Abstract
Behavioral responses to D1 and D2-dopamine agonists are enhanced when these agonists are administered systemically to 6-hydroxydopamine (6-OHDA)-lesioned rats. In the present investigation, microinjection of SKF-38393, a D1-dopamine agonist, into the nucleus accumbens of adult rats lesioned as neonates with 6-OHDA produced a dose-related increase in locomotor activity that was enhanced markedly compared to control. LY-171555, a D2-agonist, elicited less locomotor activity than did SKF-38393 after microinjection into this site. Administration of SKF-38393 or LY-171555 into the nucleus accumbens did not increase locomotion in unlesioned rats at the doses administered to lesioned animals. In adult-6-OHDA-lesioned rats, microinjection of SKF-38393 into the nucleus accumbens also increased locomotion more than did LY-171555. As described previously, systemic administration of SKF-38393 produced little locomotion in adult-6-OHDA-lesioned rats, whereas LY-171555 produced a markedly enhanced response. Administration of SKF-38393 or LY-171555 into the caudate nucleus of neonatally and adult-6-OHDA-lesioned rats produced negligible locomotor activity, but did induce stereotypic behaviors similar to those observed after systemic treatment with these drugs. Stereotypic behaviors occurred to a greater degree in the 6-OHDA-lesioned rats than in unlesioned controls. A regional specificity for certain behaviors induced by dopamine agonist administration was observed. In spite of the enhanced behavioral responses of D1 and D2-dopamine agonists after microinjection into the brain of 6-OHDA-lesioned rats, binding of [3H]spiperone (D2-receptor antagonist ligand) and [3H]SCH 23390 (D1-receptor antagonist ligand) to tissue from striatum and nucleus accumbens was not altered significantly. In contrast to this lack of change in binding characteristics in 6-OHDA-lesioned rats, blockade of dopaminergic transmission with haloperidol treatment caused an elevation of [3H]spiperone binding sites in striatum without affecting affinity for the site. However, chronic haloperidol treatment did not alter significantly [3H]SCH 23390 binding to striatal membranes. These latter findings suggest that chronic dopamine receptor blockade need not produce the same adaptive mechanisms as destruction of dopamine-containing neurons. Thus, a change in receptor characteristics as measured by dopamine antagonist binding does not account for the behavioral supersensitivity observed after D1- and D2-dopamine agonist administration to neonatally or adult-6-OHDA-treated rats.
The loss of catecholamine-containing neurons has a dramatic effect on the responsiveness of animals to dopamine agonists (Ungerstedt, 1971; Uretsky and Schoenfeld, 1981; Schoenfeld and Uretsky, 1972; Hollister et al., 1974, 1979; Breese et al., 1979; Kilts et al., 1979; Setler et al., 1978). This is true of agonists acting on either D1- or D2-dopamine receptor sites proposed by Kebabian and Calne (1979), although the age at which the lesion occurs has a dramatic effect on the degree of change (Breese et al., 1985b). For example, Breese et al. (1985b) found that locomotor activity is increased markedly after systemic administration of SKF-38393 (a D1-receptor agonist) to adult rats lesioned as neonates with 6-OHDA; less locomotor activity occurred after similar treatment with the D2-dopamine agonist, LY-171555 (Tsuruta et al., 1981). Conversely, rats lesioned as adults with 6-OHDA exhibit more locomotor activity to LY-171555 than to systemic administration of SKF-38393 (Breese et al., 1985b). The behavioral responses after SKF-38393 administration were antagonized by SCH 23390, a D1-dopamine receptor antagonist, but not by haloperidol, a D2-dopamine receptor antagonist (Breese et al., 1985a,b). These data support the conclusion that D1-dopamine receptors are distinct from D2-receptors (Arnt and Hyttel, 1984; Christensen et al., 1984; Arnt, 1985; Breese and Mueller, 1985; Breese et al., 1985b). Furthermore, these findings indicate that D1- and D2- dopamine receptor agonist administration to 6-OHDA-lesioned rats induce greater behavioral responses than seen in unlesioned controls (Breese et al., 1984a, 1985a,b; Molloy and Waddington, 1984).
Microinjection of dopamine agonists into dopamine terminal regions can produce behavioral effects. For example, administration of dopamine and D2-dopamine agonists into the nucleus accumbens is associated primarily with locomotor activity, whereas microinjection into the striatum generally causes stereotyped behaviors (Costall et al., 1975; Jackson et al., 1975; Pijnenburg et al., 1976). Such microinjection experiments have not been undertaken with SKF-38393, a D1-dopamine agonist.
A unilateral lesion of dopamine-containing neurons in adult rats increases [3H]spiperone binding in the striatum, ipsilateral to the side of the lesion (Creese et al., 1977; Goldstein, et al., 1980; Heikkela et al., 1981; Neve et al., 1984; Staunton et al., 1981). This increase in [3H]spiperone binding is presumed to reflect an increase in D2-dopamine receptors, which is suggested to be responsible for the enhanced turning observed after dopamine agonist administration to animals with unilateral lesions of dopaminergic neurons (Creese et al., 1977). Preliminary investigations of [3H]spiperone binding to striatal tissue from rats treated intracisternally with 6-OHDA failed to demonstrate a similar increase in the number of receptors in striatum (Mailman et al., 1981, 1983). To date, no literature is available concerning the effect of dopaminergic lesions on binding of [3H]SCH-23390, a marker for D1-dopamine receptor sites (Billard et al., 1984).
The goals of the present investigation were 2-fold. The first series of experiments conducted was to examine the behavioral effects of central administration of D1- and D2-dopamine agonists into nucleus accumbens and striatum to document that behavioral responses induced were enhanced after 6-OHDA treatment. A second set of experiments was developed to measure binding of [3H]spiperone and [3H]SCH 23390 to nucleus accumbens and striatal tissue to determine whether altered receptor number or affinity could be associated with the enhanced behavioral responses observed after D1- and D2-dopamine agonist administration to 6-OHDA-treated rats. Binding results for these D1- and D2-dopamine receptor antagonist ligands in neonatally and adult-6-OHDA lesioned rats were compared to binding alterations after chronic haloperidol treatment.
Methods
General
Pregnant Sprague-Dawley female and adult rats (> 225 g) were purchased from Charles River Laboratories (Wilmington, MA). Pregnant females were housed in clear plastic cages with wood chip bedding. Other rats were housed four per cage. The rats were maintained under a 7:00 a.m. to 7:00 p.m. light-dark cycle, temperature was maintained between 23–25°C and the rats had continuous access to water and food (Wayne Lab Blox laboratory chow). Adult rats received saline or 6-OHDA hydrobromide (200 µg free base in 25 µl) intracisternally 30 min after pargyline (50 mg/kg) and an additional dose of 6-OHDA (200 µg) 1 week later (Breese and Traylor, 1970). At 5 days of age, neonatal rats received saline or 6-OHDA (10 µl; 100 µg free base) intracisternally (Breese and Traylor, 1972; Smith et al., 1973). Litters were limited to 10 rats each. Adult rats sometimes required additional care to treat aphagia and adipsia (i.e., tube feeding and fruit supplement; Breese et al., 1973). Neonates treated with 6-OHDA required no special support until after weaning, at which time lesioned rats were given fruit and sunflower seeds to supplement their diet. A separate set of adult rats (equal males and females) were given haloperidol (1 mg/kg s.c. in a 0.5% tartaric acid solution) for 16 days to allow comparison of the 6-OHDA treatments with a protocol known to increase [3H]spiperone binding (Seeman, 1980).
When adult-treated rats recovered from the acute aphagia and adipsia (Breese et al., 1973), they were challenged with apomorphine hydrochloride (1 mg/kg i.p., salt) and the locomotor response measured. Once neonatal rats reached 30 days of age, they were challenged with 15 mg/kg of l-dopa (i.p.) 1 hr after the decarboxylase inhibitor, RO-4-4602 (50 mg/kg i.p.) and locomotor activity was determined. The locomotor activity had to be greater than 10,000 counts per 2 hr (i.e., a criterion for functional supersensitivity) for the rat to be retained for further testing. Those neonatally lesioned rats found to have an exaggerated locomotor response to l-dopa were subsequently given three 3-mg/kg (i.p.) doses of SKF-38393 at weekly intervals. The third locomotor response to SKF-38393 had to be in excess of 10,000 activity counts per 2 hr for the rat to be used in other investigations. Some of the neonatally lesioned rats that reached criterion were given 100 mg/kg of l-dopa after RO-4-4602 and the incidence of SMB noted (Breese et al., 1984a). Only those neonatally lesioned rats demonstrating SMB were used in experiments in which behavior was assessed after microinjection of drugs into brain. These criteria assured that the lesioned animals were behaviorally supersensitive to dopamine agonists before surgery. It has been established that the different drug regimens to test for behavioral supersensitivity in adult- and neonatal-6-OHDA-treated rats are not responsible for the different behaviors reported when these two groups are given the same treatments (Breese et al., 1984a; 1985a,b). Control unlesioned rats received treatments like those described for neonatally or adult-6-OHDA-treated rats. Behaviors observed in the present study were qualitatively the same as those described earlier by Breese et al. (1984a).
Administration of drugs into brain
In order to permit microinjection of drugs and saline into striatum and nucleus accumbens, cannulas were placed bilaterally into the brains of anesthetized rats (45 mg/kg, pentobarbital sodium) and secured to the skull with stainless-steel screws surrounded by acrylic cement. Stereotaxic coordinates with incisor bar set at 3.3 mm (flat skull) for rats weighing 270 to 350 g were: AP, +0.92; ML, +0.3; DV, −0.5 for striatum and AP, 1.12; ML, +0.13; DV, −0.53 for the nucleus accumbens with bregma serving as zero. Animals were allowed to recover for at least 10 days before microinjection of drug solutions or saline. A 10-µl syringe (Precision Instrument Co., Baton Rouge, LA) driven by a Sage infusion pump (White Plains, NY) delivered the solution to the bilateral cannulas through polyethylene tubing. Drugs were infused into brain in a volume of 0.5 µl over a 5-min period through 33-gauge injection cannulas which extended 1 mm below each of the guide tubes (Breese et al., 1984b). The animals were restrained gently by hand during the injection procedure. The bilateral injection cannulas remained in place for 1 min after the infusion. Animals were infused up to 5 times at weekly intervals. Identification of the cannula tip placements was obtained by cutting frozen sections through the site.
Evaluation of locomotor activity
Locomotor activity was measured after various doses of dopamine agonists were microinjected into the nucleus accumbens or caudate as well as after systemic administration. This activity was quantified as described previously (Hollister et al., 1974, 1979) using circular activity monitors with sensors about the perimeter. Each interruption of light beam resulted in an activity count. Rats were habituated to the chamber for 45 or 60 min before receiving the dopamine agonists. Counts were accumulated every 10 min for 2 to 3 hr depending upon the dopamine agonist being investigated. Unlesioned-control rats for the neonatal- and adult-6-OHDA groups rats did not demonstrate a difference in response to saline or to dopamine agonists and these data were combined. Data were presented for each 10-min collection period or as the total accumulated counts for the period of time that data were collected.
Evaluation of behavior
Behaviors induced by the dopamine agonists were assessed by a trained observer using the procedure described previously (Breese et al., 1984a). A second observer verified periodically behaviors scored by the primary observer. Behavior of individual rats was observed for a 1-min period every 10 min for 2 hr. Each 1-min period of observation was divided into four 15-sec intervals and occurrence of a behavior during each interval was recorded. The proportional incidence of a behavior was determined for each 1-min observation period by dividing the occurrence of a behavior during each of the observation intervals by 4 (i.e., total occurrence per 4) and scores for each behavior were summed for the 12 1-min observation periods (Breese et al., 1985a). The behaviors monitored included sniffing, rearing, grooming, head nodding, locomotion, “taffy pulling,” paw treading, self-biting, licking, jumping, digging in and eating wood chips and skin laceration. Taffy pulling is the repeated movement of the forepaws toward and away from the nose (Breese et al., 1984a, 1985b). This evaluation allowed us to compare the incidence of a behavior induced by dopamine agonists in 6-OHDA-lesioned rats with incidence in control rats (see “Statistical evaluations”).
Radioligand binding to brain tissue
The 6-OHDA-treated and unlesioned-control rats used for binding investigations were from the same treatment groups that received drugs into nucleus accumbens and caudate, but these rats chosen for the binding studies were not implanted with cannulas. Only those rats exhibiting supersensitive behavioral responses after systemic injection of dopamine agonists were included for investigation. For binding determinations, rats lesioned with 6-OHDA were drug-free for at least 3 weeks before decapitation. Haloperidol-treated rats were sacrificed 48 hr after treatment was discontinued. The whole brain was removed and placed on an ice-cold glass plate. The striatum and nucleus accumbens were dissected from brain, weighed, frozen on dry ice and stored at −70°C until radioligand binding to membranes was performed. The olfactory tubercles and posterior striatum were dissected to permit monoamine determinations on each rat. Drug binding to brain membranes was performed with [3H]SCH 23390 utilizing the method described by Billard et al. (1984) and for [3H]spiperone using the method of Hamblin et al. (1984). Tissue was homogenized in 50 mM Tris at pH 7.5 with a Beckman polytron. After centrifugation (32,000 × g for 30 min), the resulting pellet was homogenized in the same volume of 50 mM Tris-HCl and again centrifuged at 32,000 × g for 30 min. The final pellet was suspended in Tris buffer (0.12 N NaCl or as indicated in the text) to yield a tissue concentration of 0.4 mg of original tissue per ml of buffer for [3H] spiperone or 3 mg/ml for [3H]SCH 23390. Tissue in each tube corresponded to 2 mg (5-ml suspension) of the net weight of fresh brain for [3H]spiperone and 3 mg (1-ml suspension) of brain tissue for [3H]SCH 23390. Because of the low tissue weights for the nucleus accumbens, [3H]spiperone binding was performed on tissue from adubt-6-OHDA-lesioned rats and corresponding controls and [3H]SCH-23390 was performed on nucleus accumbens tissue from neonatal-6-OHDA-lesioned rats and their controls. Labeled agents and unlabeled competitors (e.g., haboperidol or SCH 23390) were added to the suspension. Appropriate preliminary studies were performed to establish that binding was at equilibrium within 10 min at 37°C. For single concentration assays, the final concentration of labeled compounds was 1.0 nM (nucleus accumbens) or 0.2 nM (striatum) for [3H]spiperone (24.5 Ci/mmol) and 0.3 nM (all tissues) for [3H]SCH 23390 (72 Ci/mmol). Concentrations of labeled compounds were varied for saturation analysis. This required pooling three striate from each group to generate a single saturation curve. All determinations were in triplicate as were the tubes for nonspecific binding (1 µM SCH-23390 for [3H]SCH 23390 and 1 µM haloperidol for [3H]spiperone). Ketanserin (20 nM) was added to tubes to eliminate an interaction of [3H]spiperone with serotonergic receptors in striatum (Hamblin et al., 1984). This was not necessary for the [3H]SCH 23390 samples because ketanserin does not alter binding of this ligand (Billard et al., 1984; Hess et al., 1986; see “Results”). At the end of the incubation, 5 ml of ice-cold buffer was added to the mixture for each sample; the contents were filtered on glass-fiber discs (Gelman, Ann Arbor, MI) and washed with 10 ml of Tris-HC1, pH 7.5, at 0°C. Filters were dried and counted in 10 ml of scintillation fluid (Scintiverse II, Fisher Scientific Co., Springfield, NJ). A Scatchard transform of the saturation analysis was used to calculate Kd and Bmax from the individual observations of three to five separate experiments.
Monoamine determinations
Concentrations of dopamine and its major metabolites were determined in brain areas associated with dopamine terminal fields (striatum and olfactory tubercles) to assess the effectiveness of the 6-OHDA treatments. Compounds were separated with high-performance liquid chromatography and detected with an electrochemical detector as described by Kilts et al. (1981).
Statistical evaluations
Locomotor responses, amine data and binding of ligands were tested for significance among groups with an analysis of variance and then compared with a Newman-Keuls or Dunnett test for post hoc comparison of means. Behavioral scores were compared using an analysis of variance. For each analysis that yielded a significant F ratio, the Newman-Keuls test was applied to allow mean comparisons of behavioral responses by various groups (Breese et al., 1985a). In cases in which only two groups were compared, a Student’s t test was used. Significance was considered to be P < .05 for all comparisons.
Results
Behavioral effects of SKF-38393 and LY-171555 administration into nucleus accumbens of neonatally 6-OHDA-lesioned rats
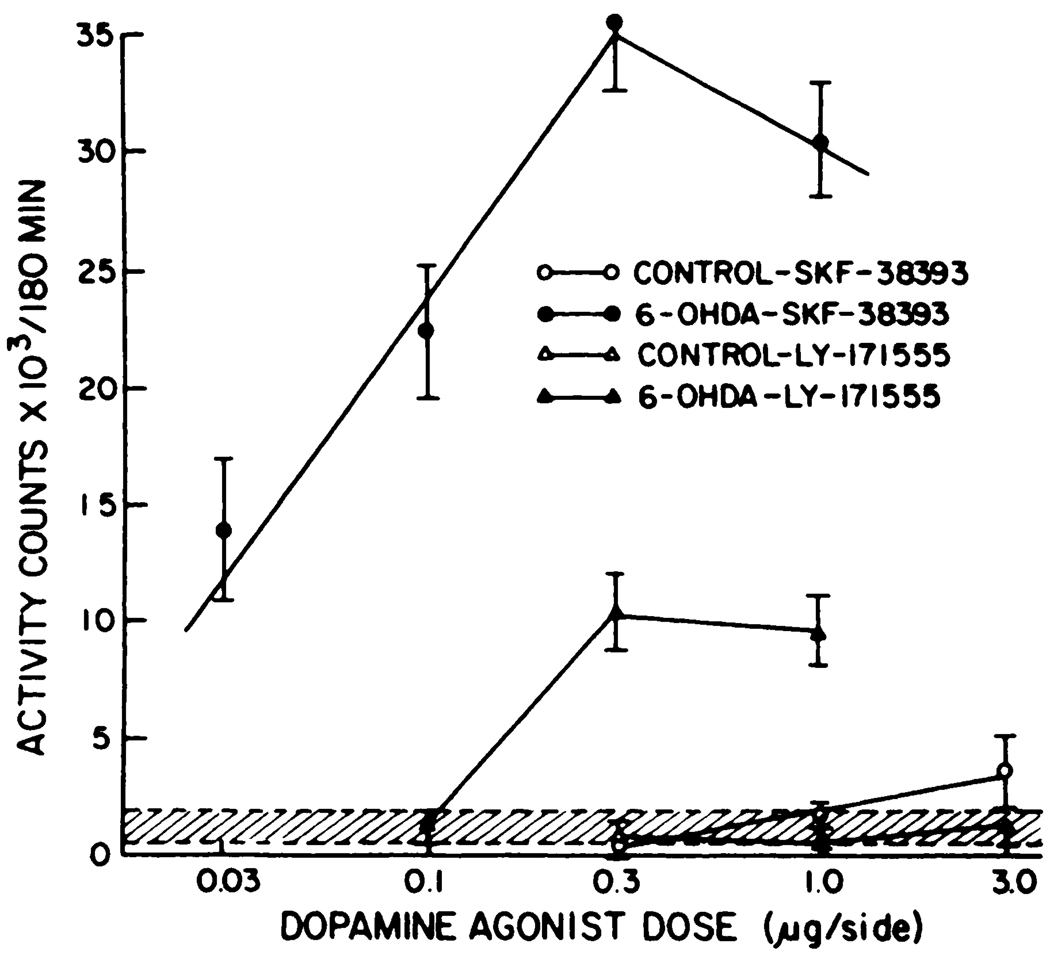
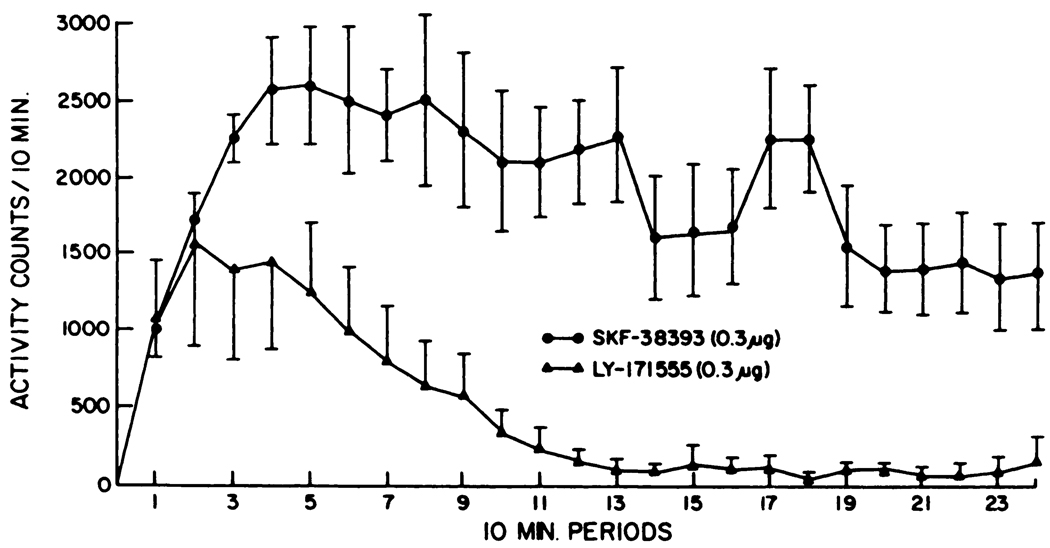
Using adult rats lesioned as neonates with 6-OHDA with proven behavioral supersensitivity to dopamine agonists after systemic (i.p.) adminsitration (see “Methods”), experiments were initiated to assess the action of SKF-38393 and LY-171555 microinjected into nucleus accumbens. Administration of SKF-38393 into the nucleus accumbens of neonatal-6-OHDA-treated rats produced a dose-related increase in locomotor activity (fig. 1). The magnitude of the activity after microinjection of SKF-38393 into the nucleus accumbens of the lesioned rats was considerably greater than that observed in unlesioned rats (see fig. 1; table 1). LY-171555 also increased locomotion, but less so than did SKF-38393 at same dose. Duration of the responses after intracerebral microinjection of SKF-38393 (0.3 µg) and of LY-171555 (0.3 µg) in neonatally 6-OHDA-treated rats is illustrated in figure 2. These data demonstrate that SKF-38393 induced a sustained level of activity for over 4 hr, whereas the duration of the response to LY-171555 was approximately 2 hr. The nucleus accumbens seemed critical for the locomotor responses induced SKF-38393, because fewer activity counts were observed when 0.3 µg of SKF-38393 was microinjected into the caudate (table 1).
Fig. 1.
SKF-38393 and LY-171555-induced locomotor activity after microinjection into the nucleus accumbens of neonatally 6-OHDA-treated rats. The dose-response curve for SKF-38393 is significantly different from that observed in control rats (P < .001). The responses obtained with the 0.3- and l.0-µg doses of LY-171555 in the neonatally lesioned rats are significantly different from control responses (P < .01). There are four to eight determinations at each dose of the drugs for the various groups.
TABLE 1. Locomotor activity induced by SKF-38393 after microinjection into nucleus accumbens or caudate of neonatally 6-OHDA-treated rats.
SKF-38393 (0.3 µg/side) was administered bilaterally into either the nucleus accumbens or the caudate after 45 min of habituation to the activity chambers. Activity counts were accumulated over a 180-min period. The dose of SKF-38393 was administered in a volume of 0.5 µl over a 5-min period. In untreated rats the response to saline administered into the nucleus accumbens was 1255 ± 317 counts per 180 min (N = 6) and 1538 ± 246 counts when administered into the caudate (N = 5). The response to saline administered into the nucleus accumbens of neonatally 6-OHDA treated rats was 1611 ± 392 counts per 180 min (N = 5). There are at least five determinations for each of the groups.
| Group | Nucleus Accumbens | Caudate |
|---|---|---|
| (Counts/180 min ± S.E.M.) | ||
| Unlesioned control | 3121 ± 974* | 1583 ± 342 |
| Neonatal-6-OHDA | 37,687 ± 4785*** | 3236 ± 1603 |
P < .05 when compared to saline treated control;
P < .001 when compared to control response.
Fig. 2.
Time course of the locomotor response to the D1- and D2-agonists after microinjection of 0.3 µg into the nucleus accumbens of neonatally 6-OHDA-treated rats. These determinations were from five rats in each group.
The frequency of behaviors induced by SKF-38393 microinjection (0.3 µg/side) into the nucleus accumbens was also measured in an open-field condition (table 2). SKF-38393 administered into the nucleus accumbens increased the incidence of locomotion, sniffing, licking and grooming without affecting the incidence of rearing, when compared to SKF-38393-induced responses in unlesioned controls (table 2). At this dose, behaviors other than these were not observed. In contrast to the lack an effect by SKF-38393 in control, unlesioned rats after i.p. administration (Breese et al., 1985a), this D1-dopamine agonist produced a small, but significant, increase in the incidence of sniffing, rearing, grooming and locomotion when administered into the nucleus accumbens of these animals.
TABLE 2. Effect of microinjection of SKF-38393 into nucleus accumbens on behavior in an open field.
Each value is the mean ± S.E.M. of the percentage of scoring intervals summed for the 12 observation periods. There are four rats in each of the groups. Saline administration into nucleus accumbens of unlesioned controls did not differ from values for saline administered to unlesioned rats (see representative values when saline injected into caudate; table 5).
| Behavior | Neonatal-6-OHDA SKF (0.3 µg/side) | Unlesioned Control SKF (0.3 µg/side) | Neonatal-6-OHDA Saline |
|---|---|---|---|
| Sniffing | 8.3 ± 1.8*,† | 4.3 ± 1.0† | 1.7 ± 0.6 |
| Rearing | 2.6 ± 0.9† | 1.7 ± 0.7† | 0.2 ± 0.2 |
| Locomotion | 8.1 ± 2.6*,† | 1.0 ± 0.4† | 0.1 ± 0.1 |
| Eating and digging in wood chips | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Grooming | 2.8 ± 0.9*,† | 1.0 ± 0.3† | 0.0 ± 0.0 |
| Taffy pulling | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Self-biting | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Paw treading | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Licking | 2.0 ± 1.2*,† | 0.0 ± 0.0 | 0.0 ± 0.0 |
P < .05 when compared to unlesioned control treated rats with SKF-38393;
P < .05 when compared to lesioned control rats that received saline.
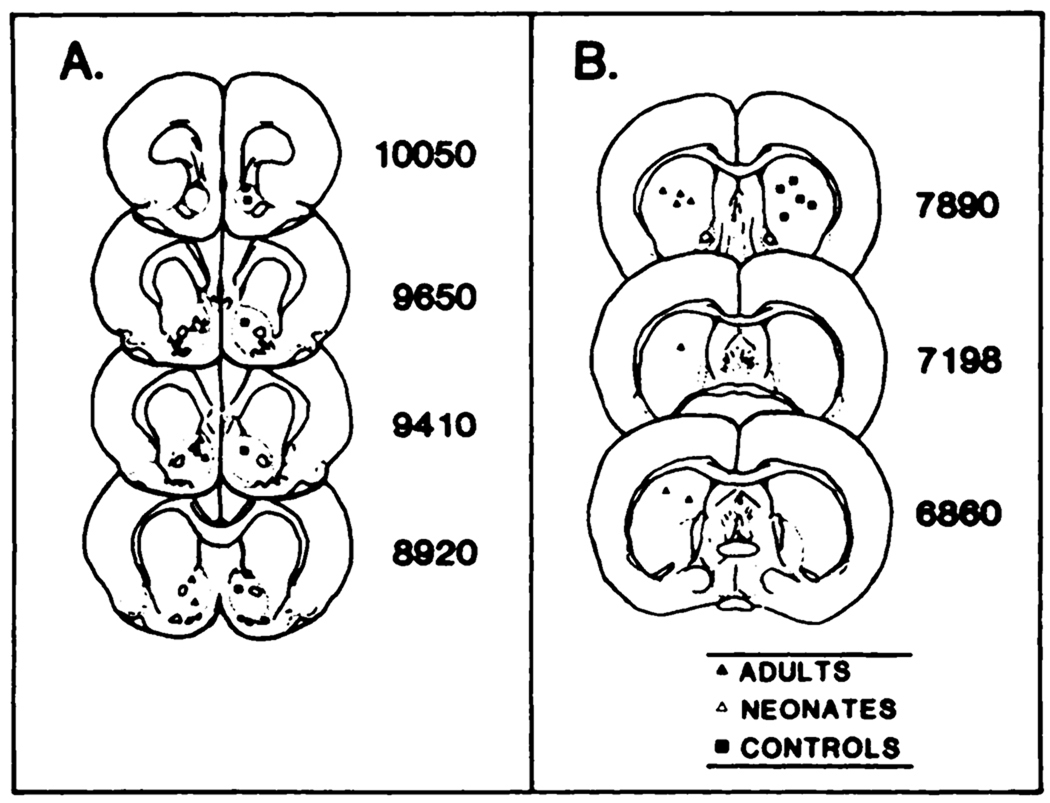
Representative placements of the injector tips aimed at the nucleus accumbens from rats used in these experiments are presented in figure 3. Depletion of dopamine in olfactory tubercle and striatum of neonatally lesioned rats is presented in table 3. The elevation of serotonin and 5-hydroxyindole-acetic acid observed previously after this treatment was also noted in these animals (table 3; see Breese et al., 1984a).
Fig. 3.
Representative placements of injector tips in 6-OHDA-treated animals and controls. Placement was determined by histological procedures performed in randomly selected rats. Each symbol represents tip placement of bilateral injections. A, nucleus accumbens placements; B, caudate nucleus placements. Numerals to the right indicate anterior/posterior position, in micrometers, according to Konig and Klippel (1963).
TABLE 3. Effect of 6-OHDA treatments on brain monoamines in olfactory tubercle and caudate.
Values are the mean nanograms per milligram of protein ± S.E.M. Adult 6-OHDA, rats treated as adults and neonatal 6-OHDA, rats treated when 5 days of age. There are at least 10 rats in each group. ND, not detectable; DOPAC, 3,4-dihydroxyphenytacetic acid; HVA, homovanillic acid; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindole-acetic acid.
| Monoamine or Metabolite | Nonlesioned Control | Adult 6-OHDA | Neonatal 6-OHDA |
|---|---|---|---|
| Olfactory tubercle (ng/mg protein ± S.E.M.) | |||
| Dopamine | 66.9 ± 5.0 | 4.5 ± 0.8*** | 6.2 ± 0.7*** |
| DOPAC | 11.0 ± 1.1 | 1.3 ± 0.6*** | 1.1 ± 0.2*** |
| HVA | 2.6 ± 0.3 | 0.2 ± 0.1*** | 0.3 ± 0.06*** |
| 5-HT | 11.7 ± 0.8 | 9.9 ± 1.9 | 13.3 ± 0.5 |
| 5-HIAA | 4.0 ± 0.6 | 4.1 ± 0.4 | 4.9 ± 0.7 |
| Caudate (ng/mg protein ± S.E.M.) | |||
| Dopamine | 100.8 ± 2.1 | 3.2 ± 1.1*** | 1.7 ± 0.7*** |
| DOPAC | 6.9 ± 0.7 | 0.7 ± 0.4*** | 1.6 ± 0.6*** |
| HVA | 2.5 ± 0.8 | 0.7 ± 0.6*** | ND*** |
| 5-HT | 5.7 ± 0.6 | 4.9 ± 0.7 | 12.5 ± 0.6*** |
| 5-HIAA | 4.3 ± 0.3 | 4.6 ± 0.7 | 7.2 ± 0.8*** |
P < .001 when compared to content in control rats.
SKF-38393- and LY-171555-induced locomotion after microinjection into nucleus accumbens of adult-6-ORDA-lesioned rats
Locomotor activity was also measured in adult-6-OHDA-lesioned rats after bilateral microinjection of SKF-38393 and LY-171555 into the nucleus accumbens (table 4). A moderate increase in locomotion was observed after LY-171555 (0.3 and 1.0 µg/side) injection into nucleus accumbens of adult-6-OHDA-lesioned rats, but a greater locomotor increase was observed when 1.0 µg of SKF-38393 was microinjected bilaterally into this brain site.
TABLE 4. Effect of SKF-38393 and LY-171555 on locomotor activity in adult-6-OHDA-treated rats after peripheral and nucleus accumbens Injections.
Animals received drugs peripherally or by central microinjection. Control refers to unlesioned rats. Rats were habituated to the chambers 45 min before drug treatments. Numbers in parentheses, number of determinations.
| Treatment, Dose | Activity ± S.E.M. (Counts/180 min) | |
|---|---|---|
| SKF-38393 | LY-171555 | |
| Peripheral injectiona | ||
| Control, 1.0 mg/kg | 1716 ± 549 (4) | 5112 ± 1365 (4) |
| Adult 6-OHDA, 1.0 mg/kg | 3299 ± 958 (6) | 30633 ± 7833*** (6) |
| Nucleus accumbens microinjectiona | ||
| Control, 0.3 µg/side | 2121 ± 974 (4) | 1799 ± 451 (4) |
| Control, 1.0 µg/side | 1451 ± 491 (5) | 1724 ± 864 (4) |
| Adult-6-OHDA, 0.3 µg/side | 2980 ± 463*** (4) | |
| Adult-6-OHDA, 1.0 µg/side | 23581 ± 7840*** (5) | 11884 ± 4827*** (5) |
Response of control rats to i.p. saline administration was 1346 ± 426 counts/180 min and after microinjection of saline into the nucleus accumbens was 1511 ± 344 counts/180 min (N = 4 for each group).
P < .001 when 6-OHDA response is compared to control.
Because adult-6-OHDA-treated rats reportedly show a greater locomotor response to a D2-dopamine agonist than to a D1-dopamine receptor agonist after systemic i.p. administration (Breese et al., 1985b), the adult-lesioned animals used for the site injections were given the D1- and D2-dopamine agonists peripherally and locomotor activity was again measured (table 4). The adult-6-OHDA-treated rats demonstrated the same characteristics as those used in earlier studies (i.e., a greater response to the D2-dopamine agonist compared to the response to the D1 agonist; see Breese et al., 1985b).
Representative placements of the injector tips within the nucleus accumbens from rats used in these investigations are illustrated in figure 3. The concentrations of dopamine and its metabolites in olfactory tubercle and striatum for control rats as well as for the adult-6-OHDA-lesioned group are presented in table 3. As expected, there were marked reductions in dopamine and its major metabolites in the adult-6-OHDA-treated rats without a change in serotonin content (Breese et al., 1984a).
Behavioral effects of SKF-38393 and LY-171555 microinjected into the caudate of 6-OHDA-lesioned rats
Administration of 3 µg/side of SKF-38393 into the caudate nucleus of rats lesioned as neonates with 6-OHDA increased the incidence of rearing, sniffing and to a lesser degree locomotion, licking and head weaving when compared to unlesioned controls that received this dose of SKF-38393 (table 5). Administration of this dose of SKF-38393 to adult-6-OHDA-treated rats produced greater sniffing than in unlesioned controls (table 5). There was no evidence of self-biting in either of the 6-OHDA treatment groups, even after the bilateral microinjection of 3 µg of SKF-38393 into the caudate (P > .1; data not presented). In unlesioned controls, microinjection of SKF-38393 produced a small increase in the incidence of rearing and grooming when compared to saline.
TABLE 5. Behavioral effects induced by SKF-38393 and LY-171555 administration into the caudate in neonatally and adult-6-OHDA-treated rats.
Behavior scores for each behavior are the mean ± S.E.M. of the percentage of scoring intervals summed for the 12 observation periods over 2 hr. See table 1 for group designations. Values Obtained after saline administration into the caudate of unlesioned rats were not different from values obtained after saline administration to unlesioned rats (P > .1). SKF-38393 was administered to adult 6-OHDA-treated rats and both were administered to unlesioned controls. There are six to eight rats in each group.
| Drug Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Behavior | Saline | SKF-38393 (3 µg/side) |
LY-171555 (3 µg/side) |
||||
| Unlesioned control |
Unlesioned control |
Neonatal 6- OHDA |
Adult 6-OHDA |
Unlesioned control |
Neonatal 6- OHDA |
Adult 6-OHDA |
|
| Sniffing | 0.9 ± 0.3 | 1.6 ± 0.5 | 7.0 ± 1.1* | 4.9 ± 1.6* | 2.8 ± 1.2† | 8.1 ± 1.3* | 5.8 ± 1.8 |
| Rearing | 0.1 ± 0.1 | 0.8 ± 0.4† | 3.5 ± 1.5* | 0.7 ± 0.1 | 0.2 ± 0.1 | 3.2 ± 1.6* | 3.8 ± 1.5* |
| Locomotion | 0.1 ± 0.1 | 0.3 ± 0.1 | 2.1 ± 0.9* | 1.5 ± 1.3 | 0.5 ± 0.4 | 8.3 ± 1.7* | 2.9 ± 1.3* |
| Eating and digging in wood chips | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Grooming | 0.0 ± 0.0 | 1.6 ± 0.5† | 1.0 ± 0.4 | 2.0 ± 0.3 | 0.3 ± 0.2 | 0.0 ± 0.0 | 0.9 ± 0.4 |
| Licking | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 0.7* | 0.4 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Head weaving | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.5* | 0.5 ± 0.5 | 0.0 ± 0.0 | 0.8 ± 0.4 | 1.9 ± 1.6 |
| Taffy pulling | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.3 ± 1.6* | 0.0 ± 0.0 |
P < .05 when compared to corresponding drug response in unlesioned control rats;
P < .05 when compared to saline response in unlesioned control.
Microinjection of 3 µg/side of LY-171555 into the caudate of neonatally 6-OHDA-treated rats resulted in an increase in sniffing, rearing, locomotion and taffy pulling (table 5). In adult-6-OHDA-treated rats, the incidence of rearing and locomotion was increased compared to the incidence of these behaviors in control-unlesioned rats (table 5). Head weaving was observed in some rats. Placements for injector tips within the caudate from selected animals used in these studies are presented in figure 3.
Effect of 6-OHDA lesions on binding of [3H]spiperone and [3H]SCH 23390 to nucleus accumbens membranes
Because of the behavioral supersensitivity to dopamine agonist microinjection into the nucleus accumbens of 6-OHDA-lesioned rats (fig. 1), the binding of [3H]spiperone and [3H]SCH 23390 to tissue from this brain region was determined (table 6). Binding for [3H]spiperone was performed on tissue from adult-6-OHDA-lesioned rats that demonstrated a supersensitive locomotor response to LY-171555 or apomorphine treatment. Binding for [3H]SCH-23390, the D1-dopamine receptor antagonist (Iorio et al., 1983), was performed on tissue from neonatal-6-OHDA-lesioned rats that demonstrated an increased locomotor response to SKF-38393 administration and SMB after treatment with l-dopa. [3H]Spiperone (the D2-dopamine receptor antagonist, Seeman, 1980) binding to the nucleus accumbens membranes was not altered significantly by the adult-6-OHDA-induced lesion when compared to unlesioned controls (table 6). Similarly, binding of [3H]SCH 23390 to nucleus accumbens membranes was not altered by the neonatal-6-OHDA-induced lesion.
TABLE 6. Effect of neonatal and adult 6-OHDA-lesions on binding of [3H]SCH-23390 and [3H]spiperone to nucleus accumbens.
Values represent the mean picomoles per gram of tissue ± S.E.M. at a single concentration of the ligand which was determined with 1.0 nM [3H]spiperone and 0.3 nM [3H]SCH-23390. Numbers in parentheses, number of determinations made in triplicate. Adult 6-OHDA-treated rats (Adult 6-OHDA) demonstrated a supersensitive locomotor response to LY-171555 or to apomorphine (1 mg/kg)and neonatal 6-OHDA-treated rats (neonatal 6-OHDA) showed an elevated locomotor response after three treatments with SKF-38393 (3 mg/kg). No significant differences were observed among the groups for either ligand (P > .1).
| Treatment | Nucleus Accumbens | |
|---|---|---|
| [3H]SCH-23390 | [3H]spiperone | |
| Control | 7.0 ± 0.9 (9) | 10.8 ± 0.9 (7) |
| Neonatal 6-OHDA | 7.8 ± 0.7 (11) | |
| Adult 6-OHDA | 9.4 ± 0.9 (8) | |
Effect of 6-OHDA lesions on binding of [3H]spiperone and [3H]SCH-23390 to striatal membranes
Because Hamblin et al. (1984) and MacKenzie and Zigmond (1984) provided evidence that [3H]spiperone can interact with serotonin-2 receptors, studies were performed to see what degree this interaction was occurring in striatal samples prepared in our laboratory, as serotonin-2 receptors in striatum are localized regionally (Altar et al., 1985). As shown in table 7, ketanserin, a serotonin-2 antagonist, displaced approximately 12% of [3H]spiperone bound specifically to striatal membranes from control or neonatally 6-OHDA-lesioned rats. However, no difference in [3H]spiperone binding was noted between control and neonatally lesioned rats in the presence or absence of ketanserin (table 7). Studies performed with [3H]SCH 23390 in the presence of ketanserin did not reveal a significant change (P > .1) in the binding of this ligand to striatal tissue (data not shown); these data are in agreement with that presented by Hess et al. (1986). Therefore, because of the results obtained with ketanserin, the scatchard analysis performed for [3H] spiperone binding in tissue from neonatal- and adult-lesioned rats was performed in the presence of ketanserin, whereas that for [3H]SCH 23390 was not.
TABLE 7. Effect of ketanserin on the binding of [3H]spiperone to striatal membranes.
“With” refers to addition of ketanserin (20 nM) to incubation from unlesioned controls and neonatally 6-OHDA-lesioned rats. See “Methods” for details. N, number of determinations. P > .1 for comparison of control with 6-OHDA-treated groups.
| Incubation | N | Control | 6-OHDA |
|---|---|---|---|
| pmol/g tissue | |||
| No ketanserin | 8 | 21.2 ± 1.0 | 21.9 ± 1.3 |
| With ketanserin | 8 | 18.8 ± 0.7 | 19.3 ± 1.1 |
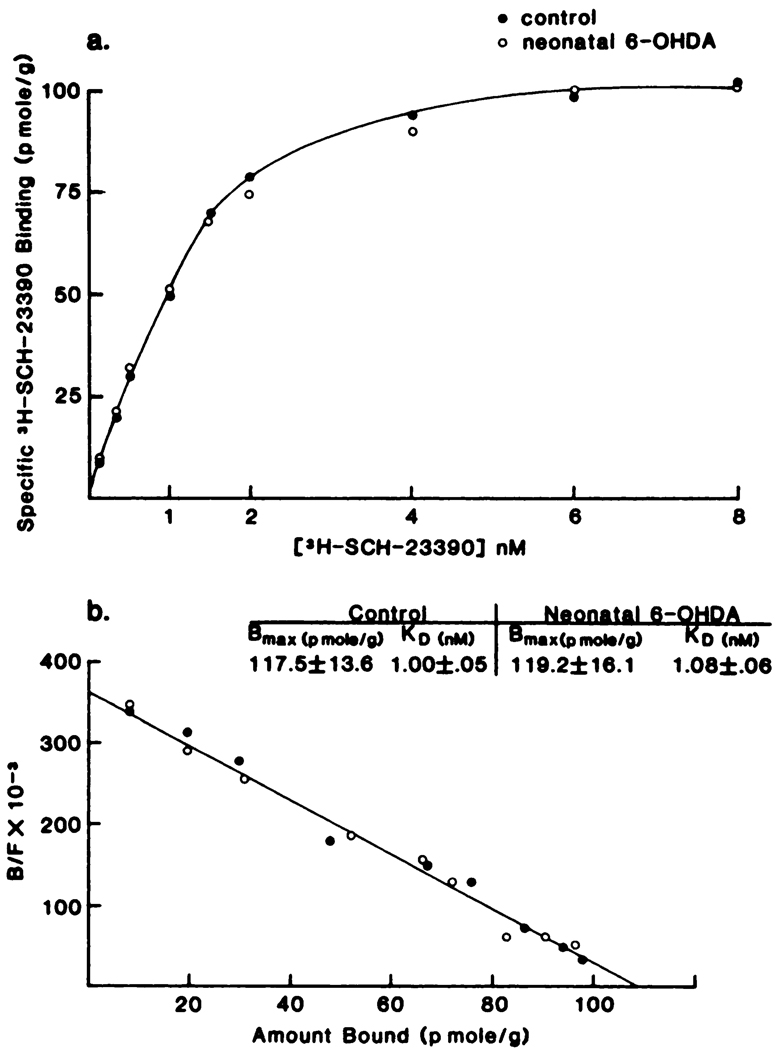
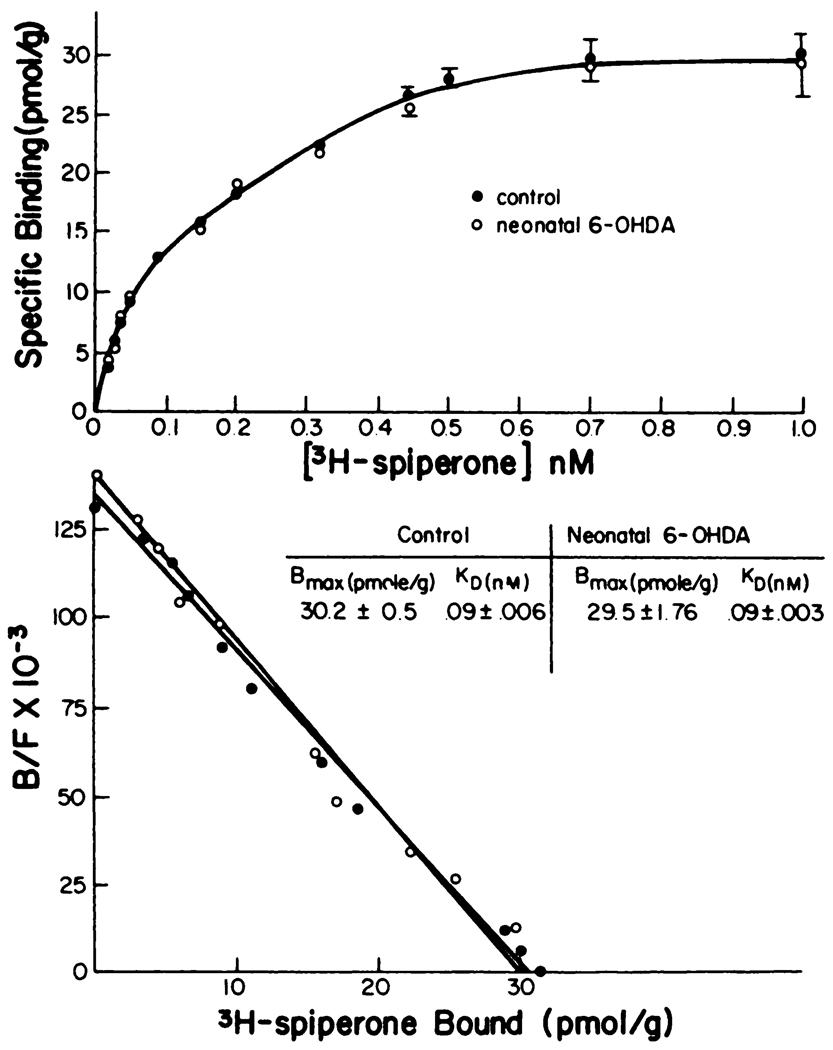
As shown in figure 4, the binding characteristics of [3H]spiperone were not altered by neonatal-6-OHDA treatment. Neither the Kd nor Bmax for spiperone binding in striatum was different from that obtained from unlesioned-control rats (fig. 4). Furthermore, binding of [3H]spiperone to striatal membranes from adult-6-OHDA-lesioned rats was not altered (table 8). In contrast to results with 6-OHDA lesioned rats, binding of [3H]spiperone to striatal membranes was elevated significantly in rats treated chronically with haloperidol (1 mg/kg/day for 16 days; see table 8). Thus, the 6-OHDA-lesioned rats do not demonstrate the change in [3H]spiperone binding seen after chronic haloperidol treatment.
Fig. 4.
Saturation analysis of [3H]spiperone binding to striatal membranes from control and neonatally 6-OHDA-treated rats. All determinations were in the presence of 0.12 M NaCl and 20 nM ketanserin. See table 8 for other values for rats lesioned with 6-OHDA when adults or rats treated chronically with haloperidol. Striatal tissue from three rats was pooled for each of the three independent determinations (i.e., total = 9). Data are presented as the mean of these separate determinations. [3H]-Spiperone binding is presented as a function of ligand concentration: a, Scatchard transformation; b, the calculated values for the KD and Bmax are provided in the insert. There were no significant differences between groups (P > .1). B/F, bound/free.
TABLE 8. Summery of binding characteristics for [3H]spiperone binding after adult 6-OHDA-lesions or haloperidol.
Adult 6-OHDA refers to rats that received 6-OHDA when adult. chronic haloperidol refers to rats that received haloperidol daily (1 mg/kg) for 16 days. Values from these experiments are the mean ± S.E.M. of three individual Scatchard determinations (See fig. 5 for value for neonatally lesioned rats and matched controls).
| Treatments | Bmax | Kd |
|---|---|---|
| pmol/g | nM | |
| Experiment I | ||
| Control | 38.3 ± 1.36 | 0.1 ± 0.005 |
| Adult 6-OHDA | 39.5 ± 1.73 | 0.09 ± 0.015 |
| Experiment II | ||
| Control | 36.2 ± 3.0 | 0.1 ± 0.008 |
| Chronic haloperidol | 46.0 ± 2.9** | 0.1 ± 0.003 |
P < .01 when compared to control.
Binding characteristics for [3H]SCH 23390 to striatal tissue from neonatally lesioned rats did not differ from control (fig. 5). Binding of [3H]SCH 23390 to striatal membranes from adult-6-OHDA-treated rats was also found not to differ from control (control, 32.8 ± 2.8 pmol/g of tissue; adult-6-OHDA, 35.2 ± 2.0 pmol/g of tissue; P > .1; N = 10 and 6, respectively, for the groups). In contrast to the elevated [3H]spiperone binding observed in rats treated chronically with haloperidol (table 8), [3H]SCH-23390 was not altered after this treatment (30.6 ± 1.3 pmol/g in controls and 33 ± 2.7 pmol/g in rats treated chronically with haloperidol; P > .1).
Fig. 5.
Saturation analysis of [3H]SCH-23390 binding to striatal membranes from neonatally 6-OHDA-treated rats. Twelve controls and 12 neonatal 6-OHDA-treated rats were used. Striatal tissue from three rats was pooled to obtain four independent determinations. All binding was performed in the presence of 0.12 M NaCl. Data are presented as the mean of four separate saturation curves. Upper, [3H]SCH-23390 binding as a function of ligand concentration. Lower, Scatchard transformation of data in A. The calculated values for the Kd and Bmax are provided in an insert. There are no significant differences between the groups (P > .1). B/F, bound/free.
Discussion
Systemic administration of D1- and D2-dopamine agonists induces behavioral responses in 6-OHDA-lesioned rats that are of a greater magnitude than that induced in unlesioned controls (Breese et al., 1985a,b; Arnt, 1985). The enhanced response to dopamine agonists in 6-OHDA-lesioned rats could be reflecting an increased number of dopamine receptors, a change in the sensitivity of secondary and tertiary messenger systems linked to the dopamine recognition sites or an alteration in nondopaminergic neural mechanisms which influence the response to dopamine receptor stimulation. A major focus for the present work was to determine if a change in D1- and D2-dopamine receptor binding explained the enhanced behavioral responses induced by D1- and D2-dopamine agonist administration to 6-OHDA-lesioned rats. In order to establish that the brain areas used for the binding studies were behaviorally sensitive to D1- and D2-dopamine agonists, SKF-38393 (D1) and LY-171555 (D2) were administered into the nucleus accumbens and the caudate, the dopamine-rich terminal regions used for the binding investigations, and behavioral responses were assessed in unlesioned controls and the 6-OHDA-lesioned rats.
Intracerebral administration of dopamine into nucleus accumbens increases locomotor activity (Costall et al., 1975; Pijnenburg et al., 1976). Receptor binding studies have demonstrated the presence of both D1- and D2-dopamine receptors in nucleus accumbens tissue (table 5; Seeman, 1980; Schulz et al., 1985). In the present study, the locomotor response induced by D1- and D2-dopamine agonist administration into nucleus accumbens increased locomotor activity more in the 6-OHDA-lesioned rats than in unlesioned controls. The locomotor response to LY-171555 was considerably less than that observed after SKF-38393 microinjection into the nucleus accumbens of both neonatally and adult-6-OHDA-lesioned rats. The observation in neonatally lesioned rats is consistent with other data obtained after i.p. administration of these drugs. In contrast, the locomotor responses observed with adult-6-OHDA-lesioned rats after injection of LY-171555 or SKF-38393 into the nucleus accumbens did not parallel locomotor responses observed after parenteral administration (Breese et al., 1985a,b). The neural basis of these apparently paradoxical findings could have relevance to why locomotor responses to the D1- and D2-dopamine agonists differ between adult- and neonatal-6-OHDA-lesioned rats after systemic (i.p.) administration. Perhaps these findings in the 6-OHDA-lesioned rats relate to evidence that striatal efferents are associated with different dopamine receptors (Herrera-Marschitz and Ungerstedt, 1984) or that D1-dopamine receptors interact with D2-dopamine receptor function (Breese and Mueller, 1985; Breese et al., 1985a,b). Regardless of the interpretation, the present data document the presence of D1- and D2-dopamine receptors in the nucleus accumbens of both 6-OHDA-lesioned groups which, when activated with selective dopamine agonists, result in enhanced behavioral responses.
Administration of dopamine agonists into the striatum has been associated with behaviors such as sniffing, biting, licking and repetitive movements (Creese and Iversen, 1975; Kelly et al., 1975). This brain area is believed to be the primary site at which these drugs act to produce stereotyped behaviors (Costall et al., 1975; Ernst and Smelik, 1966). In the present investigation, administration of SKF-38393 into the caudate produced a variety of behaviors in 6-OHDA-lesioned rats, with sniffing being the most prominent. After microinjection of LY-171555 into the caudate, sniffing, rearing and locomotion were observed in both 6-OHDA-treatment groups, whereas taffy pulling was observed only in neonatally lesioned rats. Inasmuch as some behaviors were not observed after microinjection of dopamine agonists into the caudate, it is possible that there is regional specificity within the caudate for certain behaviors such as self-biting or, alternatively, that this brain region is not involved in all behaviors elicited after systemic administration of dopamine agonists to lesioned rats. Another possibility is that higher doses must be microinjected into the caudate before such behaviors are observed. Because certain behaviors were observed when SKF-38393 was microinjected into either the caudate or the nucleus accumbens, it appears that some behaviors induced by dopamine agonists may have multiple sites of origin. Thus, these microinjection studies with dopamine agonists suggest that stimulation of dopamine receptors in the caudate nucleus contributes to some of the dopamine-agonist-induced behaviors observed after parenteral (i.p.) administration (Breese et al., 1985a,b). Furthermore, both D1- and D2-dopamine agonists are able to induce a greater incidence of behaviors after microinjection into the caudate nucleus of 6-OHDA-lesioned rats than in unlesioned controls.
Because dopamine receptors in both the nucleus accumbens and caudate of 6-OHDA-lesioned rats were found to elicit enhanced behavioral responses after microinjection of D1- and D2-dopamine agonists, investigations were undertaken to determine if an increase in receptor number could account for the behavioral supersensitivity observed in the lesioned rats. [3H]Spiperone binding to membranes from striatum or nucleus accumbens was not altered by the 6-OHDA lesions induced by intracisternal administration, a finding in agreement with earlier reports (Mailman et al., 1981, 1983). These results contrast with the increase in 3H-neuroleptic binding observed in vitro in tissue from rats with unilateral 6-OHDA lesions (Creese et al., 1977; Staunton et al., 1981). Inasmuch as the lesions in our animals were not limited to a unilateral nigrostriatal pathway, but destroyed dopaminergic neurons throughout the brain, the type of lesion is a likely explanation for the observed differences in binding characteristics for [3H]spiperone among these studies. However, Bennett and Wooten (1986) have reported that in vivo binding of [3H]spiperone is not altered after a unilateral lesion to dopamine-containing neurons.
The ability of ketanserin to displace [3H]spiperone confirms earlier work indicating that the serotonin-2 site can contribute to the binding of [3H]spiperone to brain tissue (Hamblin et al., 1984; MacKenzie and Zigmond, 1984). However, with or without the addition of ketanserin to prevent binding to this serotonin site, 6-OHDA treatment did not produce a significant increase in binding of [3H]spiperone (table 7). In contrast to the 6-OHDA treatments, chronic haloperidol treatment caused an elevation of [3H]spiperone binding in the caudate, as reported previously (see Seeman, 1980). It appears, therefore, that the enhanced behavioral responses observed in lesioned rats after administering a selective D2-dopamine agonist is not always accompanied by an increased binding of [3H]spiperone. This conclusion is supported by the work of Koller et al. (1984), who found that pergolide, a dopamine agonist, prevented the increase in [3H]spiperone binding, which accompanies chronic administration of haloperidol, but did not reduce the behavioral supersensitivity observed to a dopamine agonist after chronic exposure to a neuroleptic. It is not clear if these results are suggesting that [3H]spiperone is binding to a site which doss not represent the action of LY-171555 or whether other mechanisms responsible for the enhanced responses of this D2-dopamine agonist in 6-OHDA-lesioned rats.
Mailman et al. (1983) reported that [3H]dopamine binding to caudate was diminished in rats treated neonatally with 6-OHDA, suggesting that the [3H]dopamine was binding to a site on dopaminergic neurons. However, because this presynaptic site on dopaminergic terminals has properties of a D2-dopamine receptor (Kebabian and Calne, 1979), it is not clear why binding of [3H]spiperone is not also reduced by the 6-OHDA treatments. One possible explanation might be an equal rise in the number of postsynaptic sites associated with [3H]spiperone binding to compensate for the loss of autoreceptors (i.e., no net change compared to control). Nevertheless, this possibility raises questions about why there are differences in results depending on whether a dopamine agonist ([3H]dopamine) or dopamine antagonist ([3H]spiperone) is used to delineate receptor number.
Although functional responses to SKF-38393 were enhanced in neonatally 6-OHDA-lesioned rats, binding of [3H]SCH 23390 (a selective D1-dopamine receptor antagonist) to caudate or nucleus accumbens membranes from these animals was not altered. Binding of [3H]SCH-23390 to caudate membranes also was not affected by chronic haloperidol treatment, a finding in agreement with recent reports (MacKenzie and Zigmond, 1985; Creese and Chen, 1985). These studies indicate that the site associated with spiperone binding can adapt independently of the site at which SCH 23390 binds. Adult-6-OHDA-lesioned rats are reported to have an increase in dopamine-stimulated-adenylate cyclase (Mailman et al., 1981), whereas in neonatally 6-OHDA-lesioned rats the activity of this enzyme is unchanged (Mailman et al., 1983). Inasmuch as binding of [3H]SCH 23390 in the present report was not altered by either of these 6-OHDA treatments, it appears that a simple association between [3H]SCH 23390 binding and adenylate cyclase activity does not exist. It is not known whether these observations indicate two distinct D1-dopamine receptor sites, an alteration in cyclase reactivity without a change in receptor number or both.
Because Parkinson’s patients as well as children with Lesch-Nyhan disease have reduced brain dopamine (Hornykiewicz, 1973; Lloyd et al., 1981), binding results obtained in the 6-OHDA-lesioned rats, depending upon the age at which treatment occurred (Breese et al., 1984a), may be relevant to the neuropathological changes in these clinical disorders. Although no data are available presently on binding of dopamine receptor antagonist ligands in Lesch-Nyhan disease, there are data reported from Parkinson’s patients at autopsy. For example, [3H]haloperidol binding has been found to be elevated in putamen (Guttman and Seeman, 1985) of patients with Parkinsonism, particulary those that had not received l-dopa (Lee et al., 1978; Bokobza et al., 1984). Binding of [3H]haloperidol in caudate or putamen tissue from Parkinson’s patients has not been found to differ from normal, if the patients were being treated with l-dopa (Guttman and Seeman, 1985), although no change (Bobobza et al., 1984) and increases (Guttman and Seeman, 1985) are reported in caudate of patients not receiving this drug. In view of these clinical results, the absence of a change in [3H]spiperone receptor binding in the 6-OHDA-treated rats may be associated with the fact that these animals had been exposed to dopamine agonists. However, even if this should be the case, enhanced behavioral responses to the D2-dopamine agonist persist in the lesion rats, even though the number of dopamine antagonist binding sites are not altered.
In addition to results with 3H-neuroleptic ligands, several studies have been performed on tissue from patients with Parkinsonism to evaluate adenylate cyclase activity and [3H]SCH 23390 binding. Dopamine-stimulated adenylate cyclase activity is reported to be decreased (Shibuya, 1979) or increased (Nagatsu et al., 1978) in patients with Parkinsonism. On the other hand, [3H]SCH 23390 binding is reported to be not changed (Pimoule et al., 1985; Raisman et al., 1985) or elevated (Raisman et al., 1985) in patients with Parkinsonism. The length of time that patients are free of l-dopa was suggested as a possible reason for the conflicting results, because no change in [3H]SCH 23390 was observed in those patients that had not received l-dopa from 5 days to 4 years before death (Raisman et al., 1985). Thus, the results with [3H]SCH 23390 in patients with Parkinsonism seem consistent with the absence of a change in [3H]SCH 23390 binding to striatal tissue observed in adult-6-OHDA-lesioned rats. From the absence of a change in [3H]SCH 23390 binding in neonatally lesioned rats, it would not seem likely that a change in [3H]SCH-23390 binding will be observed in patients with Lesch-Nyhan disease.
In summary, the present investigation has demonstrated the following: 1) D1- and D2-dopamine agonists produced supersensitive behavioral responses when microinjected into the nucleus accumbens and caudate nucleus of 6-OHDA-lesioned rats; 2) locomotor responses from adult-6-OHDA-lesioned rats induced by intra-accumbens microinjection of D1- and D2-dopamine agonists were not the same as those observed after systemic administration; and 3) [3H]SCH 23390 and [3H]spiperone binding to nucleus accumbens and caudate tissue were not altered in neonatally and adult-6-OHDA-treated rats that exhibited enhanced behavioral responses to D1- and D2-dopamine agonist administration. Thus, a change in binding characteristics for the dopamine-antagonist ligands was not associated with the behavioral supersensitivity observed with 6-OHDA-lesioned rats after D1- and D2-dopamine agonist administration. The absence of an association between the enhancement of dopamine-agonist induced behavior and dopamine-antagonist binding suggest that there are deficiencies in our present understanding of dopamine receptor mechanisms. Studies of dopamine agonist binding in dopaminergic terminal areas, evaluation of neural systems that can modulate dopamine receptor function and further definition of cellular mechanisms beyond the dopamine receptor level of lesioned rats are areas for future experimentation that might suggest a molecular mechanism for the enhanced behavioral responses observed after dopamine agonist administration to rats with lesions of dopamine-containing neurons.
Acknowledgments
The authors acknowledge the excellent technical assistance of Susan Emerick, Edna Edwards and Marcine Garrison and the typing of the manuscript by Carolyn Reams.
ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- SMB
self-mutilation behavior
- Bmax
maximum number of binding sites
Footnotes
This work was supported by U.S. Public Health Service Grants MH-36294, HL-31424, NS-21345 and HD-03110.
References
- Altar CA, Kim H, Marshall JF. Computer imaging and analysis of dopamine (D2) and serotonin (S2) binding sites in rat basal ganglia or neocortex labeled by [3H]spiroperidol. J. Pharmacol. Exp. Ther. 1985;233:527–538. [PubMed] [Google Scholar]
- Arnt J. Hyperactivity induced by stimulation of separate D-1 and D-2 receptors in rats with bilateral 6-OHDA lesions. Life Sci. 1985;37:717–723. doi: 10.1016/0024-3205(85)90541-7. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Differential inhibition by dopamine D-1 and D-2 antagonists of circling behavior induced by dopamine agonists in rats with unilateral 6-hydroxydopamine lesions. Eur. J. Pharmacol. 1984;102:349–354. doi: 10.1016/0014-2999(84)90267-x. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Wooten GF. Dopamine denervation does not alter in vivo3H-spiperone binding in rat striatum: Implications for external imaging of dopamine receptors in Parkinson’s disease. Ann. Neurol. 1986;19:378–383. doi: 10.1002/ana.410190412. [DOI] [PubMed] [Google Scholar]
- Billard W, Ruperto V, Crosby G, Iorio LC, Barnett A. Characterization of the binding of 3H-Sch-23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984;35:1885–1893. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Bokobza B, Ruberg M, Scatton B, Javoy-Agid F, Agid Y. [3H] spiperone binding, dopamine and HVA concentrations in parkinson’s disease and supranuclear palsy. Eur. J. Pharmacol. 1984;99:167–175. doi: 10.1016/0014-2999(84)90238-3. [DOI] [PubMed] [Google Scholar]
- Breese GR, Baumeister AA, McCown TJ, Emerick SG, Frye GD, Crotty K, Mueller RA. Behavioral differences between neonatal and adult 6-hydroxydopamine-treated rats to dopamine agonists: Relevance to neurological symptoms in clinical syndromes with reduced brain dopamine. J. Pharmacol. Exp. Ther. 1984a;231:343–354. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Baumeister A, Napier TC, Frye GD, Mueller RA. Evidence that D-1 dopamine receptors contribute to the supersensitive behavioral responses induced by l-dihydroxyphenylalanine in rats treated neonatally with 6-hydroxydopamine. J. Pharmacol. Exp. Ther. 1985a;234:287–295. [PubMed] [Google Scholar]
- Breese CR, Frye GD, McCown TJ, Mueller RA. Comparison of the CNS effects induced by TRH and bicuculline after microinjection into various brain sites: Absence of support for a GABA antagonist action for TRH. Pharmacol. Biochem. Behav. 1984b;21:145–149. doi: 10.1016/0091-3057(84)90144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Mueller RA. SCH-23390 antagonism of a D-2 dopamine agonist depends upon catecholaminergic neurons. Eur. J. Pharmacol. 1985;113:109–114. doi: 10.1016/0014-2999(85)90349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Mueller RA, Mailman RB. Effects of dopaminergic agonists and antagonists on in vivo cyclic nucleotide content Relation of guanosine 3′5′-monophosphate (cGMP) changes in cerebellum to behavior. J. Pharmacol. Exp. Ther. 1979;209:262–270. [PubMed] [Google Scholar]
- Breese GR, Napier TC, Mueller RA. Dopamine agonist-induced locomotor activity in rats treated with 6-hydroxydopamine at differing ages: Functional supersensitivity of D-1 dopamine receptors in neonatally lesioned rats. J. Pharmacol Exp. Ther. 1985b;234:447–455. [PubMed] [Google Scholar]
- Breese GR, Smith RD, Cooper BR, Grant LD. Alterations in consummatory behavior following intracisternal injection of 6-hydroxydopamine. Pharmacol. Biochem. Behav. 1973;1:319–328. doi: 10.1016/0091-3057(73)90124-x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Traylor TD. Effects of 6-hydroxydopamine on brain norepinephrine and dopamine: Evidence for selective degeneration of catecholamine neurons. J. Pharmacol. Exp. Ther. 1970;174:413–420. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Traylor TD. Developmental characteristics of brain catecholamines and tyrosine hydroxylase in the rats: Effects of 6-hydroxydopamine. Br. J. Pharmacol. 1972;44:210–222. doi: 10.1111/j.1476-5381.1972.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Neumeyer JL. Differences in the nature of the stereotyped behavior by apomorphine derivatives in the rat and in their actions in extrapyramidal and mesolimbic brain areas. Eur. J. Pharmacol. 1975;31:1–16. doi: 10.1016/0014-2999(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Christensen AV, Arnt J, Hyttel J, Larson JJ, Svendsen O. Pharmacological effects of a specific dopamine D-1 antagonist SCH-23390 in comparison with neuroleptics. Life Sci. 1984;34:1529–1540. doi: 10.1016/0024-3205(84)90607-6. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science (Wash. DC) 1977;197:596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- Creese I, Chen A. Selective D1 dopamine receptor increase following chronic treatment with SCH 23390. Eur. J. Pharmacol. 1985;109:127–128. doi: 10.1016/0014-2999(85)90549-7. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Ernst AM, Smelik DG. Site of action of dopamine and apomorphine on compulsive gnawing behaviour in rats. Experientia (Basel) 1966;22:837–838. doi: 10.1007/BF01897450. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Law TY, Asano T, Weta K. Alterations in dopamine receptors. Effects of lesions and haloperidol treatment. Commun. Psychopharmacol. 1980;4:21–25. [PubMed] [Google Scholar]
- Guttman M, Seeman P. L-DOPA reverses the elevated density of D2-dopamine receptors in Parkinson’s diseased striatum. J. Neural Transm. 1985;64:93–103. doi: 10.1007/BF01245971. [DOI] [PubMed] [Google Scholar]
- Hamblin MW, Leff SE, Creese I. Interactions of agonists with D-2 dopamine receptors: Evidence for a single receptor population existing in multiple agonist affinity-states in rat striatal membranes. Biochem. Pharmacol. 1984;33:877–887. doi: 10.1016/0006-2952(84)90441-6. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Shapiro BS, Duvoisin RC. The relationship between loss of dopamine nerve terminals, striatel [3H]-spiroperidol binding and rotational behavior in unilaterally 6-hydroxydopamine-lesioned rats. Brain Res. 1981;211:285–292. doi: 10.1016/0006-8993(81)90614-4. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Battaglia G, Norman AB, Iorio LC, Creese I. Guanine nucleotide regulation of agonist interactions of 3H-SCH 28396-labelled D1-dopamine receptors in rat striatum. Eur. J. Pharmacol. 1986;121:31–38. doi: 10.1016/0014-2999(86)90389-4. [DOI] [PubMed] [Google Scholar]
- Herrera-marschitz M, Ungerstedt U. Evidence that striatal efferents relate to different dopamine receptors. Brain Res. 1984;323:269–278. doi: 10.1016/0006-8993(84)90297-x. [DOI] [PubMed] [Google Scholar]
- Hollister AS, Breese GR, Cooper BR. Comparison of tyrosine hydroxylase and dopamine-β-hydroxylase inhibition with the effects of various 6-hydroxydopamine treatments on d-amphetamine induced motor activity. Psychopharinacologia. 1974;36:1–16. doi: 10.1007/BF00441377. [DOI] [PubMed] [Google Scholar]
- Hollister AS, Breese GR, Mueller RA. Role of monoamine neural systems in l-dihydroxyphenylalanine stimulated activity. J. Pharmacol. Exp. Ther. 1979;208:37–43. [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson’s disease: From brain homogenate to treatment. Fed. Proc. 1973;32:183–190. [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Lettz FH, Houser VP, Korduba A. SCH-23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J. Pharmacol. Exp. Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- Jackson DM, Anden N-E, Dahlstrom A. A functional effect of dopamine in the nucleus accumbens and in some dopamine-rich areas of the rat brain. Psychopharmacologia. 1975;45:139–149. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature (Lond) 1979;227:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Breese GR, Mailman RB. Simultaneous quantification of dopamine, 5-hydroxytryptamine, and four metabolically related compounds by means of reverse phase HPLC with electrochemical detection. J. Chromatogr. Biol. Med. Appl. 1981;225:347–357. doi: 10.1016/s0378-4347(00)80283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Smith DA, Ondrusek MG, Mailman RB, Mueller RA, Breese GR. Differential effects of “dopaminergic agonist” on measures of dopaminergic function. Soc. Neurosci. Abstr. 1979;5:562. [Google Scholar]
- Koller WC, Cortin JC, Fields JZ. Pergolide down-regulates D-2 dopamine receptors but fails to block haloperidol induced behavioral supersensitivity. Soc. Neurosci. Abstr. 1984;10:1136. [Google Scholar]
- Konig JFR, Klippel RA. The Rat Brain: A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. R. E. Krieger Publishing Co.; 1963. pp. 1–162. [Google Scholar]
- Lee T, Seeman P, Rajput A, Farley IJ, Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson’s disease. Nature (Lond.) 1978;273:59–61. doi: 10.1038/273059a0. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH. Biochemical evidence of dysfunction of brain neurotransmitters in the Loach-Nyhan syndrome. N. Engl. J. Med. 1981;305:1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- MacKenzie RG, Zigmond MJ. High and low-affinity states of striatal D-2 receptors are not affected by 6-hydroxydopamine or chronic haloperidol treatment. J. Neurochem. 1984;43:1310–1318. doi: 10.1111/j.1471-4159.1984.tb05388.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie RG, Zigmond MJ. Chronic neuroleptic treatment increases D2 but not D1 receptors in rat striatum. Eur. J. Pharmacol. 1985;113:159–165. doi: 10.1016/0014-2999(85)90732-0. [DOI] [PubMed] [Google Scholar]
- Mailman RB, Kilts CD, Beaumont K, Breese GR. “Supersensitivity“ of dopamine systems: Comparisons between haloperidol withdrawal, intracisternal and Unilateral 6-hydroxydopamine (6-OHDA) treatments. Fed. Proc. 1981;40:291. [Google Scholar]
- Mailman RB, Towle A, Schulz DW, Lewis MH, Breese GR, Dehaven DH, Krigman MR. Neonatal 6-OHDA treatment of rats Changes in dopamine (DA) receptors, striatal neurochemistry and anatomy. Soc. Neurosci. Abstr. 1983;9:932. [Google Scholar]
- Molloy AG, Waddington TL. Dopaminergic behavior stereospecifically promoted by the D1 agonist R-SKF-38393 and selectively blocked by the D1 antagonist SCH 23390. Psychopharmacology. 1984;82:409–410. doi: 10.1007/BF00427697. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Kanamori T, Kato R, Iizuka R, Narabayashi H. Dopamine-stimulated adenylate cyclase activity in the human brain. Changes in Parkinsonism. Biochem. Med. 1978;19:360–365. doi: 10.1016/0006-2944(78)90036-4. [DOI] [PubMed] [Google Scholar]
- Neve KA, Altar CA, Wong CA, Marshall TF. Quantitative analysis of (3H)spiroperidol binding to rat forebrain sections: Plasticity of neostriatal dopamine receptors after nigrostriatal injury. Brain Res. 1984;302:9–18. doi: 10.1016/0006-8993(84)91280-0. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJJ, Honig WMM, Van Dee Heyden JAM, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur. J. Pharmacol. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Pimoule C, Schoemaker H, Reynolds GP, Langer SZ. [3H] SCH 23390 labeled D1-dopamine receptors are unchanged in schizophrenia and Parkinson’s disease. Eur. J. Pharmacol. 1985;114:235–237. doi: 10.1016/0014-2999(85)90634-x. [DOI] [PubMed] [Google Scholar]
- Raisman R, Cash R, Ruberg M, Javoy-Agid F, Agid Y. Binding of [3H]SCH 23390 to D-1 receptors in the putamen of control and Parkinsonian subjects. Eur. J. Pharmacol. 1985;113:467–468. doi: 10.1016/0014-2999(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Schoenfeld R, Uretsky N. Altered response to apomorphine in 6-hydroxydopamine-treated rats. Eur. J. Pharmacol. 1972;19:115–118. doi: 10.1016/0014-2999(72)90085-4. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Stanford EJ, Wyrick SW, Mailman RB. Binding of 3H-SCH 23390 in rat brain Regional distribution and effects of assay conditions and GTP suggest interactions at a D1-like dopamine receptor. J. Neurochem. 1985;45:1601–1611. doi: 10.1111/j.1471-4159.1985.tb07233.x. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol. Rev. 1980;32:229–313. [PubMed] [Google Scholar]
- Setler PE, Sarau HM, Zirkle CL, Saunders HL. The central effects of a novel dopamine agonist. Eur. J. Pharmacol. 1978;50:419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Dopamine-sensitive adenylate cyclase activity in the striatum in Parkinson’s disease. J. Neural Transm. 1979;44:287–295. doi: 10.1007/BF01250323. [DOI] [PubMed] [Google Scholar]
- Smith RD, Cooper BR, Breese GR. Growth and behavioral changes in developing rats treated intracisternally with 6-hydroxydopamine: Evidence for involvement of brain dopamine. J. Pharmacol. Exp. Ther. 1973;185:609–619. [PubMed] [Google Scholar]
- Staunton DA, Wolfe BB, Groves PM, Molinoff PB. Dopamine receptor changes following destruction of the nigrostriatal pathway: Lack of a relationship to rotation behavior. Brain Res. 1981;211:315–327. doi: 10.1016/0006-8993(81)90704-6. [DOI] [PubMed] [Google Scholar]
- Tsuruta K, Frey EA, Grewe CW, Cote TE, Eskay RL, Kebabian TW. Evidence that LY-141865 specifically stimulates the D-2 dopamine receptor. Nature (Lond.) 1981;292:463–465. doi: 10.1038/292463a0. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acts Physiol. Scand. 1971;367 suppl.:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Uretsky NJ, Schoenfeld RI. Effect of L-DOPA on the locomotor activity of rats pretreated with 6-hydroxydopamine. Nat. New Biol. 1981;234:157–159. doi: 10.1038/newbio234157a0. [DOI] [PubMed] [Google Scholar]