrease in the proportion of sideroblasts, because too little iron is available to support siderotic granule formation.
Lower and upper GI tract evaluation is recommended to diagnose the cause of IDA, particularly in men >50 and in post-menopausal women, in whom IDA is suspected to occur from a bleeding lesion. GI evaluation can be endoscopic and radiographic. Asymptomatic colonic and gastric carcinoma may present with IDA and exclusion of these conditions is of prime concern. The upper endoscopic evaluation should include random gastric antral and fundic biopsies in addition to duodenal biopsies in order to assess the histological changes of atrophic gastritis and celiac disease 10. Upper GI endoscopy can be expected to reveal a cause in between 30 and 50% of patients. Small bowel biopsies should be taken during this endoscopy as 2-3% of patients presenting with IDA have coeliac disease 3-6, 11. Iron deficiency anemia is considered as an alarm sign for the presence of possible GI malignancies, and inadequate evaluation of patients with IDA may delay the diagnosis of GI tumors especially colorectal cancer 12.
In this study, we aimed to evaluate the diagnostic yield of endoscopy in patients with IDA and to define predictive factors of gastrointestinal (GI) lesions causing IDA and identify clinical and biochemical variables that predict the outcome of upper/lower endoscopy in outpatients with iron deficiency anemia. The aim of our study was to investigate the incidence of GI pathological findings in symptomatic and asymptomatic patients with IDA and to identify the predictive factors for such lesions.
Patients and Methods
From March 2006 to July 2007, 91 patients who visited our hematology or gastroenterology out-patient clinics with a diagnosis of IDA were consecutively enrolled into the present study after patient consent was obtained. Our study is prospective.
The criteria for enrollment were as follows:
Hemoglobin concentration ≤13 g/dl for men and ≤12 g/dl for women.
Age > 18 years.
With at least one of the following laboratory values consistent with iron deficiency: a serum iron concentration < 10 µg/ml with a transferrin saturation ≤ 20 percent, mean corpuscular volume (MCV) < 80 fL and a serum ferritin concentration ≤ 30 ng/ml.
No other associated disease that could contribute to anemia other than iron deficiency.
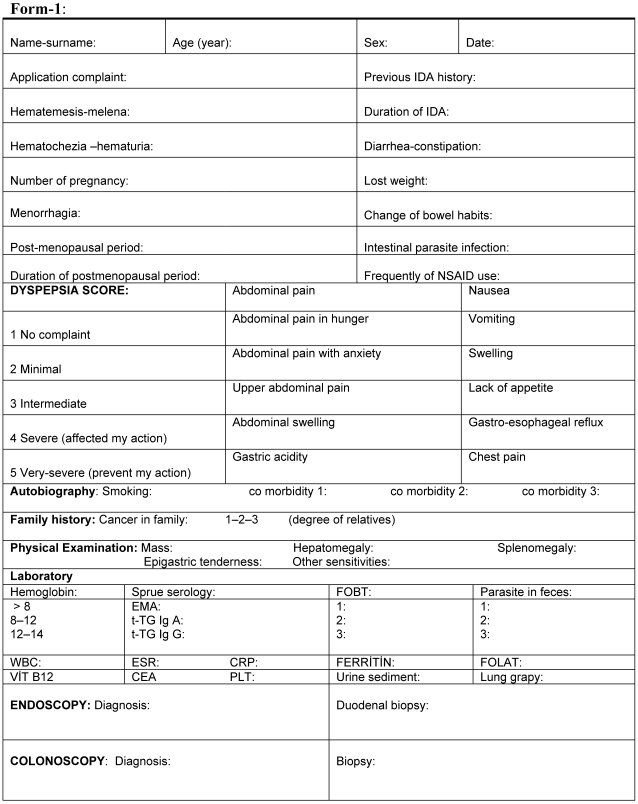
All patients were interrogated and examined according to Form-1 in Figure 1. This form developed by us for this study. The presence of dyspeptic complaint and its severity calculated by presence of abdominal pain, abdominal pain with hungry and anxiety, abdominal distension, nausea, vomiting, poor appetite and symptoms of gastroesophageal reflux. All patients were graded 1 to 5 for these symptoms. Dyspepsia score of patients were minimally 12 and maximally 60. The patients investigated previous smoking history, coronary artery disease, diabetes mellitus and malignancy history in their family.
Figure 1.
Form-1 used in patients.
Statistical Analysis
Data files were analyzed initially with Access and SPSS (version 13.0). Chi-square (x2) tests were performed to determine whether the clinical and biochemical variables were associated with a GI lesion. A multivariate analysis was applied to identify variables significantly related with the outcome of the GI lesions. Multiple analyses were performed with Cox regression analysis. P < 0.05 was considered significant in statistical analysis.
Results
Ninety-one patients fulfilled the entry criteria and were enrolled. Their mean age was 43.3 (19-81) years. 71 were patient aged under 50 and 20 were over 50 years. 77 were female and 14 were male. Sixty-six of women were pre-menopausal and 11 were post-menopausal. Presence or absence of GI symptoms was evaluated in every patient. Table 1 describes the frequency predictive signs for possible gastrointestinal lesions in iron deficiency anemia patients.
Table 1.
Frequency predictive signs for possible gastrointestinal lesions in iron deficiency anemia patients.
| Yes (%) | No (%) | |
|---|---|---|
| Hematemesis | 0 (0) | 91 (100) |
| Melena | 4 (4.4) | 87 (95.6) |
| Hematochezia | 8 ( 8.8) | 83 (91.2) |
| Hematuria | 2 ( 2.2) | 89 (97.8) |
| Menorrhagia | 20 (30.7) | 46 (69.7) |
| Diarrhea | 3 (3.3) | 88 (96.7) |
| Constipation | 39 (42.9) | 52 (57.1) |
| Change of bowel habits | 5 (5.5) | 86 (94.5) |
| Lost weight | 4 (4.4) | 87 (95.6) |
| Frequently of NSAID1 use | 3 (14.3) | 88 (96.7) |
| Intestinal parasite infection | 7 (7.7) | 84 (92.3) |
| Previous IDA2 history | 45 (49.5) | 46 (50.5) |
| Smoking | 23 (25.3) | 68 (74.7) |
| Cancer in first degree relatives | 22 (24.2) | 69 (75.8) |
| Cancer in family | 28 (30.7) | 63 ( 69.3) |
1Nonsteroidal anti-inflammatory drugs, 2Iron deficiency anemia
Clinically, significant predictive signs for possible gastrointestinal lesions were demonstrated in 11 patients. 8 patients had hematochezia and 4 had melena. Only 2 patients had hematuria, 39 had constipation, 3 had diarrhea. 45 patients had been found to be iron deficiency anemia previously. 20/66 pre-menopausal women had heavy menstrual bleeding. 28/91 patients had cancer in their family. At admission, significant physical examination findings of 91 patients; 2 had hepatomegaly, 1 had splenomegaly and 8 had epigastric sensitivity (Table 2).
Table 2.
Significant physical examination findings in iron deficiency anemia patients
| Yes (%) | No (%) | |
|---|---|---|
| Hepatosplenomegaly | 3 (3.3) | 88 (96.7) |
| Abdominal mass | 0 (0) | 91 (100) |
| Epigastric sensitivity | 8 (8.8) | 83 (91.2) |
18 of 89 patients had fecal occult blood test positive, 6 patients had parasite in feces, 7 had microscopic hematuria and 3 had positive sprue serological (antiendomysium antibodies IgA and tissue transglutaminase antibodies). 55 patients had no additional systemic disease, 13 patients had thyroid diseases (8 had hypothyroidism, 5 had hyperthyroidism), 9 patients had diabetes mellitus (7 had diabetes mellitus type 2, 2 had diabetes mellitus type 1), 8 patients had hypertension, 3 had coronary artery disease, 2 had collagen tissue disease, 2 had immune thrombocytopenic purpura, 2 had hypophysial adenoma and 1 had Parkinson disease (Table 4). Table 5 shows biochemical characteristics of patients. Their mean hemoglobin level was 10.2 g/dl (range 6.4-12.7), mean white blood cell count was 7095 l/mm3 (range 3100-16900), mean platelet count was 326x103/mm3 (range 74-669), mean ferritin level was 7.5 ng/ml (range 1.38-28).
Table 4.
The additional systemic disease in IDA patients
| Patients number | % | |
|---|---|---|
| Absent | 55 | 60.4 |
| Thyroid diseases | 13 | 14.2 |
| Diabetes Mellitus | 9 | 9.8 |
| Hypertension | 8 | 8.8 |
| Coronary artery disease | 3 | 3.2 |
| Collagen tissue disease | 2 | 2.1 |
| Immune thrombocytopenic purpura | 2 | 2.1 |
| Hypophysial adenoma | 2 | 2.1 |
| Parkinson disease | 1 | 2.1 |
Table 5.
Biochemical variables of patients with iron deficiency anemia
| Patients Number | Mean | Range | Normal lab. range | |
|---|---|---|---|---|
| Hb1 (gr/dl) | 91 | 10.2 | 6.4-12.7 | 12-14/women 14-15/men |
| WBC2 (l/mm3) | 91 | 7095 | 3100-16900 | 4.8-10.8 |
| Plt3 (x103/mm3) | 91 | 326 | 74-669 | 150-400 |
| Ferritin (ng/ml) | 91 | 7.5 | 1.38-28 | 10-291/women 22-322/men |
| CRP4 (gr/dl) | 83 | 0.66 | 0.1-7.6 | 0-5 |
| ESR5 (mm/h) | 80 | 17.2 | 2-75 | 0-20 |
| CEA6 (ng/ml) | 77 | 3.4 | 0.25-97 | 0-7 |
1Hemoglobin, 2White blood cell, 3Platelets, 4C reactive protein, 5Erythrocyte sedimentation rate, 6Carcinoembryonic antigen
86 patients underwent upper gastrointestinal tract endoscopies and 62 patients underwent upper and lower gastrointestinal tract endoscopies. An upper GI finding, mainly antral gastritis was the most common pathologic finding (n=23, 26.7 %). The abnormalities considered as possible causes of upper gastrointestinal lesions were Helicobacter pylori (HP) gastritis (n=18), duodenitis (n=12), pangastritis (n=11), coeliac disease (n=3), gastric ulcer (n=2), duodenal ulcer (n=2), erosive gastritis (n=1) and gastric tumor (n=1). The lower gastrointestinal tract lesions regarded as possible causes of IDA included hemorrhoid (n=19), chronic colitis (n=2), inflammatory intestinal disease (n=2), interstitial colitis (n=1) and colorectal cancer (n=1) (Table 6).
Table 6.
Pathological conditions of the GI tract in iron deficiency anemia patients
| Diagnosis | Frequency | Result/Number of process, (%) |
|---|---|---|
| Non-diagnostic | 12 | 12/86, (13.9) |
| Antral gastritis | 23 | 23/86, (26.7) |
| Hemorrhoid | 19 | 19/66, (28.7) |
| H.1 pylori gastritis | 18 | 18/86, (20.9) |
| Duodenitis | 12 | 12/86, (13.9) |
| Pangastritis | 11 | 11/86, (12.7) |
| Anal fissure | 5 | 5/66, (7.5) |
| Colonic polyp | 4 | 4/66, (6.0) |
| Diverticulitis | 3 | 3/66, (4.5) |
| Coeliac disease | 3 | 3/86, (3.4) |
| Gastric ulcer | 2 | 2/86, (2.3) |
| Duodenal ulcer | 2 | 2/86, (2.3) |
| Chronic colitis | 2 | 2/66, (3.0) |
| IID2 | 2 | 2/66, (3.0) |
| Atrophic gastritis | 2 | 2/86, (2.3) |
| Interstitial colitis | 1 | 1/86, (1.1) |
| Gastric polyp | 1 | 1/86, (1.1) |
| Erosive gastritis | 1 | 1/86, (1.1) |
| Gastric cancer | 1 | 1/86, (1.1) |
| Colonic cancer | 1 | 1/66, (1.5) |
1Helicobacter, 2Inflammatory intestinal disease
A list of the upper and lower GI pathological conditions associated with IDA is included in Table 7. The patients were interviewed and responded to a questionnaire that included clinical and biochemical variables. Table 8 is shown that rate of clinically significant lesions in IDA with positively symptoms-sign or laboratory results. The presence of advanced age (>50 years), male gender, diarrhea, lost weight, change of bowel habits, epigastric tenderness, positively serological sprue, hemoglobin levels less than 10 g/dl and high CEA level (>5 pg/ml) were associated with an increased likelihood of significant gastrointestinal lesions (p<0.05); melena, constipation, cancer in first degree relatives, fecal occult test positivity, high C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) level were associated with limited positively findings (p≤ 0.19).
Table 7.
Pathological conditions of the GI tract associated with iron deficiency (Clinically meaningful lesions)
| Patients number | |
|---|---|
| Celiac disease (villous atrophy) | 3 |
| Erosive gastritis | 1 |
| Peptic ulcer | 3 |
| IID*/chronic colitis | 4 |
| Diverticulitis | 3 |
| Gastric cancer | 1 |
| Colon cancer | 1 |
| Familial polyposis | 1 |
| Helicobacter pylori gastritis | 18 |
*Inflammatory intestinal disease (Not: Hemorrhoid did not considerate due to coincidentally lesions.
Table 8.
Rate of clinically significant lesions in IDA with positively symptoms-sign or laboratory results
| Symptoms, sign or laboratory results | Existence of significant lesion | Absence of significant lesion | P value |
|---|---|---|---|
| Age> 50 | 8 | 12 | 0.010 |
| Sex (Male) | 7 | 7 | 0.004 |
| Diarrhea | 3 | 0 | 0.006 |
| Lost weight | 3 | 1 | 0.020 |
| Change of bowel habits | 3 | 2 | 0.043 |
| Epigastric tenderness | 4 | 4 | 0.037 |
| Serological of sprue | 2 | 1 | 0.074 |
| Hb level | 7 | 50 | 0.054 |
| High CEA level | 3 | 2 | 0.039 |
| Melena | 2 | 2 | 0.157 |
| Constipation | 10 | 29 | 0.178 |
| Cancer in first degree relatives | 7 | 15 | 0.112 |
| Fecal occult blood test positiviy | 5 | 11 | 0.178 |
| High CRP level | 3 | 6 | 0.173 |
| High ESR level | 6 | 13 | 0.174 |
| Whatever positively in general evaluation | 17 | 53 | 0.010 |
The risk factors for finding GI lesions causing IDA were as follows: male gender (p= 0.004), advanced age (p= 0.010), weight loss (p= 0.020), chronic diarrhea (p= 0.006), change of bowel habits (p= 0.043), epigastric tenderness (p= 0.037), raised CEA level (p= 0.039), < 10 gr/dl Hb level (p=0.054). None of these risk factors had been present in 21 (23%) women younger than 51 years. In this group, no patient had any GI lesion likely to cause IDA (negative predictive value= 100%). In multivariate analysis, advanced age (p=0.017), male gender (p< 0.01) and weight lost (p=0.012) found that associated with GI lesions in all patients.
In addition, we determine the yield of endoscopy evaluations in pre-menopausal and age < 50 women with iron deficiency anemia but without any clinically significant sign-symptoms and laboratory findings. There were 21 patients had these criteria but none of them had any endoscopic significant lesions.
Discussion
Iron deficiency is the most common hematological disorder encountered in general practice and iron-deficiency anemia is the most frequently cause of anemia worldwide 13. Blood loss is a major cause of iron-deficiency anemia 14. However, the commonest cause of IDA in developing countries is still nutritional deficiency. In some instances, an insufficient supply of iron may contribute to the development of iron deficiency. The consumption of an iron-deficient diet, such as occurs in strict vegans, can deplete iron stores if the diet is adhered to for three or more years in the absence of excessive losses 15.
Iron-deficiency anemia is not a disease itself but a manifestation of an underlying disease, searching for the latter is therefore crucial and may be of far greater importance to the ultimate well-being of the patient than repleting iron stores. This is particularly important, because a large proportion of patients with IDA does not undergo endoscopy or are incompletely evaluated, despite specific guidelines 16-17. These procedures are not cost-effective for each IDA patients. In fact due to economic or practical consideration, not all iron deficiency patients could be fully investigated. And, in 20% of patients with IDA a routine upper and lower GI endoscopy may not ascertain GI cause during hospital admission 18. Cancer was diagnosed in 13.1% and gastrointestinal cancer in 11.2% of patients with IDA. But two studies reported that IDA was one of the predictive factors of colorectal cancer and small intestinal cancer 19-20. The standard procedure for investigating the source of IDA among men and postmenopausal women is to rule out gastrointestinal tract pathology and a nutritional cause 17-18, 21-23. In pre-menopausal women, iron deficiency anemia is common and menstrual flow is often held responsible, but it is not clear whether these women should be submitted to gastrointestinal (GI) evaluation.
Iron deficiency anemia results from chronic occult gastrointestinal bleeding. Endoscopic evaluation of the gastrointestinal tract is commonly performed to evaluate iron deficiency. Most of patients with iron deficiency in, whom gastrointestinal or systemic signs or symptoms are absent have an underlying gastrointestinal lesion 24. Idiopathic iron-deficiency anemia in adults is widely believed to result from chronic colonic blood loss due to mass lesions. A thorough examination of the gastrointestinal tract, particularly the colon, has become standard practice, previously 25-26.
The most of studies shown that substantial gastrointestinal lesions, particularly those of the upper gastrointestinal tract, are common in patients with iron deficiency anemia. Cook et al. 4 shown that 40% of patients had upper GI tract lesions and Kepczyk et al. 3 showed that 55% of patients had upper GI tract lesions. In generally, 41% of patients had upper GI tract lesions 7. In our study, 52 of 91 patients (57%) had upper GI tract conditions. The most common abnormality in the upper gastrointestinal tract was esophagitis, gastritis or duodenitis, gastric ulcer or duodenal ulcer and gastric cancer, in these studies. In our study, most common abnormality in upper gastrointestinal tract was antral gastritis, H. pylori gastritis, duodenitis and pangastritis. Two patients had gastric ulcer, 2 had duodenal ulcer and 1 patient had gastric cancer. The rate of lower gastrointestinal tract abnormality in iron deficiency anemia patients was 13.5-30% in literature 7 and 30% in our study. Cancer was the most common lesion in the colon. However, only one patient had colon cancer in our study. Because of postmenopausal women and men patients' number were higher in literature than our study. For example, Rockey et al. study had 9/100 pre-menopausal women but our study had 66/91 pre-menopausal women patients.
Many of the causes identified in our study, particularly in the upper GI tract have similar treatment. Further, we identified 1 gastric and 1 colon cancer patient in our study. An early gastric cancer was diagnosed on biopsy of a suspicious ulcerated area in a 45-year-old man patient. Partial gastrectomy was successful and remains well. An 82-year-old man was diagnosed adenocarcinoma by endoscopic biopsy. A right hemicolectomy was performed, and the patient had no any metastasis. Three years after surgery, he is alive without any symptoms.
The standard diagnostic procedure for men and postmenopausal women with iron deficiency is to investigate gastrointestinal tract (upper and lower) pathology as well as rule out a nutritional cause 27-28. The diagnostic value of endoscopy was 58% in these conditions 3, 29-32. Endoscopy demonstrated a lesion in 7 of the 11 pre-menopausal women patients and 12 of the 14 men patients; significant risk factors for gastrointestinal lesions in these patients were older age and male sex (p value; 0.010-0.004, respectively). The prevalence of gastrointestinal malignancy was 6-23% in these group patients 24, 28-29, 3 but only two men patients (2 of the 14 patients) had gastrointestinal malignancy.
The ability to predict the site of GI lesions that cause IDA could optimize the endoscopic approach. But, the previous studies have found that symptoms and signs are poor indicators of the site of lesions causing IDA and, thus, are not helpful in choosing appropriate investigative tests 34-35. Capurso et al. suggested that accurate initial assessment of patient characteristics, clinical history, and certain laboratory data may guide the choice of which endoscopic investigation to perform first in patients with IDA, thereby, potentially reducing the frequency of negative findings. By using multiple logistic regression analysis, no statistically significant risk factor for the presence of upper-GI tract diseases likely to cause IDA was identified. None of the variables investigated were predictive of upper-GI tract lesions 36. In our study, no statistically significant association for the presence of dyspepsia score between organic lesions was identified. But, the only statistically significant risk factors for the presence of GI tract disease likely to cause IDA were the following; diarrhea, weight loss, change of bowel habits and epigastric tenderness in our study. The statistically limited association for the presence of GI tract lesions were following; constipation, melena and a family history of a first-degree relative with GI cancer.
Capurso et al. demonstrated that a positive FOBT and older age were associated with the presence of GI tract organic lesions 36. In our study, predictive risk factors for GI tract lesions to cause of IDA were older age (>50 years) and positive FOBT. Capurso et al. showed that the risk factors for GI malignancies were: male gender (p < 0.01), advanced age (p < 0.01), and lower mean corpuscular volume (p < 0.002).
The standard diagnostic procedure for men and postmenopausal women with iron deficiency is to investigate gastrointestinal tract (upper and lower) pathology. The cause of iron deficiency anemia (IDA) in pre-menopausal women is often presumed to be menstrual blood loss. There are sparse data on gastrointestinal investigations in pre-menopausal women who have IDA, but significant gastrointestinal pathology was detected in published studies 29, 37-39. Significant upper gastrointestinal disease is identifiable among most pre-menopausal women with IDA (18 of 19 or 95%), even when careful evaluation by a specialist in gynecology suggests a gynecological source 38. Upper endoscopy should be considered in the evaluation of all pre-menopausal women with IDA expressing digestive complaints or in those with IDA refractory to iron supplementation. Lower endoscopic examination may be reserved for those women with symptoms or signs suggestive of colorectal disorders 38. Nahon et al. aimed to evaluate the diagnostic yield of endoscopy in women with IDA and to define predictive factors of a GI lesion. 241 consecutive women had endoscopies for IDA. Predictive factors of GI lesions diagnosed by endoscopy were abdominal symptoms, age > 50 years, and Hb < 9 g/dl. They suggested that endoscopic investigation should be avoided in women without these three predictive factors 39. In our study, none of these risk factors had been present in 21 (23%) women younger than 51 years. In this group, no patient had any GI lesion likely to cause IDA (negative predictive value= 100%). Pre-menopausal women and young patients with IDA may also provide unique diagnostic challenges. The accurate initial assessment of patient characteristics, clinical history, and certain laboratory data may guide the choice of which endoscopic investigation to perform first in patients with IDA (especially in pre-menopausal women), thereby, potentially reducing the frequency of negative findings. It may be an appropriate clinical approach to consider these risk factors when deciding for gastrointestinal endoscopic evaluation in iron deficiency anemia.
Helicobacter pylori infection has been implicated in several recent studies as a cause of IDA refractory to oral iron treatment with a favorable response to H. pylori eradication 40-46. In our study, 18 of 91 IDA patients (19.8%) had Helicobacter pylori gastritis. Hershko et al. showed that H. pylori infection was the only finding in 29 of 150 patients (19%), but was a common co-existing finding in 77 (51%) of the entire group 47. The celiac disease as a possible cause of IDA refractory to oral iron treatment, without other apparent manifestations of malabsorption syndrome is increasingly being recognized. Celiac disease should be included and routinely looked for in the differential diagnosis of adult patients with IDA. Grisolano et al. showed that the celiac disease prevalence was 8.7% in IDA patients 48. Three patients had celiac disease in our study.
In conclusion, our study demonstrated that it may be an appropriate clinical approach to consider these risk factors when deciding for gastrointestinal endoscopic evaluation in iron deficiency anemia. But, the sample size of this study was too small to draw any reasonable conclusions.
Table 3.
Laboratory findings related to iron deficiency in IDA patients.
| Positive (Positive / totally, %) | Negative (%) | |
|---|---|---|
| Fecal Occult Blood | 16 (16/89, 18) | 73 (82) |
| Parasite in feces | 6 (6/81, 7.4) | 75 (92.6) |
| Microscopic hematuria | 7 (7/91, 7.6) | 84 (93.4) |
| Lungs film | 2 (2/88, 2.2) | 86 (97.8) |
| Sprue serological | 3 (3/82, 3.6) | 79 (96.4) |
References
- 1.Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–7. doi: 10.1136/gut.2003.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008;23:128–41. doi: 10.1177/0884533608314536. [DOI] [PubMed] [Google Scholar]
- 3.Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig Dis Sci. 1995;40:1283–9. doi: 10.1007/BF02065539. [DOI] [PubMed] [Google Scholar]
- 4.Cook IJ, Pavli P, Riley JW. et al. Gastrointestinal investigation of iron deficiency anaemia. BMJ. 1986;292:1380–2. doi: 10.1136/bmj.292.6532.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuckerman G, Benitez J. A prospective study of bidirectional endoscopy (colonoscopy and upper endoscopy) in the evaluation of patients with occult gastrointestinal bleeding. Am J Gastroenterol. 1992;87:62–6. [PubMed] [Google Scholar]
- 6.Hardwick RH, Armstrong CP. Synchronous upper and lower gastrointestinal endoscopy is an effective method of investigating iron-deficiency anaemia. Br J Surg. 1997;84:1725–8. [PubMed] [Google Scholar]
- 7.Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34:699–718. doi: 10.1016/j.gtc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Powell N, McNair A. Gastrointestinal evaluation of anaemic patients without evidence of iron deficiency. Eur J Gastroenterol Hepatol. 2008;20:1094–100. doi: 10.1097/MEG.0b013e328304d621. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Ali M. et al. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145–53. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 10.Dickey W, McMillan SA, McCrum EE, Evans AE. Association between serum levels of total IgA and IgA class endomysial and antigliadin antibodies: implications for coeliac disease screening. Eur J Gastroenterol Hepatol. 1997;9:559–62. doi: 10.1097/00042737-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut. 1993;34:1102–7. doi: 10.1136/gut.34.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsay JO, Robinson SD, Jackson JE, Walters JR. The investigation of iron deficiency anemia - a hospital based audit. Hepatogastroenterology. 1999;46:2887–90. [PubMed] [Google Scholar]
- 13.Majid S, Salih M, Wasaya R, Jafri W. Predictors of gastrointestinal lesions on endoscopy in iron deficiency anemia without gastrointestinal symptoms. BMC Gastroenterology. 2008;8:52. doi: 10.1186/1471-230X-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55:548–59. doi: 10.1007/s10620-009-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008;121:943–8. doi: 10.1016/j.amjmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JG, Sahagun G, Oehlke MA, Lieberman DA. Serious gastrointestinal pathology found in patients with serum ferritin values #50 ng/ ml. Am J Gastroenterol. 1998;93:772–6. [PubMed] [Google Scholar]
- 17.Ioannou GN, Spector J, Scott K, Rockey DC. Prospective evaluation of a clinical guideline for the diagnosis and management of iron deficiency anemia. Am J Med. 2002;113:281–7. doi: 10.1016/s0002-9343(02)01226-3. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman GR, Prakash C, Askin MP, Lewis BS. American Gastroenterological Association medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:197–201. doi: 10.1016/s0016-5085(00)70429-x. [DOI] [PubMed] [Google Scholar]
- 19.Tan YM, Rosmawati M, Ranjeev P, Goh KL. Predictive factors by multivariate analysis for colorectal cancer in Malaysian patients undergoing colonoscopy. J Gastroenterol Hepatol. 2002;97:590–3. doi: 10.1046/j.1440-1746.2002.02694.x. [DOI] [PubMed] [Google Scholar]
- 20.Sworczak K, Siekierska HM, Drobinska A. et al. Iron deficiency anemia as the sole symptom of small intestine carcinoma. Med Sci Monit. 2001;7:457–60. [PubMed] [Google Scholar]
- 21.Rockey DC. Gastrointestinal bleeding. In: Feldman M, Friedman LS, Sleisneger MH, editors. Gastrointestinal and Liver Disease; 7th edn, vol 1. Philadelphia: WB Saunders; 2002. pp. 221–48. [Google Scholar]
- 22.Gordon SR, Smith R, Power GC. The role of endoscopy in the evaluation of iron deficiency anemia in patients over the age of 50. Am J Gastroenterol. 1994;89:1963–7. [PubMed] [Google Scholar]
- 23.Fireman Z, Gurevich V, Coscas D. et al. Results of gastrointestinal evaluation of hospitalized patients with iron deficiency anemia. IMAJ. 1999;1:232–5. [PubMed] [Google Scholar]
- 24.Wilcox CM, Alexander LN, Clark WS. Prospective evaluation of the gastrointestinal tract in patients with iron deficiency and no systemic or gastrointestinal symptoms or signs. Am J Med. 1997;103:405–9. doi: 10.1016/s0002-9343(97)00168-x. [DOI] [PubMed] [Google Scholar]
- 25.Peterson WL. Gastrointestinal bleeding. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal disease; 4th ed; Vol 1. Philadelphia: WB Saunders; 1989. pp. 415–6. [Google Scholar]
- 26.Ahlquist DA. Approach to the patient with occult gastrointestinal bleeding. In: Yamada T, editor. Textbook of gastroenterology; Vol 1. Philadelphia: JB Lippincott; 1991. pp. 616–33. [Google Scholar]
- 27.Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anemia for the British Society of Gastroenterology. Gut. 2000;46:1–5. doi: 10.1136/gut.46.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockey DC, Cello JP. Evaluation of gastrointestinal tract in patients with iron deficiency anemia. N Engl J Med. 1993;329:1691–5. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 29.Bini EJ, Micale PL, Weinshel EH. Evaluation of the gastrointestinal tract in premenopausal women with iron deficiency anemia. Am J Med. 1998;105:281–6. doi: 10.1016/s0002-9343(98)00260-5. [DOI] [PubMed] [Google Scholar]
- 30.Rockey DC. Occult gastrointestinal bleeding. N Engl J Med. 1999;341:38–48. doi: 10.1056/NEJM199907013410107. [DOI] [PubMed] [Google Scholar]
- 31.Bampton PA, Holloway RH. A prospective study of the gastroenterological causes of iron deficiency anaemia in a general hospital. Aust N Z J Med. 1996;26:793–9. doi: 10.1111/j.1445-5994.1996.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 32.Till SH, Grundman MJ. Prevalence of concomitant disease in patients with iron deficiency anaemia. Br Med J. 1997;314:206–8. doi: 10.1136/bmj.314.7075.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: A population-based cohort study. Am J Med. 2002;113:276–80. doi: 10.1016/s0002-9343(02)01214-7. [DOI] [PubMed] [Google Scholar]
- 34.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anemia. Gut. 1993;34:1102–7. doi: 10.1136/gut.34.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joosten E, Ghesquiere B, Linthoudt H. et al. Upper and lower gastrointestinal evaluation of elderly inpatients who are iron deficient. Am J Med. 1999;107:24–9. doi: 10.1016/s0002-9343(99)00162-x. [DOI] [PubMed] [Google Scholar]
- 36.Capurso G, Baccini F, Osborn J. et al. Can patient characteristics predict the outcome of endoscopic evaluation of iron deficiency anemia: a multiple logistic regression analysis. Gastrointest Endosc. 2004;59:766–71. doi: 10.1016/s0016-5107(04)00348-7. [DOI] [PubMed] [Google Scholar]
- 37.Green BT, Rockey DC. Gastrointestinal endoscopic evaluation of premenopausal women with iron deficiency anemia. J Clin Gastroenterol. 2004;38:104–9. doi: 10.1097/00004836-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kepczyk T, Cremins JE, Long BD. et al. A prospective, multidisciplinary evaluation of premenopausal women with iron-deficiency anemia. Am J Gastroenterol. 1999;94:109–15. doi: 10.1111/j.1572-0241.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 39.Nahon S, Lahmek P, Lesgourgues B. et al. Predictive factors of GI lesions in 241 women with iron deficiency anemia. Am J Gastroenterol. 2002;97:590–653. doi: 10.1111/j.1572-0241.2002.05534.x. [DOI] [PubMed] [Google Scholar]
- 40.Konno M, Muraoka S, Takahashi M, Imai T. Iron-deficiency anemia associated with Helicobacter pylori gastritis. J Ped Gastr Nutr. 2000;31:52–6. doi: 10.1097/00005176-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Choe YH, Kim SK, Son BK. et al. Randomized placebo controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999;4:135–9. doi: 10.1046/j.1523-5378.1999.98066.x. [DOI] [PubMed] [Google Scholar]
- 42.Choe YH, Lee JE, Kim SK. Effect of helicobacter pylori eradication on sideropenic refractory anaemia in adolescent girls with Helicobacter pylori infection. Acta Paediatr. 2000;89:154–7. doi: 10.1080/080352500750028753. [DOI] [PubMed] [Google Scholar]
- 43.Choe YH, Hwang TS, Kim HJ. et al. A possible relation of the Helicobacter pylori Pfr gene to iron deficiency anemia? Helicobacter. 2001;6:55–9. doi: 10.1046/j.1523-5378.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 44.Choe YH, Kwon YS, Jung MK. et al. Helicobacter pylori-associated iron-deficiency anemia in adolescent female athletes. J Pediatr. 2001;139:100–4. doi: 10.1067/mpd.2001.114700. [DOI] [PubMed] [Google Scholar]
- 45.Ashorn M, Ruuska T, Makipernaa A. Helicobacter pylori and iron deficiency anaemia in children. Scand J Gastr. 2001;36:701–5. doi: 10.1080/003655201300191950. [DOI] [PubMed] [Google Scholar]
- 46.Annibale B, Marignani M, Monarca B. et al. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med. 1999;131:668–72. doi: 10.7326/0003-4819-131-9-199911020-00006. [DOI] [PubMed] [Google Scholar]
- 47.Hershko C, Hoffbrand AV, Keret D. et al. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica. 2005;90:585–95. [PubMed] [Google Scholar]
- 48.Grisolano SW, Oxentenko AS, Murray JA. et al. The usefulness of routine small bowel biopsies in evaluation of iron deficiency anemia. J Clin Gastroenterol. 2004;38:756–60. doi: 10.1097/01.mcg.0000139034.38568.51. [DOI] [PubMed] [Google Scholar]