Abstract
Evidence is provided in this manuscript that ethanol acts directly on neurons in the medial septal area (MSA). Initially, the electrophysiological characteristics of MSA neurons in freely moving rats were characterized and found similar to that observed in rats anesthetized with urethane, but not chloral hydrate. Therefore, urethane was used to evaluate the effects of ethanol in anesthetized rats. The conclusion that ethanol influences neural function in the MSA is based on electrophysiological data that ethanol (0.75–3.0 g/kg i.p.) suppresses neural firing of medial septal cells in urethane-anesthetized as well as in unanesthetized rats in a dose-related fashion. Concurrent with the suppression of firing rate, the rhythmic bursting pattern of activity of MSA neurons is disrupted by ethanol. The changes observed in the MSA could not be attributed to an indirect action of ethanol on afferents from the lateral septum to the MSA, because ethanol did not alter neural activity of cells in the lateral septum. These data indicate that ethanol does not have a common action on all neurons. Neural activity in the MSA recovered from the acute action of ethanol at a time when blood ethanol levels were near maximal, indicating an acute tolerance to this effect of ethanol. The time course of change in neural activity in the MSA was highly correlated with the time course of a measure of behavioral sedation, but not the hypothermia produced by ethanol. Thus, the work in this manuscript supports the view that ethanol has selective actions on MSA neurons in the rat septal area and that these actions may influence the behavioral sedation induced by ethanol.
The complex cognitive and behavioral effects of ethanol are believed to result from disruption of normal neural processing in the central nervous system. However, the specific brain regions responsible for the deficiencies produced by ethanol have been poorly defined. Identification of brain regions critical for ethanol-induced impairment of specific functions should allow better resolution of the potential neural mechanisms involved in central nervous system changes produced by ethanol (Breese et al., 1988).
One such approach to define sites of action for ethanol has been to microinject drugs that enhance or antagonize specific actions of ethanol into selected brain regions. It has been established that drugs altering GABA function can influence ethanol-induced sedation (Frye and Breese, 1982; Lilequist and Engel, 1982; Hartz et al., 1983). By defining sites involved in the effects of these drugs on specific impairments produced by ethanol, it can be inferred that ethanol may act by altering its neural function at this site. Microinjection of bicuculline and thyrotropin-releasing hormone into the MSA will antagonize and microinjection of GABAmimetic drugs into the MSA will enhance ethanol-induced sedation (Breese et al., 1984; McCown et al., 1986; Givens and Breese, 1989). Such evidence supports the hypothesis that the MSA plays a critical role in this action of ethanol (Breese et al., 1988).
The purpose of the present study was to investigate further the potential role of the MSA in the sedative actions of ethanol. The approach taken was to examine the effects of ethanol on neural activity in the MSA. In order to establish the most suitable anesthetic to investigate this action of ethanol, the effects of urethane and chloral hydrate on neural activity were compared to recordings in unanesthetized rats. Next, the effects of ethanol on neural activity in the MSA were examined in both anesthetized and unanesthetized rats. The time course of changes in neural activity produced by ethanol in the MSA was compared with the time course for ethanol-induced sedation, hypothermia and changes in blood-ethanol levels. Because the lateral septum provides the major source of afferents to the MSA (Swanson and Cowan, 1979), the effect of ethanol on neural activity in the lateral septum was determined to evaluate the possibility that a change in neural function in the lateral septum was responsible for changes induced by ethanol in the MSA. Collectively, these experiments will demonstrate that ethanol has a selective effect on neurons of the MSA without affecting neural activity in the lateral septum.
Methods
Animals
Male Sprague-Dawley rats, purchased from Charles River Laboratory (Raleigh, NC), weighing 300 to 400 g were used in all experiments. The animals were group housed (four rats per cage) in a room that was maintained at 25°C with a light/dark cycle of 12/12 hr. Food and water were available ad libitum in the home cage.
Electrophysiological analysis: animal preparation
Two types of animal preparations were used in the experimental procedures to evaluate the effects of ethanol on neural activity. In one of the approaches the rats were anesthetized and in the other the rats were free moving (i.e., unanesthetized). In initial experiments, rats were anesthetized with either urethane (1.5 g/kg i.p.) or chloral hydrate (400 mg/kg) to evaluate the effects of these anesthetics on neural activity so the most appropriate anesthetic for investigating the action of ethanol on neural activity could be established. Once the rats were anesthetized, the scalp was retracted and burr holes placed in the skull above the MSA (cells of the medial septal nucleus and the vertical limb of the nucleus of the diagonal band). These rats were then placed in a stereotaxic instrument. For recording in anesthetized rats, electrodes were lowered into the MSA or lateral septal regions at a 15° angle. The coordinates for the point of entry into the brain were 0.6 mm anterior to bregma and 1.5 mm lateral to the midsaggital suture. The electrode was advanced into the MSA by means of a hydraulic microdrive (Trent Wells, Inc., South Gate, CA). Lateral septal neurons were found 4.5 to 5.5 mm ventral and MSA neurons 6.0 to 7.0 mm ventral to the surface of the brain. Body temperature of anesthetized rats was monitored continuously with a rectal probe and was maintained with a hot water bottle within 37 ± 0.5°C.
To evaluate the effects of ethanol in freely moving rats, a threaded base (onto which the microdrive is mounted) was implanted onto the skull of rats anesthetized with sodium pentobarbital (40 mg/kg). The base was attached to the skull at a point 1.5 lateral to midline, 0.6 mm anterior to bregma at a 15° angle toward midline according to the atlas of Paxinos and Watson (1986). A threaded cap was placed on the base. A stainless-steel reference wire (120 μm diameter, insulated except at tip) was lowered into the frontal lobe of the cortex and soldered to a 3-pin connector that was secured to the skull by dental acrylic. The apparatus used for chronic single unit recording was a large circular container (52 cm × 34 cm diameter) with a 1.5 cm diameter rod (35 cm above the floor) extended through the container walls. In the center of the rod was a wire-covered cylinder (13 cm ×7.5 cm diameter). The rod and cylinder assembly ran perpendicular to the base of the container and was connected by a belt system to a motor that rotated the cylinder at a speed of 3 revolutions per min. Rats were placed on top of the apparatus and were required to walk in order to avoid a 35 cm drop to the floor of the container. Before surgery, rats were acclimated to the testing apparatus by walking on the cylinder for 60 min a day for 5 days. The animals were allowed 7 days to recover from the surgical procedure before neural activity was recorded.
Electrophysiological analysis: single unit recording procedure
Extracellular electrode potentials were amplified via a Grass P15 high impedance preamplifier (Quincy, MA) and a secondary amplifier, and monitored with an Tektronix oscilloscope and audiomoniter (Portland, OR). These signals were filtered by the preamplifier (300 Hz 1/2 amplitude low and a 10 kHz 1/2 amplitude high filter cutoffs). Individual spikes were digitized by a window comparator with the 0.5 msec square pulse output fed into an IBM PC XT which generated ratemeter, interspike interval and peristimulus interval histograms. The analog raw signal, a voice channel and stimulus synchronized pulses were recorded on magnetic tape by two dual channel tape recorders for offline analysis.
Action potentials isolated from background activity with at least a 3-to- 1 signal-to-noise ratio and a constant duration and configuration were defined as a single neuron. MSA neurons were identified by their rhythmically bursting pattern of activity. Bursts were defined as groups of 2 to 18 spikes, with a duration of 50 to 200 msec that occurred at a frequency of 3 to 8 Hz. Lateral septal neurons were identified by their location and rate, which was generally slower than that for MSA cells.
After encountering an active cell, the spontaneous firing rate was monitored for at least a 20-min base-line period. Once a stable base line was established for neurons in the MSA, ethanol (10% prepared in 0.9% NaCl) at doses of 0.75, 1.5 or 3.0 g/kg or an equivalent volume of saline was administered i.p. over a 1-min period. Only the 1.5-g/kg dose was administered to rats from which neural activity was recorded in the lateral septum. The unit activity was monitored for 2 to 3 hr. ISIHs were generated from 2-min spike trains and compared pre- and post-ethanol. Rhythmically firing MSA neurons have a characteristic ISIH which has a large number of spikes which occur in the 0 to 20 msec range and a second mode in the histogram which occurs after the interburst interval (70–110 msec range). The effect of ethanol on the rhythmic bursting pattern of activity was assessed by calculating the percentage of spikes occurring during the interburst interval (30–60 msec) before and after ethanol administration. The entire experiment was recorded on magnetic tape so that the following characteristics could be determined off-line: the action potential configuration and duration, the burst frequency and duration, the overall firing frequency and the number of spikes per burst.
Recordings from MSA neurons were made in 26 freely moving rats in an isolated, sound attenuated recording room. A head-mounted movable microdrive (Deadwyler et al., 1979) containing a glass single barrel micropipette was attached to the threaded base. The microdrive was advanced until a single unit was encountered. Rats were required to walk on the rotating cylinder throughout the experiment except during the base-line period when the single unit activity was characterized. Thus, the rotating cylinder standardized the behavior of the rats and prevented them from performing behaviors that could influence single unit activity of cells in the medial septum from which recordings were being made. Only MSA neurons that remained rhythmically bursting when the rotating cylinder was turned off were used in the analysis. This yielded recordings from 52 MSA cells. After the 20 min base-line period, the rotating cylinder was turned off, the animal was picked up, injected with saline or ethanol and then placed back on the cylinder. The cylinder was again activated. Animals that received saline or 0.75 g/kg were able to continue walking on the rotorod. Some animals that received 1.5 g/kg of ethanol had difficulty during the first 15 min; therefore, the cylinder rotation was slowed enough to allow them to remain on the rotating cylinder. Animals receiving 3.0 g/kg of ethanol were unable to stay atop the rotating cylinder and were placed on a stationary platform for continued recording. After 30 min, and for every 10 min thereafter, these rats were placed on the cylinder until able to return to walking at full speed. Neural characteristics were evaluated as described for anesthetized rats.
Measurement of aerial righting reflex and rectal temperature
The sedative effects of ethanol were assessed using the “aerial righting reflex” (Frye and Breese, 1982). This investigation was performed in a separate set of rats from those used in the electrophysiological studies. For measuring aerial righting, a meter stick mounted vertically behind a 10 cm thick foam rubber pad allowed measurement of the height at which righting could occur. Rats were held by the back of the neck and the base of the tail in an inverted position at specific heights above a foam pad and released. A successful righting required that the rat land with all four feet on the foam rubber pad at the time of first contact on two of three consecutive releases. Each test began at 5 cm, the height rats given saline or no treatment generally required for a successful landing. Animals were never released from heights greater than 55 cm. The minimum height required for successful righting was used as an index of sedation. Animals received an injection of ethanol or saline at time 0 and were then tested for aerial righting ability after the injections every 10 min for 60 min and then every 30 min. Ethanol was administered in a 10% solution over a 1-min period by i.p. injection at a dose of 0.75, 1.5 or 3.0 g/kg b.wt. In addition to aerial righting, body temperature was recorded with a rectal thermometer (Yellow Springs Instrument Co., Yellow Springs, OH) at 30-min intervals throughout the experiment.
Blood ethanol concentration
The concentration of ethanol in venous blood was determined for each animal that received ethanol (Frye et al., 1981). In most electrophysiological experiments and in the rats used for the aerial righting study, tail blood (20 μl) was drawn 60 min after ethanol administration. In the experiment to determine the time course of blood ethanol concentration, the blood samples from a separate group of rats were drawn before the ethanol injection and 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 120, 180 and 240 min after the injection of ethanol. Blood samples (20 μl) collected from the tail vein were diluted immediately with 180 μl of ice-cold distilled water containing 0.2 μg/ml of tert-butanol as an internal standard. The samples were centrifuged (10,000 × g for 10 min) and aliquots (10 μl) of the supernatant were injected into a gas chromatograph (Varian Associates, Palo Alto, CA) that used flame-ionization detection. A glass column packed with 0.2% Carbowax was used to separate ethanol and tert-butanol. Chromatographic conditions were He = 30 ml/min; H2 = 30 ml/min; air = 250 ml/min; injector = 155°C; column = 130°C and detector = 185°C.
Histology
For histological verification of the recording site, an anodal d.c. current of 20 μA was passed for 45 sec through the recording pipette that contained a 2 M NaCl solution saturated with sky blue dye. A cathodal 20 μA current was applied to the stimulation electrode for 45 sec. The animal was then perfused with a 10% formalin solution. The brain was removed, stored in formalin for 3 days and then freeze mounted onto a microtome chuck. With the use of a cryostat and microtome (Damon/IEC Division, Needham Hts., MA), 50-μm slices were sectioned, mounted onto slides and stained with cresyl violet (Fisher Scientific, Pittsburgh, PA). After representative sections were obtained for the recording sites, placements were determined routinely with the use of fresh frozen sections. Rats were overdosed with chloral hydrate, their brains removed, placed on a microtome chuck, then put into powdered dry ice. The brain was sectioned until the electrode tip location was determined. The location of each recording site was logged onto rat brain atlas maps.
Statistical analysis
An analysis of variance for repeated measures was used to analyze the time course of single unit firing rates, blood ethanol concentration and righting reflex after systemic ethanol and saline administration. Analysis of variance also was used to compare electrophysiological characteristics between freely moving and those animals that received anesthesia and data comparing saline with various doses of ethanol. Significant treatment effects were followed with a Newman-Kuhls multiple comparison test where appropriate. Comparisons between time courses for unit activity, aerial righting, blood ethanol levels and body temperature were accomplished with the Pearson’s product-moment correlation analysis.
Results
Effect of anesthesia on neural activity in the MSA
In order to select an anesthetic most appropriate for investigating the electrophysiological actions of ethanol in the MSA, the effects on neural activity of two commonly used anesthetics, chloral hydrate and urethane, were compared with base-line neural activity observed in unanesthetized rats. Whereas MSA neurons under all three conditions fired action potentials in a rhythmic bursting pattern (fig. 1), the burst frequency was significantly slower in rats anesthetized with chloral hydrate (table 1). The base-line firing rate of MSA cells in rats anesthetized with chloral hydrate was slower than in rats anesthetized with urethane, or in freely moving rats (table 1). In addition, MSA cells from chloral hydrate-anesthetized rats were less stable in their rhythmic bursting pattern than in MSA cells from urethane-anesthetized rats. During a given electrode penetration, fewer active MSA cells were encountered in rats anesthetized with chloral hydrate than in freely behaving rats, whereas a greater number were found in rats under urethane anesthesia (table 1). Other basic electrophysiological characteristics of MSA cells under these conditions of anesthesia were not altered. The distribution of recording sites for the anesthetized rats is presented in figure 2.
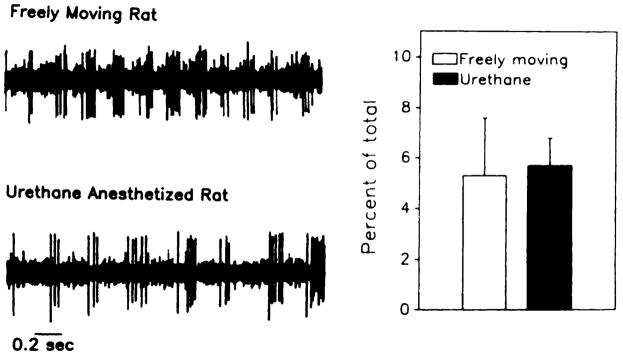
Fig. 1.

Oscilloscope traces of single unit activity from rhythmically bursting neurons of the MSA in unanesthetized (free-moving) rats and in rats anesthetized with urethane or chloral hydrate.
TABLE 1. Comparison of the electrophysiological characteristics of MSA cells in freely moving rats and in rats under urethane or chloral hydrate anesthesia.
Dose of urethane was 1.5 g/kg and the dose of chloral hydrate was 400 mg/kg. Determinations were made 60 min after administration. n, number of determinations.
| Measurement | Freely moving | Urethane | Chloral Hydrate |
|---|---|---|---|
| n = 8 | n = 12 | n = 7 | |
| Firing rate (spikes/sec) | 30.4 ± 3.6 | 27.1 ± 2.8 | 18.4 ± 3.6* |
| Burst frequency (burst/sec) | 6.2 ± 0.2 | 4.1 ± 0.2* | 3.8 ± 0.4* |
| Burst duration (msec) | 71.0 ± 5.5 | 89.0 ± 5.8 | 86.0 ± 10.4 |
| Spikes/burst | 5.9 ± 0.5 | 6.7 ± 0.9 | 4.9 ± 1.1 |
| No. of cells(per track) | 3.1 ± 0.4 | 6.4 ± 0.6* | 1.1 ±0.2* |
P < .05when compared to response measurement in the freely moving rat.
Fig. 2.
Histological location of the tips of electrodes that recorded neurons in the MSA of rats anesthetized with urethane (●)or chloral hydrate (■). The coronal sections also include the location of the electrode tips at which LSI single units were recorded (▼). cc, corpus callosum; cp, caudate-putamen; Is, lateral septum; ac, anterior commissure; ms, medial septum; db, diagonal band (vertical limb).
To determine the relationship between the population of rhythmically bursting MSA neurons in the freely moving rats and the corresponding population in the urethane-anesthetized rat, an additional experiment was performed. MSA neural activity was monitored in freely moving rats, then urethane was administered such that the same single unit could be followed from the unanesthetized to the anesthetized state within a rat. These results, shown in figure 3, demonstrated that the rhythmically bursting pattern of activity in MSA cells remained rhythmically bursting after the induction of urethane anesthesia. From these data, urethane was chosen as the preferred anesthetic to evaluate the actions of ethanol on medial and lateral septal function.
Fig. 3.
Oscilloscope traces of a rhythmically bursting MSA cell before and after the administration of urethane in a freely moving rat. In 6 of 6 cells tested (right side), the rhythmic bursting pattern remained unchanged after urethane. Rhythmicity was determined by the percentage of the total number of spikes that occur with an interspike interval between 40 and 60 msec(P > .1).
Response of MSA cells to ethanol in anesthetized rats
In the first of a series of experiments to evaluate the effects of ethanol on MSA neural activity, the response of rhythmically bursting MSA cells to ethanol was assessed in rats anesthetized with urethane. After ethanol administration, there was an initial increase in neuronal firing (fig. 4A). Because this increase was also observed after saline administration (fig. 4B), this change is probably the result of the injection procedure, as noxious stimuli enhance the neural activity of MSA cells (Dutar et al., 1985). Following this transient change after ethanol, the spontaneous activity of MSA neurons in rats under urethane anesthesia was suppressed (fig. 4).
Fig. 4.
Representative histogram of the effect of (A) ethanol (1.5 g/kg) or (B) saline on the spontaneous activity of a rhythmically bursting MSA neuron in a urethane-anesthetized rat.
The ethanol-induced suppression of spontaneous activity of medial septal cells was found to be dose related (fig. 5). The suppression of activity observed after ethanol did not occur after saline administration suggesting that this change in neural function was an effect of ethanol and not of the injection procedure, per se. The time course of the response to various doses of ethanol under urethane anesthesia conditions demonstrated that ethanol caused not only a dose- but also a time dependent suppression of MSA neural activity (fig. 5A). The blood ethanol concentration in a separate set of rats anesthetized with urethane is presented in figure 5B. Notice that firing rate recovered in the presence of significant ethanol levels in blood.
Fig. 5.
A, effect of ethanol on the firing rate of MSA neurons over time (0–1 20 min) in the urethane-anesthetized rat. Numbers of animals for each treatment was as follows: 0.75 g/kg (n= 13), 1.5 g/kg (n = 9), 3.0 g/kg (n = 7) for ethanol treatment groups, and for the saline group (n = 6). *P < .05 when compared to saline. B, the time course of blood ethanol concentrations after 0.75, 1.5 and 3.0 g/kg of ethanol i.p. in the urethane-anesthetized rat. (n = 4 at each point). All blood ethanol concentrations points beyond 0 min after ethanol are elevated significantly through 120 min; blood ethanol concentration is elevated for the 1.5- and 3.0-g/kg doses of ethanol through 240 min (P < .05). The blood ethanol concentration determinations were made in a group of rats anesthetized with urethane and given ethanol. These data are in a group of rats different from those used to collect the electrophysiological data.
The rhythmic pattern of activity was disrupted by 1.5 g/kg of ethanol in 8 of 12 cells tested. Upon recovery of rhythmic bursting, the burst frequency was reduced from 4.1 ± 0.1 to 3.4 ± 0.3 and 3.2 ± 0.2 bursts per sec by 1.5 and 3.0 g/kg of ethanol (P < .05), respectively, whereas 0.75 g/kg of ethanol had no reliable effect on burst frequency. Under no condition was a prolonged increase in the mean spontaneous discharge rate observed in MSA cells after ethanol administration.
Response of single neurons in the MSA to ethanol in the freely moving rat
In order to test whether the response of MSA neurons to ethanol observed in rats anesthetized with urethane has general validity, an additional series of experiments using chronic single unit recording was carried out in unanesthetized rats walking at a constant rate on a rotating cylinder (see “Methods”). All data concerning recordings from the MSA in these unanesthetized freely moving rats were derived from neurons that fired action potentials in a rhythmically bursting pattern. Furthermore, only those neurons which did not change their rate when the rotating cylinder was turned off were used, eliminating any involvement of motor function in the effects of ethanol on the recording parameters (see “Methods”).
The characteristics of MSA unit activity were remarkably consistent both across and within rats and were similar to the characteristics observed in anesthetized rats (see fig. 1; table 1). MSA cells in the unanesthetized rats fired a burst of 2 to 10 action potentials followed by a silent interburst interval. The mean spontaneous discharge rate of these cells was 31.1 ± 3.6 spikes/sec and the burst frequency was 6.3 ± 0.8 bursts/second (table 2). Histological examination in these rats revealed that all rhythmically bursting cells were located in the MSA, resembling the distribution in the recording sites seen in anesthetized rats (see fig. 2). Cells in the medial septal nucleus were located on the midline, whereas those in the diagonal band were located bilaterally along the lateral border of the nucleus. There were no striking differences in the location of the cells based on response properties, although the more stable rhythmic cells tended to be found in the vertical limb of the diagonal band nucleus.
TABLE 2. Effect of ethanol on the electrophysiological characteristics of MSA neurons in the freely moving rat.
Determinations were made 60 min after ethanol administration. n, number of determinations.
| Measurea | Saline n = 9 | Dose of Ethanol |
||
|---|---|---|---|---|
| 0.75 g/kg | 1.5 g/kg | 3.0 g/kg | ||
| n = 12 | n = 12 | n = 9 | ||
| Burst frequency (bursts/sec) | 6.3 ± 0.8 | 5.6 ± 0.2 | 4.9 ± 0.3* | 4.7 ± 0.3* |
| Burst duration (msec) | 65 ± 8.9 | 72 ± 6.3 | 63 ± 7.3 | 59 ± 6.3 |
| Spikes/burst | 5.6 ± 0.8 | 6.8 ± 1.0 | 7.2 ± 0.8 | 7.4 ± 1.0 |
P < .05 when compared to saline.
A representative histogram of the effect of ethanol (1.5 g/kg) on spontaneous activity of rhythmically bursting neurons in the MSA of a freely moving rat is presented in figure 6. Typically, there was a initial, marked increase in firing rate that was followed immediately by a profound suppression of spontaneous activity that lasted 30 to 120 min. As noted in anesthetized rats (fig. 4), systemic injections of physiological saline also produced the transient excitation observed with ethanol within the first 5 min after the injection, but saline treatment had no other reliable effect on MSA cell activity.
Fig. 6.
Representative histogram of the effect of ethanol (1.5 g/kg) on the spontaneous activity of a rhythmically bursting MSA neuron in a freely moving rat.
Ethanol caused a dose- and time-dependent suppression of MSA neural activity in 21 of the 24 (87%) neurons tested in the freely moving rat (fig. 7A). The response of MSA neurons measured 30-min postinjection at doses of 0.75, 1.5 or 3.0 g/kg of ethanol was 21, 49 and 62% below the base-line firing frequency, respectively. The suppressant effect of ethanol peaked within the first 30 min and then gradually returned to base line. Whereas the rats were somewhat ataxic after the 0.75- and 1.5-g/kg doses of ethanol, they were not affected to the point that they could not continue walking on the rotorod. However, the high dose of ethanol (3.0 g/kg) rendered the rats unable to maintain their position atop the rotating cylinder and necessitated placing these animals on a platform for continued recording. After the 3.0-g/kg dose of ethanol, single unit activity was severely disrupted during the behavioral sedation, then returned to base-line levels with behavioral recovery. Blood levels over the time course of the recording period are presented in figure 7B. As noted in the anesthetized rats, firing rate of the cells in the MSA recovered before blood ethanol levels returned to base line.
Fig. 7.
A, time course of the electrophysiological response of MSA neurons to ethanol and saline in the freely moving rat. The number of rats responsible for the electrophysiological data was as follows: saline (n = 6) and 0.75 g/kg (n = 9), 1.5 g/kg (n = 8) and 3.0 g/kg of ethanol (n = 7). *P < .05 when compared to change in saline-treated rat. B, time course of the blood ethanol concentration after 3 doses of ethanol (0.75, 1.5 and 3.0 g/kg) in freely moving rats. Blood ethanol concentration was elevated significantly by all ethanol doses and except for the 0.75-g/kg dose, remained elevated through 240 min (P < .05). The blood ethanol concentration determinations were in a separate group of rats from those used for the electrophysiological recordings.
In addition to changes in the firing rate, the pattern of neural activity in the unanesthetized rats was also significantly affected by ethanol. In 71% (17 of 24) of cells tested at the 0.75- and 1.5-g/kg dose, the rhythmic pattern of firing was disrupted shortly after the ethanol injection, concurrent with the decrease in spontaneous activity. In 2 of 14 cells studied, the 3.0- g/kg dose did not affect the rhythmic pattern or the firing rate, although the rats were clearly sedated. The neural activity remained irregular until the firing frequency began to return to pre-ethanol base-line levels. This effect can be appreciated by comparing the ISIH of a cell before and after ethanol (fig. 8). After the cell returned to a normal firing rate (ca. 90 min postinjection), the rhythmically bursting pattern of activity also had returned to base line. However, the burst frequency was reduced in a dose-dependent manner from 6.3 bursts/sec during the base-line period to 4.7 to 5.6 bursts/sec after ethanol.
Fig. 8.
ISIH (A) for a rhythmically firing MSA neuron before and 30 min after the administration of ethanol and (B) the effect of 0.75, 1.5 and 3.0 g/kg of ethanol (n = 6 for each dose) on the rhythmicity of MSA neurons in freely moving rats. Rhythmicity was determined by the percentage of the total number of spikes that occur between 40 and 60 msec. P < .05 when compared to base line.
The effect of ethanol on the firing characteristics of lateral septal cells in anesthetized rats
Activation of cells in the lateral septum is followed by an inhibition in the firing of cells in the MSA (McLennan and Miller, 1974). To test the hypothesis that the decrease in MSA unit activity by ethanol results from a change in the activity of neurons in the lateral septum, single unit studies were carried out in urethane-anesthetized rats in the LSi (fig. 2). The base-line firing rate of LSi neurons was much slower than for cells in the MSA (13.4 spikes/sec vs. 27.1 spikes/sec) and cells were encountered less frequently (1.2 cells/track vs. 6.4 cells/track). The response of LSi neurons to ethanol was examined by monitoring the change in single unit activity over time after ethanol administration (see fig. 9). The firing rates of LSi cells exhibited more variability after ethanol (1.5 g/kg), but nonetheless remained similar to the firing rates of LSi cells after saline (fig. 9). The variability is accounted for by the slight increase in firing rate observed in three cells and a slight decrease in two cells after systemic administration of 1.5 g/kg of ethanol. The firing rate of the remaining six cells was essentially unchanged during the 2-hr postinjection period. In no case was a profound suppression of activity observed in these LSi cells, as was the case in MSA cells. Thus, in marked contrast to MSA cells, ethanol at a dose of 1.5 g/kg did not affect the normal electrophysiological characteristics of cells in the lateral septum (fig. 9).
Fig. 9.
Representative histogram of the effect of ethanol (1.5 g/kg) on the spontaneous activity of a neuron in the lateral septum of an anesthetized rat (A) and the course of the single unit response of cells in the latter septum (LSi) to ethanol (n = 11) or saline (n = 5) (B). P < .1 when ethanol treatment on LSI neural activity is compared to the saline effect.
Comparison of the time course of ethanol-induced changes in behavioral, functional and electrophysiological parameters with blood ethanol
In order to determine the time course of the behavioral effects of ethanol in rats, changes in ethanol-induced sedation were assessed. The aerial righting reflex was used as a behavioral measure of ethanol-induced sedation because it is sensitive to manipulations of the MSA (Breese et al., 1984). Rats that received a 1.5-g/kg dose of ethanol rapidly developed an impairment in aerial righting ability that remained maximal for 20 min then gradually recovered to normal over the next 60 min (fig. 10). In addition to measuring a behavioral change, changes over time in blood ethanol content and body temperature were measured after ethanol administration. From these measurements in unanesthetized rats, a time course of the changes for these parameters were plotted for the 3-hr postinjection time period after ethanol administration. Blood ethanol levels increased rapidly during the initial 40 min after ethanol administration then slowly decreased (fig. 10). Likewise, rectal temperature decreased after ethanol administration (1.5 g/kg) in freely moving rats over 60 min by 2.1 ± 0.21°C, but did not return to resting levels until after 150 min (data not shown). However, in contrast to the slow return of these latter measures to base line, MSA single unit activity had recovered by 90 min after ethanol administration (fig. 10; also fig. 7).
Fig. 10.
Comparison of the time course for aerial righting reflex (top), blood ethanol concentration (middle) and MSA single unit activity (bottom) after a systemic injection of 1.5 g/kg of ethanol. All blood ethanol concentration values are significant elevated over base line (P < .01). Values for aerial righting are elevated significantly (P < .05), except for the value at 120 min. Values for the percentage of inhibition are elevated significantly between 0 to 55 mm (P < .05), but not at 60 min and beyond (P < .1). See table 3 for analysis.
The correlation in the change over time between the various parameters was calculated using the Pearson’s correlation statistic. As can be seen in table 3, there was a significant correlation between blood ethanol levels and body temperature. The changes in both of these parameters developed slowly, reaching a maximum at approximately 60 min, then slowly returned to base-line levels. The lack of correlation between MSA unit activity and blood ethanol levels is notable. Although the depression of MSA neuronal activity lasts an average of 90 min, the blood ethanol concentration remains elevated long after the neurons return to a normal rate and pattern of firing. Thus, MSA cell activity is highly susceptible to disruption by ethanol, but this effect is only transient and then becomes tolerant to the presence of ethanol Finally, a significant correlation emerged from the comparison between single unit activity in the MSA and the height of the aerial righting reflex after 1.5 g/kg of ethanol. Approximately 92% of the aerial righting reflex impairment could be statistically accounted for by changes in single unit activity in the MSA.
TABLE 3. Pearson’s r coefficient for correlation of changes over time in behavioral and physiological measures after ethanol exposure.
There were six to eight rats in each of the categories.
| Category | Aerial Righting | Blood Ethanol | Rectal Temperature | Single Unit Activity |
|---|---|---|---|---|
| Aerial righting | 1.000 | |||
| Blood ethanol | 0.344 | 1.000 | ||
| Rectal temperature | −0.521 | −0.915* | 1.000 | |
| Single unit activity | −0.981** | −0.675 | 0.547 | 1.000 |
P< .05;
P < .01.
Discussion
Earlier work from our laboratory demonstrated that microinjection of bicuculline into the MSA would antagonize ethanol-induced sedation at a lower dose than when given systemically (Breese et al., 1984). The same is true for thyrotropin-releasing factor (Breese et al., 1984). Muscimol (30 ng) administered into the MSA enhanced ethanol-induced sleep-time (McCown et al., 1986), a change like that seen when a larger dose of muscimol was administered into the ventricular system of rats (Frye et al., 1982). Such data indicate that this is a site at which these drugs act to influence the action of ethanol and provided strong inferential evidence that the MSA is an important site in the sedation produced by ethanol administration. The results from the present series of experiments demonstrate that ethanol has well-defined effects on the neural activity of MSA cells in support of this conclusion.
Initial studies to examine the effects of ethanol on electrophysiological activity in the MSA were performed in anesthetized rats to allow comparison with previous data obtained from anesthetized rats. In order to assure the use of an anesthetic having minimal effects on MSA neural function, the action of two commonly used anesthetics on MSA activity were compared. This work demonstrated that urethane was superior to chloral hydrate for evaluating MSA neural activity. Another practical aspect of this latter work was that the data collected established a useful anesthetic for investigations to evaluate the effects of various neurotransmitters applied to cells in the MSA by iontophoresis with and without ethanol treatment (see Givens and Breese, 1988, 1989, 1990).
In the anesthetized rat, subsequent studies demonstrated that ethanol suppressed spontaneous discharge and disrupted the rhythmically bursting pattern of neurons in the MSA in a dose-dependent manner. In order to assure that the anesthetic was not influencing the changes in MSA neural activity caused by ethanol, recordings were made from the MSA in unanesthetized rats with and without ethanol treatment. Because similar effects of ethanol were observed in both urethane-anesthetized and unanesthetized rats, the effects of ethanol observed on neural activity in the MSA in anesthetized rats cannot be attributed to an interaction with the anesthetic.
In contrast to the reduced neural activity induced by ethanol in the MSA, ethanol produced no reliable change in the spontaneous firing rate or in the pattern of firing of neurons in the LSi in rats anesthetized with urethane. Previous studies have established that activation of afferent inputs to the lateral septum can inhibit neural activity in the MSA (McLennan and Miller, 1974). Thus, the lack of effect of ethanol on cellular activity in the lateral septum indicates that ethanol does not act to suppress neural activity in the MSA by increasing neural activity in the lateral septum. Givens and Breese (1989, 1990) reported recently that ethanol enhances GABA inhibition in the MSA but not in the lateral septum. These data demonstrating an absence of action of ethanol in the lateral septum with potent effects on the function of neurons in the MSA reinforce the view that ethanol can have differing effects in these two brain areas.
Unit activity in the MSA was correlated over time with the behavioral sedation, but not with the hypothermia produced by ethanol. These data provide support for the view that the brain site responsible for ethanol-induced hypothermia is different from that responsible for sedation. The close correlation between unit activity in the MSA and behavioral sedation is consistent with the view that the MSA modulates this behavioral manifestation of ethanol intoxication. Therefore, if the central actions of ethanol are to be defined, it is essential to investigate the effect of ethanol at a site in brain at which ethanol has a defined action and which has a relation to a deficiency caused by ethanol.
In a few of the rhythmically bursting cells studied, the rhythmic pattern of activity was not altered by ethanol. These cells were located primarily in the vertical limb of the diagonal band (data not presented). Because the rhythmic firing pattern of these cells was very stable, it is possible that these cells correspond to a subpopulation of cells in the MSA described by Brazhnik et al. (1985) as hyperstable. These particular cells found to resist ethanol are proposed to be the bursting pacemaker cells that induce bursting activity in a larger population of cells in the MSA. If indeed this is the case, it could be concluded that ethanol is affecting the function of the MSA by acting directly on the larger population of cells in the MSA or by disrupting the synaptic network linking these units to the pacemaker cells. Once “disengaged” from the network by ethanol the cells sensitive to ethanol have an irregular firing pattern. Additional work will be required to resolve this issue.
There are several reports indicating that blood ethanol levels do not always relate to the degree of functional disruption (i.e., Le Blanc et al., 1975). A similar phenomenon of “acute tolerance” to ethanol was observed in this investigation. Blood ethanol levels remained elevated at a time when righting reflex and single unit activity had recovered from the ethanol treatment. Although the action of ethanol on firing rate and pattern showed acute tolerance to ethanol, no such tolerance to the effect of ethanol on burst frequency was observed and tolerance to the hypothermia produced by ethanol was not apparent. This acute tolerance of neural activity to ethanol was apparent in both anesthetized and unanesthetized rats. It would be of considerable interest to know the neural basis of this acute tolerance to ethanol and to determine whether this change contributes to the behavioral tolerance associated with chronic ethanol administration.
Rhythmically bursting neurons of the MSA have a modulatory influence on the physiology of the hippocampus and other brain regions (Lamour et al., 1984). Traditionally, the MSA has been associated with behavioral “arousal,” in that it mediates the synchronized hippocampal EEG activity that characterizes waking alert states and REM sleep stages (Green and Arduini, 1954). The septohippocampal pathway has also been implicated in a number of important behavioral phenomena including learning and memory (Olton, 1977) as well as behavioral inhibition (Gray, 1982). Normal activity in the MSA is critically important to proper functioning in the hippocampus and in other limbic target sites. Ethanol-induced impairments in behaviors mediated by these systems (i.e., sedation, reactivity, memory and behavioral inhibition) may result from a suppression of the phasic drive initiated by the MSA cells, in as much as the present investigation demonstrated that ethanol has a potent action on the cells in the MSA. This possibility should receive attention in future experiments, as ethanol is known to influence many of these behavioral responses associated with MSA function.
Acknowledgments
The authors acknowledge the excellent manuscript preparation by Ms. Doris Lee and the advice of Drs. Celeste Napier, Thomas McCown and Hugh Criswell during the course of these investigations.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- MSA
medial septal area
- ISIH
interspike interval histogram
- LSi
intermediate zone of the lateral septum
Footnotes
This work was supported by North Carolina Alcoholism Research Authority Fellowship No. 8611 and U.S. Public Health Service Grant NS-21345. Preliminary reports were presented at the Annual Meeting of the Society for Neuroscience (1988) and the Research Society on Alcoholism (1989).
References
- Brazhnik ES, Vingradova OS, Karanov AM. Frequency modulation of neuronal theta-bursts in rabbits septum by low-frequency repetitive stimulation of the afferent pathways. Neuroscience. 1985;14:501–508. doi: 10.1016/0306-4522(85)90305-7. [DOI] [PubMed] [Google Scholar]
- Breese GR, Frye GD, McCown TJ, Mueller RA. Comparison of CNS effects induced by TRH and bicuculline after microinjection into medial septum, substantia nigra and inferior colliculus: Absence of support for a GABA antagonist action for TRH. Pharmacol Biochem Behav. 1984;21:145–149. doi: 10.1016/0091-3057(84)90144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Givens BS, McCown TJ, Criswell HE. Strategy for investigating functions altered by ethanol at specific sites in brain: Evidence that ethanol influences GABA-benzodiazepine receptor complex function. In: Kuriyma K, Takada A, Ishii H, editors. Biomedical and Social Aspects of Alcohol and Alcoholism. Elsevier; Amsterdam: 1988. pp. 273–276. [Google Scholar]
- Deadwyler SA, Biela J, Rose G, West M, Lynch GA. A microdrive for use with glass or metal microelectrodes in recording from freely moving rats. Electroencephalogr Clin Neurophysiol. 1979;47:752–754. doi: 10.1016/0013-4694(79)90304-3. [DOI] [PubMed] [Google Scholar]
- Dutar P, Lamour Y, Jobert A. Activation of identified septo-hippocampal neurons by noxious peripheral stimulation. Brain Res. 1985;328:15–21. doi: 10.1016/0006-8993(85)91317-4. [DOI] [PubMed] [Google Scholar]
- Frye GD, Breese GR. GABAergic modulation of ethanol-induced motor impairment. J Pharmacol Exp Ther. 1982;223:750–756. [PMC free article] [PubMed] [Google Scholar]
- Frye GD, Chaplin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: A comparison with ethanol. J Pharmacol Exp Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- Givens BS, Breese GR. Ethanol suppresses activity of rhythmically bursting neurons of the medial septal area in freely moving rats. Soc Neurosci Abstr. 1988;14:193. [Google Scholar]
- Givens BS, Breese GR. Enhancement of GABA-mediated inhibition by ethanol occurs selectively in the medial septal area. Alcohol Clin Exp Res. 1989;12:309. [Google Scholar]
- Givens BS, Breese GR. Site-specific enhancement of GABA-mediated inhibition of neural activity by ethanol in the rat medial septal area. J Pharmacol Exp Ther. 1990 in press. [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The Neuropsychology of Anxiety: An Inquiry into the Functions of the Septo-Hippocampal System. Oxford University Press; New Tork: 1982. pp. 102–114. [Google Scholar]
- Green JD, Arduini A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A. Septo-hippocampal and other medial septum-diagonal band neurons: Electrophysiological and pharmacological properties. Brain Res. 1984;309:227–239. doi: 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Kalant H, Gibbins RJ. Acute tolerance to ethanol in the rat. Psychopharmacology. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- Lilequist S, Engel J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology. 1982;78:71–75. doi: 10.1007/BF00470592. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of γ-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Frye GD, Breese GR. Evidence for site specific ethanol actions In the CNS. Alcohol Drug Res. 1986;6:423–429. [PubMed] [Google Scholar]
- McLennan H, Miller JJ. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol (Lond) 1974;237:607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region of the rat. J Comp Neurol. 1979;188:621–656. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]