Abstract
The first total synthesis of the marine cyclopropane fatty acid (±)-17-methyltrans- 4,5-methyleneoctadecanoic acid was accomplished in 8 steps and in 9.1% overall yield starting from 1-bromo-12-methyltridecane. The cis analog (±)-17- methyl-cis-4,5-methyleneoctadecanoic acid was also synthesized but in 7 steps and in 16.4% overall yield. With the two isomeric cyclopropane fatty acids at hand it was possible to unequivocally corroborate the trans relative configuration of the naturally occurring fatty acid by gas chromatographic co-elution of the corresponding methyl esters. The cis isomer was cytotoxic to Leishmania donovani promastigotes with an IC50 of 300.2 ± 4.2 µM.
Keywords: Antileishmanial activity, Cyclopropane fatty acids, Sponges, Synthesis
Cyclopropane fatty acids (CFAs) are widespread in nature and they have been identified in many organisms ranging from bacteria to seed oils.1 The earliest known example is lactobacillic acid (cis-11,12-methyleneoctadecanoic acid), but several structural variants have been isolated since.1 One interesting compound is the 17- methyl-cis-9,10-methyleneoctadecanoic acid, from the protozoan Herpetomonas megaseliae, which incorporates both methyl and cyclopropyl branching in the chain.2 While most of the known CFAs incorporate a cis cyclopropyl group in the acyl chain, just a few trans CFAs are known, such as the recently discovered 17- methyl-trans-4,5-methyleneoctadecanoic acid (1a) and the 18-methyl-trans-4,5- methylenenonadecanoic acid, which were identified in the phospholipids of the Caribbean sponge Pseudospongosorites suberitoides.3 These marine fatty acids are quite interesting since they incorporate an unusual trans 4,5-cyclopropane in addition to iso methyl branching. However, the characterization of 1a in the sponge extract was done by gas chromatography-mass spectrometry on suitable volatile derivatives followed by 1H NMR of the total mixture of fatty acids. Therefore, a more rigorous confirmation of the structure of 1a is warranted. For this purpose, a total synthesis of 1a would not only serve to confirm the unusual trans cyclopropyl arrangement of the natural fatty acid, but also to report the total characterization of 1a, as well as to provide the necessary expertise to synthesize analogs for biological screening. Therefore, herein we report the first total synthesis of both the naturally occurring (±)-17-methyl-trans-4,5-methyleneoctadecanoic acid (1a) and the corresponding cis analog 1b together with the first studies of the antileishmanial activity of these CFAs.
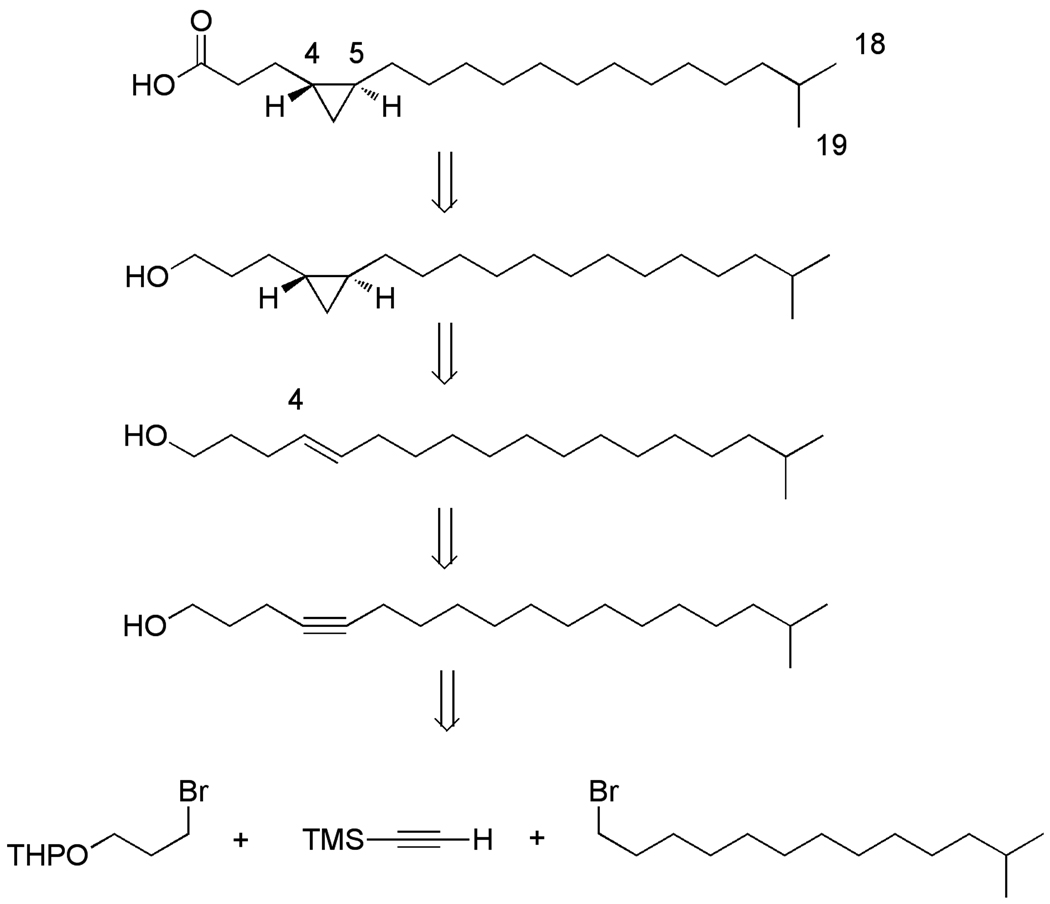
A retrosynthetic analysis aimed at the synthesis of 1a is outlined is Scheme 1. The trans cyclopropane fatty was envisioned as arising from a trans olefin with the right chain length via a Simmons-Smith reaction.4 The trans olefin, on the other hand, can be made from the corresponding alkyne using the standard sodium (Na) in ammonia (NH3) reduction. A more elaborate construction is expected to be the introduction of the iso functionality in 1a by means of the 1-bromo-12- methyltridecane, but the latter compound has been synthesized before in two steps starting from 2-bromopropane.5
Scheme 1.
Retrosynthetic analysis towards the (±)-17-methyl-trans-4,5- methyleneoctadecanoic acid.
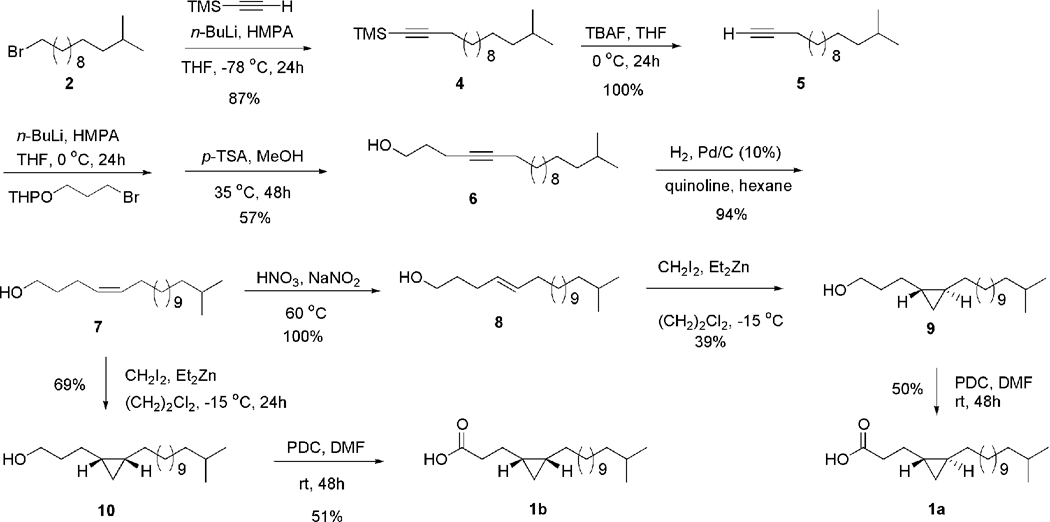
Our synthesis for the (±)-17-methyl-trans-4,5-methyleneoctadecanoic acid (1a) from the known 1-bromo-12-methyltridecane (2) is shown in Scheme 2. The first part of the synthesis called for the preparation of the key intermediate 17- methyloctadec-4-yn-1-ol (6), which can serve as precursor for both the trans and cis cyclopropane fatty acids 1a and 1b (Scheme 2). For the introduction of the unsaturation at C-4, the (trimethylsilyl)acetylene was again used and it was coupled to 2 using n-BuLi in THF-HMPA at -78°C resulting in the trimethyl(14- methylpentadec-1-ynyl)silane 4 in an 87% yield. Desilylation of 4 with TBAF in THF at 0 °C yielded, in an almost quantitative yield, 14-methylpentadec-1-yne (5). The next step was the introduction of the precursor of the carboxy group at C-1 by coupling 5 with the 2-(3-bromopropyloxy)-tetrahydro-2H-pyran by using n-BuLi in THF-HMPA at 0 °C (higher temperature for solubility reasons). In a subsequent step the tetrahydropyranyl group was removed using the standard procedure of catalytic amounts of p-TSA in methanol at 35 °C for 48 h, which yielded the desired 17- methyloctadec-4-yn-1-ol (6) in a 57% yield for the two latter steps.
Scheme 2.
Synthesis of the (±)-17-methyl-trans-4,5-methyleneoctadecanoic acid (1a) and the (±)-17-methyl-cis-4,5-methyleneoctadecanoic acid (1b).
The final steps of the synthetic plan required using the alkyne in 6 to introduce both the trans and cis double bonds needed for the synthesis of the cyclopropanes 1a and 1b. Initially, the transformation of 6 into the (E)-17-methyloctadec-4-en-1-ol (8) was attempted with the classical dissolving metal reduction conditions of Na in liquid NH3. However, all attempts to effectively carry out this transformation resulted in partial conversion of 6 into 8, probably due to the long alkyl chains. Failure to achieve a 100% reduction of 6 resulted in the need to effect a very difficult chromatographic separation of 6 and 8, which was not practical. It was then decided to take a different route. Compound 6 was hydrogenated in hexane using H2 under Lindlar catalysis, which afforded the (Z)-17-methyloctadec-4-en-1-ol (7) in a 94% yield. The desired (E)-17-methyloctadec-4-en-1-ol (8) was effectively obtained by stereomutation of 7 with sodium nitrite-nitric acid in water at 60 °C.6 This stereomutation worked quite well for this substrate and resulted in a quantitative yield of 8 from 7. Alkenol 7 will also be used to prepare the corresponding cis cyclopropane fatty acid 1b.
With the needed alkenols 7 and 8 at hand the cyclopropane ring was incorporated into the acyl chain by using the Simmons-Smith protocol, i.e., diethyl zinc and diiodomethane in 1,2-dichloroethane under an argon atmosphere at -15 °C.4 Under these conditions the 17-methyl-trans-4,5-methyleneoctadecan-1-ol (9) was obtained in a 39% yield from 8. The low yield in this reaction was due to the side-reaction of methylation of the alcohol resulting in the undesired methoxylated product. Attempts to protect the alcohol functionality in 8 with silyl protecting groups resulted in no reaction or very low yields of cyclopropanation. However, enough material of 9 was obtained by direct cyclopropanation of 8 to pursue the synthetic plan further. Final oxidation of 9 with pyridinium dichromate (PDC) in dimethylformamide (DMF) under an argon atmosphere resulted in a 50% yield of the desired trans acid 1a.7 Identical conditions were also used to obtain 1b from 7. This means that cyclopropanation of 7 under the same Simmons-Smith conditions described above resulted in a 69% yield of the 17-methyl-cis-4,5- methyleneoctadecan-1-ol (10) and further oxidation to the acid with PDC in DMF yielded the expected (±)-17-methyl-cis-4,5-methyleneoctadecanoic acid (1b) in a 51% yield.8
With both acids 1a and 1b at hand we were in a good position to unequivocally corroborate the relative trans cyclopropane stereochemistry as well as the structure of the natural fatty acid 1a that was assigned on the basis of 1H-NMR spectroscopy on the whole fatty acid mixture from the sponge P. suberitoides.3 This was done by gas chromatographic co-injection of the corresponding methyl esters of 1a and 1b, prepared from the acids by esterification with MeOH and catalytic amounts of HCl, with the fatty acid methyl ester mixture from the phospholipids of the sponge P. suberitoides.3 In this experiment the methyl ester of synthetic 1a co-eluted (in a HP- 5MS capillary column) with the natural cyclopropane methyl ester (ECL = 19.15), thus unequivocally confirming the structure of the natural fatty acid as well as its trans 4,5-cyclopropane stereochemistry.
We had previously shown that the iso methyl-branched monounsaturated fatty acid (Z)-17-methyl-13-octadecenoic acid displays antileishmanial activity towards Leishmania donovani promastigotes with an EC50 = 19.8 ± 7.0 µg/ml and, as a probable intramolecular target, inhibits the leishmania DNA topoisomerase IB enzyme at concentrations of 50 µM.9 Given these previous results we decided to test the cis cyclopropane fatty acid 1b against L. donovani promastigotes and establish how cyclopropane substitution compares to monunsaturation in determining the antileishmanial activity of these iso-C18 fatty acids.10 It was found that acid 1b was cytotoxic to L. donovani promastigotes at an IC50 = 300.2 ± 4.2 µM and it did not inhibit the leishmania DNA topoisomerase IB enzyme. Therefore, monounsaturation is more effective than cyclopropanation with respect to increasing the cytotoxicity of these iso-C18 fatty acids towards L. donovani. It is important to mention that chain length also plays a role in the antileishmanial activity of these CFAs. The longer chain analog (±)-18-methyl-cis-4,5-methylenenonadecanoic acid, also synthesized by us following a similar route as that described in Scheme 2, displayed no activity against the L. donovani promastigotes (IC50 > 1000 µM). Therefore, other shorter chain analogs could be synthesized in order to find the optimum chain length for antileishmanial activity. The synthetic route reported herein will facilitate the preparation of these analogs.
Acknowledgements
The project described was supported by Award Number SC1GM084708 from the National Institutes of General Medical Sciences of the NIH. We thank Dr. Fred Strobel (Emory University) for the high resolution mass spectral data. This research was also partially supported by a grant (Gr238) from Junta de Castilla y León, PS09/00448, and the Tropical Diseases Network (RICET) from Ministerio de Salud y Consumo (SPAIN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Grogan DW, Cronan JE., Jr Microbiol. Mol. Biol. Rev. 1997;61:429. doi: 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fish WR, Holz GG, Jr, Beach DH, Owen E, Anekwe GE. Mol. Biochem. Parasitol. 1981;3:103. doi: 10.1016/0166-6851(81)90010-4. [DOI] [PubMed] [Google Scholar]; (c) Gontier E, Boussouel N, Terrasse C, Jannoyer M, Menard M, Thomasset B, Bourgaud F. Biochem. Soc. Trans. 2000;28:578. [PubMed] [Google Scholar]; (d) Cox AD, Wilkinson SG. Biochim. Biophys. Acta. 1989;1001:60. doi: 10.1016/0005-2760(89)90307-x. [DOI] [PubMed] [Google Scholar]
- 2.Holz GG, Jr, Beach DH, Singh BN, Fish WR. Lipids. 1983;18:607. doi: 10.1007/BF02534670. [DOI] [PubMed] [Google Scholar]
- 3.Carballeira NM, Montano N, Vicente J, Rodriguez AD. Lipids. 2007;42:519. doi: 10.1007/s11745-007-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Simmons HE, Smith RD. J. Am. Chem. Soc. 1958;80:5323. [Google Scholar]; (b) Simmons HE, Smith RD. J. Am. Chem. Soc. 1959;81:4256. [Google Scholar]
- 5.Mun JY, Onorato A, Nichols FC, Morton MD, Saleh AI, Welzel M, Smith MB. Org. Biomol. Chem. 2007;5:3826. doi: 10.1039/b712707c. [DOI] [PubMed] [Google Scholar]
- 6.(a) Bumpus FM, Taylor WR, Strong FM. J. Am. Chem. Soc. 1950;72:2116. [Google Scholar]; (b) Duffy PE, Quinn SM, Roche HM, Evans P. Tetrahedron. 2006;62:4838. [Google Scholar]
- 7.Spectral data for the (±)-17-methyl-trans-4,5-methyleneoctadecanoic acid (1a): transparent oil, IR (neat) νmax 3500-2500, 2923, 2853, 1711 (C=O), 1464, 1383, 1365, 1274, 1120, 1073, 1039, 737 cm−1; 1H NMR (CDCl3, 300 MHz) δ 2.42 (2H, t, J = 7.4 Hz, H-2), 1.65-1.51 (3H, m, H-3, H-17), 1.27 (21H, m, -CH2-), 1.16 (2H, m, - CH2-, H-16), 0.86 (6H, d, J = 6.6 Hz, H-18, H-19), 0.44 (2H, m, H-4, H-5), 0.21 (2H, t, J = 6.1 Hz, CH2 in cp ring); 13C NMR (CDCl3, 75 MHz) δ 177.63 (s, C-1), 39.04 (t, C-16), 34.09 (t, C-6), 30.33 (t, C-2), 29.94 (t), 29.68 (t), 29.55 (t), 29.51 (t), 29.90 (t), 27.95 (d, C-17), 27.41 (t), 25.43 (t), 22.65 (q, C-18, C-19), 18.90 (d, C-4), 11 18.06 (d, C-5), 11.78 (t, CH2 in cp ring). HRMS (APCI): calcd for C20 H37O2 [M+ −1] 309.2799, found 309.2798.
- 8.Spectral data for the (±)-17-methyl-cis-4,5-methyleneoctadecanoic acid (1b): transparent oil, IR (neat) νmax 3500-2500, 2922, 2852, 1709 (C=O), 1459, 1382, 1365, 1274, 1078, 1039, 721 cm−1; 1H NMR (CDCl3, 300MHz) δ 2.45 (2H, t, J = 7.6 Hz, H-2), 1.71 (1H, m, H-18), 1.51 (2H, m, H-3), 1.26 (21H, m, -CH2-), 1.15 (2H, m, -CH2-, H-16), 0.86 (6H, d, J = 6.6Hz, H-18, H-19), 0.71 (2H, m, H-4, H-5), 0.60 (1H, m, one CH2 in cp ring), -0.26 (1H, m, one CH2 in cp ring); 13C NMR (CDCl3, 75MHz) δ 179.19 (s, C-1), 39.05 (t, C-16), 34.50 (t, C-6), 30.33 (t, C-2), 30.15 (t, C-3), 29.94 (t), 29.78 (t), 29.70 (t), 29.68 (t), 28.57 (t), 27.96 (d, C-17), 27.42 (t, C-7), 24.10 (t), 22.66 (q, C-18, C-19), 15.97 (d, C-4), 15.10 (d, C-5), 10.77 (t, CH2 in cp ring). HRMS (APCI): calcd for C20 H37O2 [M+ −1] 309.2799, found 309.2798.
- 9.Carballeira NM, Montano N, Balaña-Fouce R, Fernández Prada C. Chem. Phys. Lipids. 2009;161:38. doi: 10.1016/j.chemphyslip.2009.06.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For experimental details on the antileishmanial testing on L. donovani (MHOM/ET67/L82 strain) promastigotes see reference 9 above.